Preliminary Phytochemical and Biological Evaluation of Rudbeckia hirta Flowers
Abstract
1. Introduction
2. Results
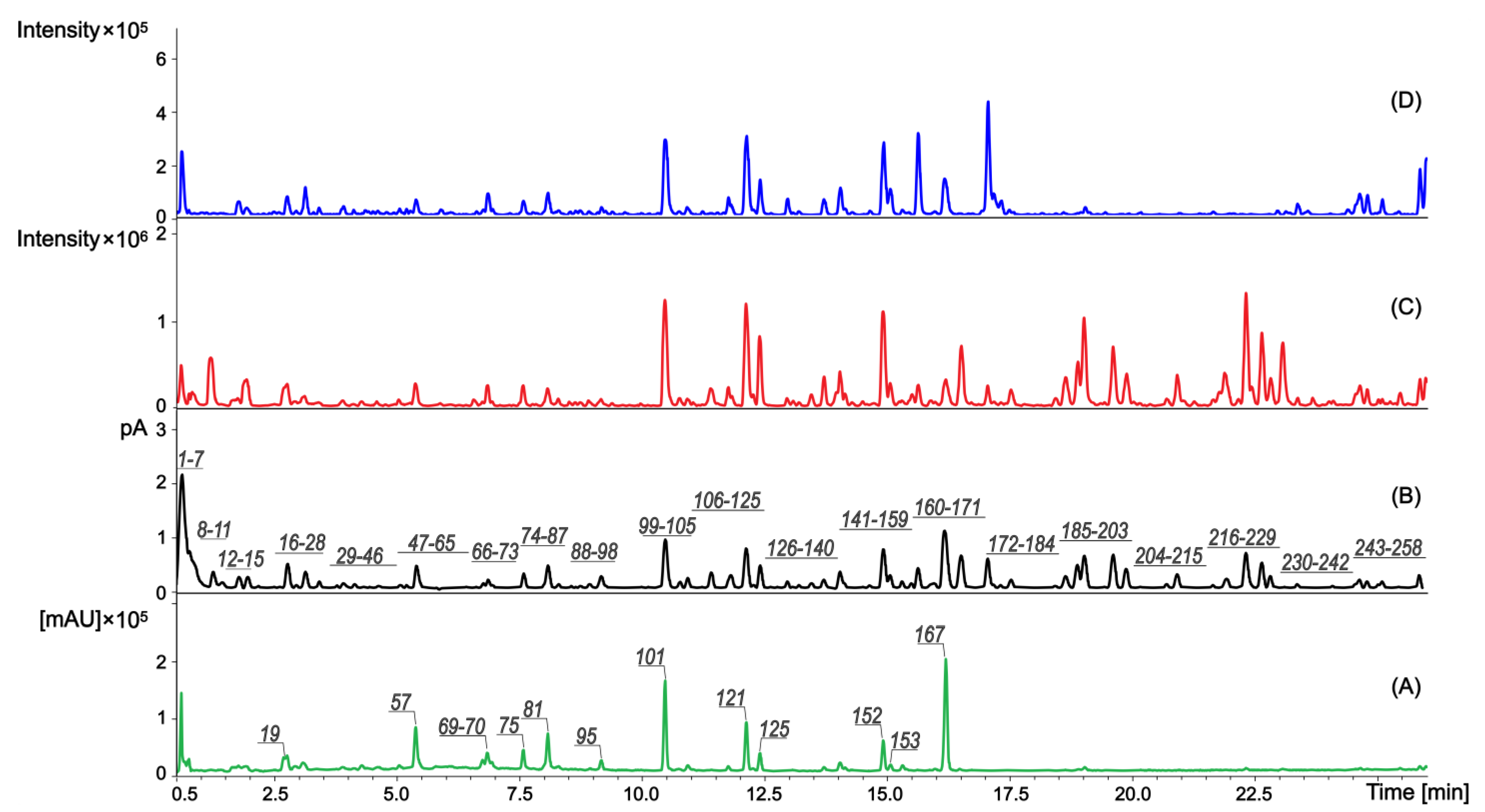
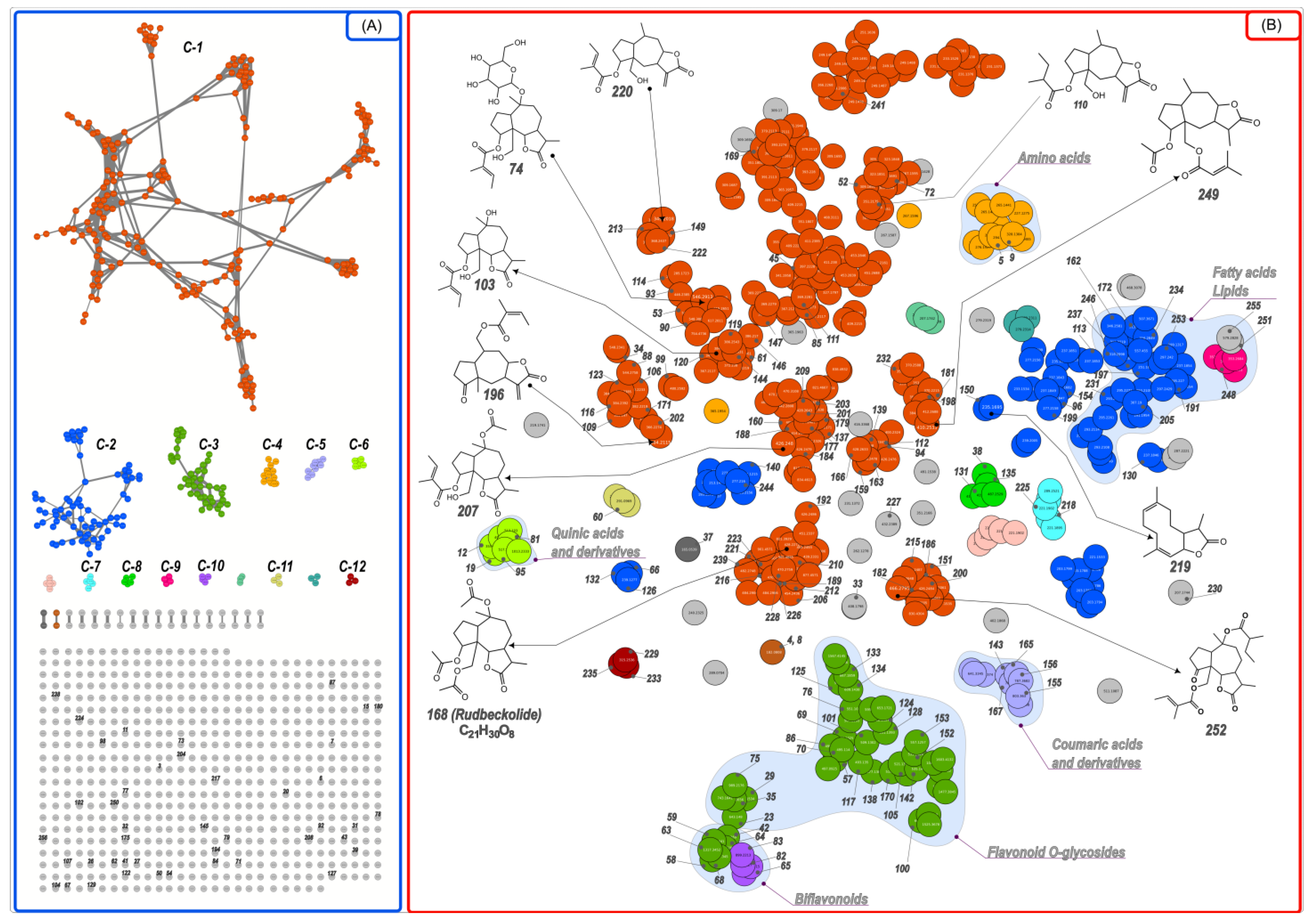
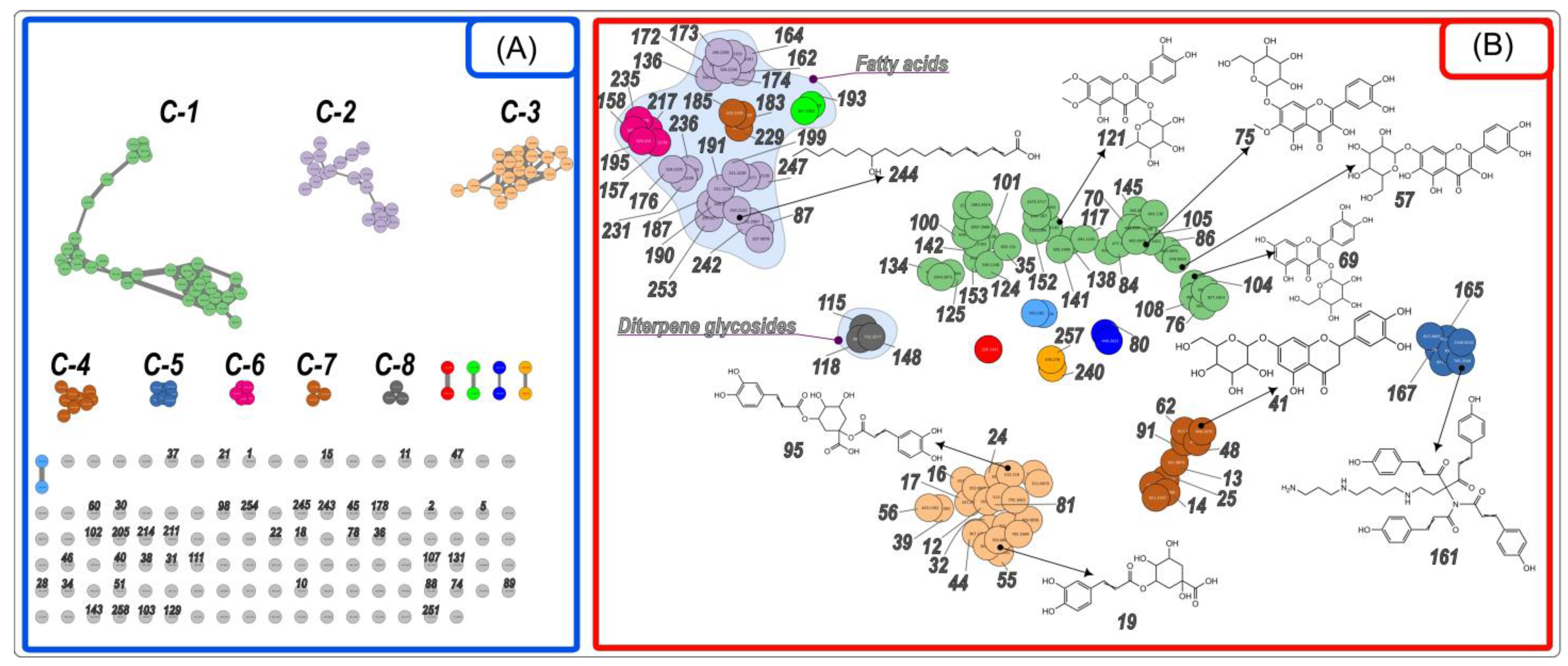
2.1. Identification of Compounds Found in the Extract
2.2. Antioxidant Assays
2.3. Antimicrobial Testing
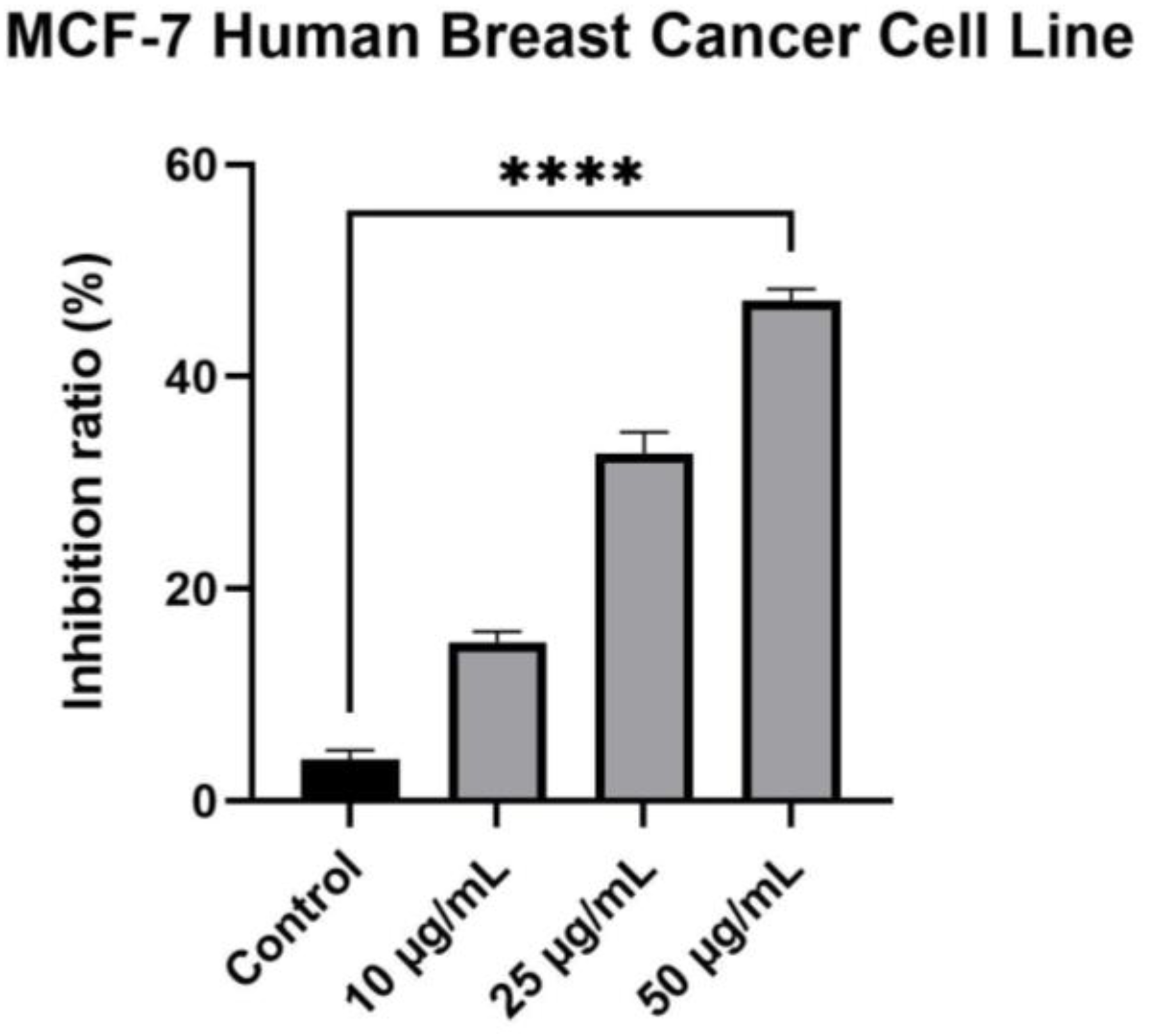
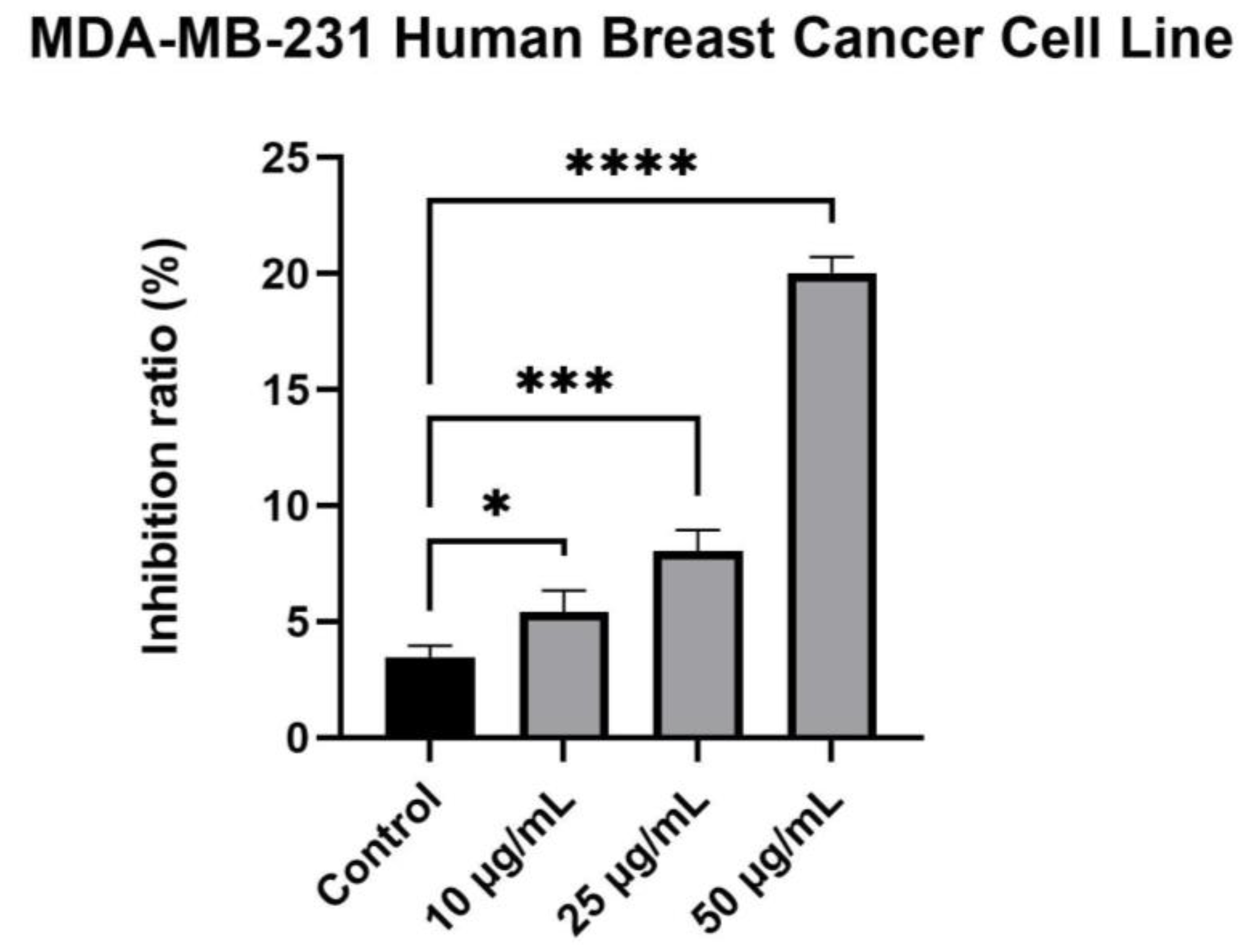
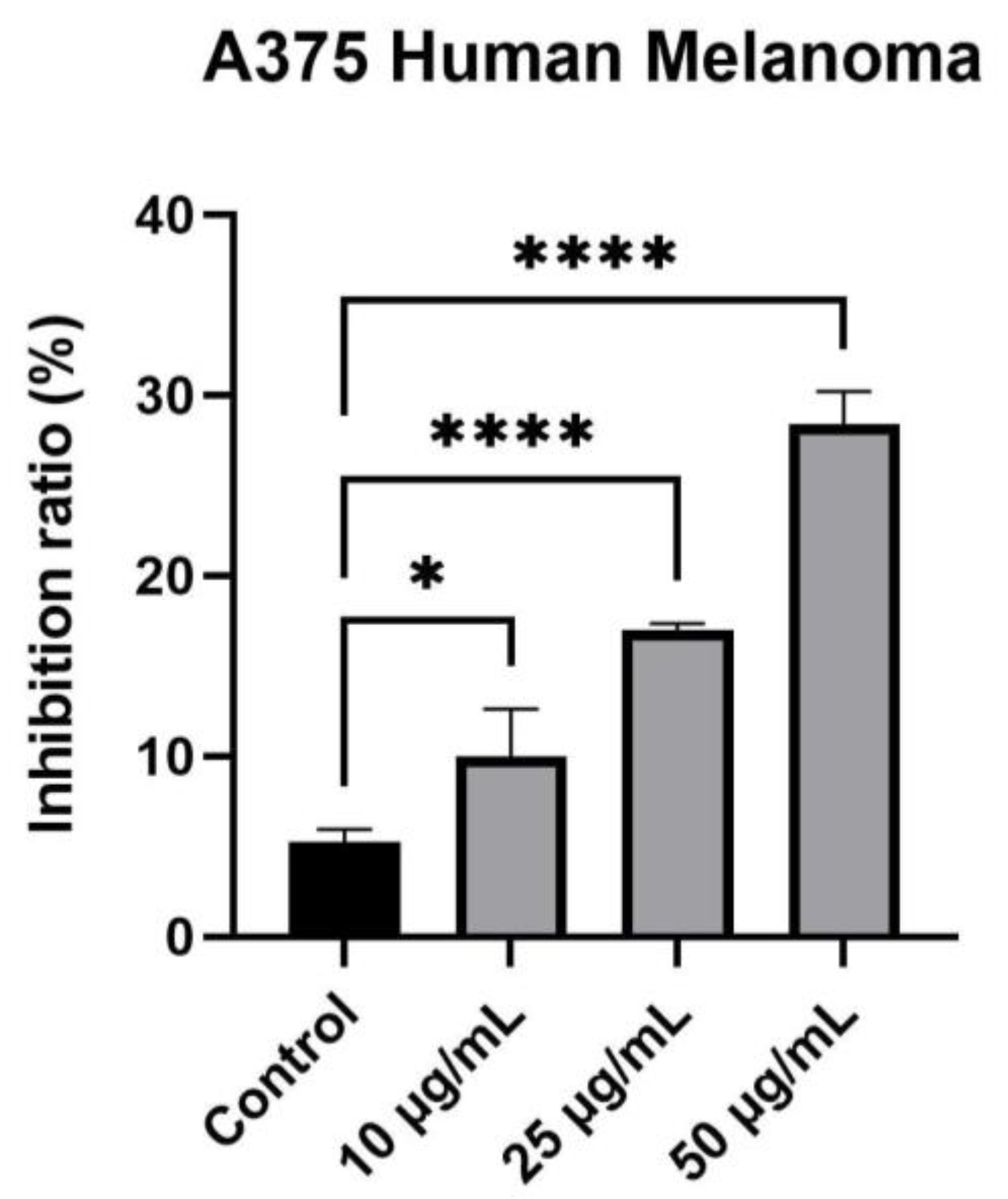
2.4. Cytotoxicity Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extract Preparation
4.3. LC-MS and Molecular Networking
4.4. Antioxidant Activity Assays
4.4.1. Lipoxygenase Inhibition
4.4.2. Iron Chelation Capacity
4.5. Antimicrobial Testing
4.5.1. Antimicrobial Susceptibility Testing
4.5.2. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
4.6. Cytotoxicity Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CAD | Charged aerosol detector |
| DAD | Diode array detector |
| DMSO | Dimethyl sulfoxide |
| EC50 | Efficient concentration 50 |
| EGF | Epidermal growth factor |
| ER | Estrogen receptor |
| ESI | Electrospray Ionisation |
| GNPS | Global Natural Products Social |
| HRMS | high-resolution mass spectrometry |
| IC50 | Inhibition concentration 50 |
| LC-MS | Liquid chromatography–mass spectrometry |
| LOX | Lipoxygenase |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MS | Mass spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PDA | Photodiode Array Detector |
| Q-TOF | Quadrupole Time-of-Flight |
| t-SNE | T-distributed stochastic neighbor embedding |
| UHPLC-HR-MS | Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry |
References
- Integrated Taxonomic Information System (ITIS). Available online: http://www.itis.gov (accessed on 23 January 2023).
- Royal Horticultural Society. RHS Encyclopedia of Plants and Flowers, 5th ed.; Brickell, C., Ed.; Dorling Kindersley: London, UK, 2010. [Google Scholar]
- Wells, D. 100 Flowers and How They Got Their Names; Algonquin Books: Chapel Hill, NC, USA, 1997. [Google Scholar]
- Greenhouse, J.; Markos, S.; Moe, R.L.; Simono, S.; Wetherwax, M.; Vorobik, L.A. The Digital Jepson Manual, 2nd ed.; Baldwin, B.G., Goldman, D.H., Keil, D.J., Patterson, R., Rosatti, T.J., Wilken, D.H., Eds.; University of California Press: Oakland, CA, USA, 2012; ISBN 9780520253124. [Google Scholar]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the Potential of Under-Utilised Plants from the Asteraceae Family as a Source of Natural Antimicrobial and Antioxidant Extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L. Asteraceae. In Identification and Control of Common Weeds: Volume 3; Springer: Singapore, 2017; pp. 441–721. ISBN 978-981-10-5402-0. [Google Scholar]
- Harkess, R.L.; Lyons, R.E. Rudbeckia hirta L.: A Versatile North American Wildflower. Hortscience 1994, 29, 226–227. [Google Scholar] [CrossRef]
- USDA, NRCS. The PLANTS. National Plant Data Team, Greensboro, NC USA. 2023. Available online: https://plants.usda.gov/home/plantProfile?symbol=RUHI2 (accessed on 15 March 2023).
- Glimn-Lacy, J.; Kaufman, P.B. Botany Illustrated, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-0-387-28870-3. [Google Scholar]
- Branhagen, A. Native Plants of the Midwest: A Comprehensive Guide to the Best 500 Species for the Garden; Timber Press: Portland, OR, USA, 2016. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4200-8044-5. [Google Scholar]
- Native American Ethnobotany. A Database of Foods, Drugs, Dyes and Fibers of Native American Peoples, Derived from Plants. Available online: http://naeb.brit.org/uses/species/3476/ (accessed on 15 March 2023).
- Setzer, W. The Phytochemistry of Cherokee Aromatic Medicinal Plants. Medicines 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Mircea, C.; Cioancă, O.; Draghia, L.; Hăncianu, M. Morphological Characteristics and Polyphenol Variations in Rudbeckia hirta L. Rom. Biotechnol. Lett. 2015, 20, 10688–10695. [Google Scholar]
- Guillet, G.; Philogène, B.J.R.; O’Meara, J.; Durst, T.; Arnason, J.T. Multiple Modes of Insecticidal Action of Three Classes of Polyacetylene Derivatives from Rudbeckia hirta. Phytochemistry 1997, 46, 495–498. [Google Scholar] [CrossRef]
- Constabel, C.P.; Balza, F.; Towers, G.H.N. Dithiacyclohexadienes and Thiophenes of Rudbeckia hirta. Phytochemistry 1988, 27, 3533–3535. [Google Scholar] [CrossRef]
- Michael, B.R.; Gedara, S.R.; Amer, M.M.A.; Stevenson, L.; Ahmed, A.F. A New Highly Oxygenated Pseudoguaianolide with 5-LOX Inhibitory Activity from Rudbeckia hirta L. Flowers. Nat. Prod. Res. 2013, 27, 2281–2285. [Google Scholar] [CrossRef]
- Vardeman, E.; Lyles, J.T.; Quave, C.L. The Genus Rudbeckia: A Critical Review of Its Traditional Medicinal Uses, Phytochemistry, and Pharmacology. J. Herb. Med. 2022, 31, 100530. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Olivon, F.; Elie, N.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MetGem Software for the Generation of Molecular Networks Based on the T-SNE Algorithm. Anal. Chem. 2018, 90, 13900–13908. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- El-Din, M.I.G.; Fahmy, N.M.; Wu, F.; Salem, M.M.; Khattab, O.M.; El-Seedi, H.R.; Korinek, M.; Hwang, T.-L.; Osman, A.K.; El-Shazly, M.; et al. Comparative LC–LTQ–MS–MS Analysis of the Leaf Extracts of Lantana camara and Lantana montevidensis Growing in Egypt with Insights into Their Antioxidant, Anti-Inflammatory, and Cytotoxic Activities. Plants 2022, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.; Macias, F.A.; Urbatsch, L.E.; Fischeri, N.H. Sesquiterpenes from Rudebeckia grandiflora. Phytochemistry 1988, 27, 2195–2198. [Google Scholar] [CrossRef]
- Herz, W.; Kumar, N.; Blount, J.F. Antileukemic C-15-Functionalized Ambrosanolides from Rudbeckia mollis. J. Org. Chem. 1981, 46, 1356–1361. [Google Scholar] [CrossRef]
- Vasquez, M.; Quijano, L.; Urbatsch, L.E.; Fischer, N.H. Sesquiterpene Lactones and Other Constituents from Rudbeckia mollis. Phytochemistry 1992, 31, 2051–2054. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhu, X.F.; Wang, X.N.; Shen, T.; Xiang, F.; Lou, H.X. Flavonoids from Malus hupehensis and Their Cardioprotective Effects against Doxorubicin-Induced Toxicity in H9c2 Cells. Phytochemistry 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Gujer, R.; Magnolato, D.; Self, R. Glucosylated Flavonoids and Other Phenolic Compounds from Sorghum. Phytochemistry 1986, 25, 1431–1436. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, J.M.; He, Y.N.; Lin, Z.W.; Sun, H.D. Eleven New Eudesmane Derivatives from Laggera pterodonta. J. Nat. Prod. 1997, 60, 545–549. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Iscan, G.; Hashimoto, T.; Demirci, F.; Noma, Y.; Asakawa, Y. The Essential Oil Constituents and Antimicrobial Activity of Anthemis aciphylla BOISS. Var. Discoidea BOISS. Pharm. Bull. 2006, 54, 222–225. [Google Scholar] [CrossRef]
- Łuczkiewicz, M.; Cisowski, W.; Majewska, E. High-Performance Liquid Chromatographic Determination of Polymethoxylated Flavonols in Soil-Grown Plant and In Vitro Cultures of Rudbeckia hirta L. Acta Pol. Pharm.—Drug Res. 1998, 55, 143–147. [Google Scholar]
- Herz, W.; Kulanthaivel, P. Trihydroxy-C18-Acids and a Labdane from Rudbeckia fulgida. Phytochemistry 1985, 24, 89–91. [Google Scholar] [CrossRef]
- Mahmoud, I.I.; Marzouk, M.S.; Moharram, F.A.; El-Gindi, M.R.; Hassan, A.M. Acylated Flavonol Glycosides from Eugenia jambolana Leaves. Phytochemistry 2001, 58, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Mirzaei, M.; Ghanadian, M.; Fallah, M.; Mahboodi, R. 6-Methoxylated Flavonoids: Jacein, and 3-Demethyljacein from Centaurea Schmidii with Their Endoplasmic Reticulum Stress and Apoptotic Cell Death in Breast Cancer Cells Along with In-Silico Analysis. Iran. J. Pharm. Res. 2021, 20, 417–432. [Google Scholar] [CrossRef]
- Schlangen, K.; Miosic, S.; Castro, A.; Freudmann, K.; Luczkiewicz, M.; Vitzthum, F.; Schwab, W.; Gamsjäger, S.; Musso, M.; Halbwirth, H. Formation of UV-Honey Guides in Rudbeckia hirta. Phytochemistry 2009, 70, 889–898. [Google Scholar] [CrossRef]
- Michael, B.R.; Gedara, S.R.; Amer, M.M.; Stevenson, L.; Ahmed, A.F. Evidence-Based Medicinal Value of Rudbeckia hirta L. Flowers. Nat. Prod. Res. 2014, 28, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.-T. Chemistry and Bioactivity of Nobiletin and Its Metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Holeman, M.; Ilidrissi, A.; Berrada, M. Flavonoids and Sesquiterpene Lactones of Artemisia mesatlantica. Planta Med. 1991, 57, 198–199. [Google Scholar] [CrossRef]
- Bokern, M.; Heuer, S.; Wray, V.; Witte, L.; Macek, T.; Vanek, T.; Strack, D. Ferulic Acid Conjugates and Betacyanins from Cell Cultures of Beta vulgaris. Phytochemistry 1991, 30, 3261–3265. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Kozachok, S.; Pecio, Ł.; Marchyshyn, S.; Oleszek, W. Determination of Phenolic Profiles of Herniaria polygama and Herniaria incana Fractions and Their in Vitro Antioxidant and Anti-Inflammatory Effects. Phytochemistry 2021, 190, 112861. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, L.; Gong, K.; Li, K.; Sun, L.; Cai, W. Identification and Quantification of Chlorogenic Acids from the Root Bark of Acanthopanax gracilistylus by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. ACS Omega 2022, 7, 25675–25685. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Deshpande, S.; Kuhnert, N. Profling the Chlorogenic Acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae Leavesby LC-MSN. Phytochem. Anal. 2011, 22, 432–441. [Google Scholar] [CrossRef]
- Guerreiro, E. Heliangolides and Acyclic Diterpene from Viguiera gilliesii. Phytochemistry 1986, 25, 748–750. [Google Scholar] [CrossRef]
- Lai, Y.; Xue, Y.; Zhang, M.; Zhang, J.; Tang, W.; Liu, J.; Lei, L.; Yan, J.; Luo, Z.; Zuo, J.; et al. Scapiformolactones A-I: Germacrane Sesquiterpenoids with an Unusual Δ3-15,6-Lactone Moiety from Salvia scapiformis. Phytochemistry 2013, 96, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Skaltsa, H.; Lazari, D.; Garcia, B.; Pedro, J.R.; Sokovic, M.; Constantinidis, T. Sesquiterpene Lactones from Centaurea achaia, a Greek Endemic Species: Antifungal Activity. Zeitschrift Für Naturforsch. C 2000, 55, 534–539. [Google Scholar] [CrossRef]
- Lazari, D.; Garcia, B.; Skaltsa, H.; Pedro, J.; Harvala, C. Sesquiterpene Lactones from Onopordon laconicum and O. sibthorpianum. Phytochemistry 1998, 47, 415–422. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Pseudoguaianolides and Other Sesquiterpene Lactones from Gaillardia Species. Phytochemistry 1984, 23, 1979–1988. [Google Scholar] [CrossRef]
- Smítalová, Z.; Buděšínský, M.; Šaman, D.; Vašíčková, S.; Holub, M. Components of the Extract from the Underground Parts of Laserpitium siler L. of Slovenian Origin, Mainly Sesquiterpenic Lactones. Collect. Czechoslov. Chem. Commun. 1984, 49, 852–870. [Google Scholar] [CrossRef]
- Imakura, Y.; Lee, K.-H.; Sims, D.; Wu, R.-Y.; Hall, I.H.; Furukawa, H.; Itoigawa, M.; Yonaha, K. Antitumor Agents XXXVI: Structural Elucidation of Sesquiterpene Lactones Microhelenins-A, B, and C, Microlenin Acetate, and Plenolin from Helenium microcephalum. J. Pharm. Sci. 1980, 69, 1044–1049. [Google Scholar] [CrossRef]
- Pan, E.; Gorka, A.P.; Alumasa, J.N.; Slebodnick, C.; Harinantenaina, L.; Brodie, P.J.; Roepe, P.D.; Randrianaivo, R.; Birkinshaw, C.; Kingston, D.G.I. Antiplasmodial and Antiproliferative Pseudoguaianolides of Athroisma proteiforme from the Madagascar Dry Forest. J. Nat. Prod. 2011, 74, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Mustakerova, E.; Todorova, M.; Tsankova, E. Sesquiterpene Lactones from Achillea collina Becker. Zeitschrift Für Naturforsch. C 2002, 57, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Singh, P.; Jakupovic, J. New Germacranolides and Other Sesquiterpene Lactones from Dicoma Species. Phytochemistry 1982, 21, 2029–2033. [Google Scholar] [CrossRef]
- Harmatha, J.; Budešínský, M.; Vokáč, K.; Kostecká, P.; Kmoníčková, E.; Zídek, Z. Trilobolide and Related Sesquiterpene Lactones from Laser trilobum Possessing Immunobiological Properties. Fitoterapia 2013, 89, 157–166. [Google Scholar] [CrossRef]
- Silva, G.L.; Pacciaroni, A.d.V.; Oberti, J.C.; Espinar, L.A.; Diáz, J.G.; Herz, W. Helenanolides, Guaianolide Glucosides and Other Constituents of Two Helenium donianum Varieties. Phytochemistry 1992, 31, 1621–1630. [Google Scholar] [CrossRef]
- Shimizu, S.; Ishihara, N.; Umehara, K.; Miyase, T.; Ueno, A. Sesquiterpene Glycosides and Saponins from Cynara cardunculus L. Chem. Pharm. Bull. 1988, 36, 2466–2474. [Google Scholar] [CrossRef][Green Version]
- Vasquez, M.; Quijano, L.; Fronczek, F.R.; Macias, F.A.; Urbatsch, L.E.; Cox, P.B.; Fischer, N.H. Sesquiterpene Lactones and Lignanes from Rudbeckia Species. Phytochemistry 1990, 29, 561–565. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, H.; Horikawa, S.; Murakami, T.; Shimoda, H.; Yamahara, J.; Matsuda, H. Bioactive Constituents of Chinese Natural Medicines. IV. Rhodiolae Radix. (2).: On the Histamine Release Inhibitors from the Underground Part of Rhodiola sacra (PRAIN Ex HAMET) S. H. Fu (Crassulaceae): Chemical Structures of Rhodiocyanoside. Chem. Pharm. Bull. 1997, 45, 1498–1503. [Google Scholar] [CrossRef]
- Yokoyama, R.; Huang, J.-M.; Hosoda, A.; Kino, K.; Yang, C.-S.; Fukuyama, Y. Seco-Prezizaane-Type Sesquiterpenes and an Abietane-Type Diterpene from Illicium minwanense. J. Nat. Prod. 2003, 66, 799–803. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.; Knigh, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acid. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, Y.J.; Choi, S.U.; Lee, K.R. A New Flavonol Glycoside from the Aerial Part of Rudbeckia laciniata. Arch. Pharm. Res. 2014, 37, 834–838. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the Interglycosidic Linkage in Di-, Tri-, Tetra- and Pentaglycosylated Flavonoids and Differentiation of Positional Isomers by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outliningtheir Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and Free Radical Scavenging Activity of Iron Chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Thakur, K.; Wei, C.-K.; Wang, H.; Zhang, J.-G.; Wei, Z.-J. Evaluation of Inhibitory Activity of Natural Plant Polyphenols on Soybean Lipoxygenase by UFLC-Mass Spectrometry. S. Afr. J. Bot. 2019, 120, 179–185. [Google Scholar] [CrossRef]
- Orafaie, A.; Matin, M.M.; Sadeghian, H. The Importance of 15-Lipoxygenase Inhibitors in Cancer Treatment. Cancer Metastasis Rev. 2018, 37, 397–408. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-Lipoxygenase from Orange Peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Dihydrolipoic Acid Inhibits 15-Lipoxygenase-Dependent Lipid Peroxidation. Free Radic. Biol. Med. 2003, 35, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.D.; Jones, C.D.; Setzer, W.N. Leaf Essential Oil Compositions of Rudbeckia fulgida Aiton, Rudbeckia hirta L., and Symphyotrichum novaeangliae (L.) G.L. Nesom (Asteraceae). Am. J. Essent. Oils Nat. Prod. 2014, 2, 36–38. [Google Scholar]
- Burlec, A.F.; Cioanca, O.; Enache, L.; Hancianu, M. Promising Biological Activities of Sesquiterpene Lactones. Med. -Surg. J. 2017, 121, 645–652. [Google Scholar]
- Nohara, K.; Wang, F.; Spiegel, S. Glycosphingolipid Composition of MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines. Breast Cancer Res. Treat. 1998, 48, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 997–1018. [Google Scholar]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What Made Sesquiterpene Lactones Reach Cancer Clinical Trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Qin, J.-J.; Jin, H.-Z.; Huang, Y.; Zhang, S.-D.; Shan, L.; Voruganti, S.; Nag, S.; Wang, W.; Zhang, W.-D.; Zhang, R. Selective Cytotoxicity, Inhibition of Cell Cycle Progression, and Induction of Apoptosis in Human Breast Cancer Cells by Sesquiterpenoids from Inula lineariifolia Turcz. Eur. J. Med. Chem. 2013, 68, 473–481. [Google Scholar] [CrossRef]
- Gohari, A.R.; Mosaddegh, M.; Naghibi, F.; Eslami-Tehrani, B.; Pirani, A.; Hamzeloo-Moghadam, M.; Read, R.W. Cytotoxic Sesquiterpene Lactones from the Aerial Parts of Inula aucheriana. An. Acad. Bras. Cienc. 2015, 87, 777–785. [Google Scholar] [CrossRef]
- Beekman, A.C.; Woerdenbag, H.J.; van Uden, W.; Pras, N.; Konings, A.W.T.; Wikström, H.V.; Schmidt, T.J. Structure−Cytotoxicity Relationships of Some Helenanolide-Type Sesquiterpene Lactones. J. Nat. Prod. 1997, 60, 252–257. [Google Scholar] [CrossRef]
- Mohamed, A.; Hassan, M.H.A.; Gouda, A.M.; AbouZid, S.; El Amir, D. Docking Studies of Sesquiterpene Lactones Isolated from Ambrosia maritima L. Reveals Their Potential Anti-Inflammatory and Cytotoxic Activities. Nat. Prod. Res. 2022, 36, 1078–1083. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Heilmann, J. Quantitative Structure-Cytotoxicity Relationships of Sesquiterpene Lactones Derived from Partial Charge (Q)-Based Fractional Accessible Surface Area Descriptors (Q_frASAs). Quant. Struct. Relatsh. 2002, 21, 276–287. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative Effect of Flavonoids and Sesquiterpenoids from Achillea millefolium s.l. on Cultured Human Tumour Cell Lines. Phyther. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Hussien, T.A.; El-toumy, S.A.; Hassan, H.M.; Hetta, M.H. Cytotoxic And Antioxidant Activities Of Secondary Metabolites from Pulicaria undulata. Int. J. Pharm. Pharm. Sci. 2016, 8, 150. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Alqarni, A.A.; Abdallah, H.M.; Omar, A.M.; Ibrahim, S.R. Cytotoxic and Alpha Amylase-Inhibitory Metabolites from Tagetes minuta: In Vitro Evaluation and Docking Studies. Trop. J. Pharm. Res. 2023, 22, 795–804. [Google Scholar] [CrossRef]
- Mircea, C.-C. Cercetări Privind Utilizarea și Valorificarea Plantelor Ornamentale cu Proprietăţi Alergenice și Toxice; Iasi University of Life Sciences: Iași, Romania, 2015. [Google Scholar]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of Polyphenols Extraction from Dried Chokeberry Using Maceration as Traditional Technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereștiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Mircea, C.; Cioancă, O.; Corciovă, A.; Nicolescu, A.; Oleszek, W.; Hăncianu, M. Chemical Profile and Antioxidant Activity of Zinnia elegans Jacq. Fractions. Molecules 2019, 24, 2934. [Google Scholar] [CrossRef] [PubMed]
- Batir-Marin, D.; Boev, M.; Cioanca, O.; Mircea, C.; Burlec, A.F.; Beppe, G.J.; Spac, A.; Corciova, A.; Hritcu, L.; Hancianu, M. Neuroprotective and Antioxidant Enhancing Properties of Selective Equisetum Extracts. Molecules 2021, 26, 2565. [Google Scholar] [CrossRef]
- Venditti, E.; Bacchetti, T.; Tiano, L.; Carloni, P.; Greci, L.; Damiani, E. Hot vs. Cold Water Steeping of Different Teas: Do They Affect Antioxidant Activity? Food Chem. 2010, 119, 1597–1604. [Google Scholar] [CrossRef]
- Burlec, A.F.; Cioancă, O.; Mircea, C.; Arsene, C.; Tuchiluş, C.; Corciovă, A.; Hăncianu, M. Antioxidant and Antimicrobial Properties of Chrysanthemum and Tagetes Selective Extracts. Farmacia 2019, 67, 405–410. [Google Scholar] [CrossRef]
- Danciu, C.; Zupko, I.; Bor, A.; Schwiebs, A.; Radeke, H.; Hancianu, M.; Cioanca, O.; Alexa, E.; Oprean, C.; Bojin, F.; et al. Botanical Therapeutics: Phytochemical Screening and Biological Assessment of Chamomile, Parsley and Celery Extracts against A375 Human Melanoma and Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 3624. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Muntean, D.; Alexa, E.; Farcas, C.; Oprean, C.; Zupko, I.; Bor, A.; Minda, D.; Proks, M.; Buda, V.; et al. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and Pro-Apoptotic Potential of Ephedra alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 2018, 24, 13. [Google Scholar] [CrossRef] [PubMed]


| Sample/ Control | Concentration (mg/mL) | EC50 (mg/mL Final Solution) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.15625 | 0.3125 | 0.625 | 1.25 | 2.5 | 5 | 10 | ||
| Rh-MeOH | 6.23 ± 0.05 | 11.65 ± 0.03 | 18.02 ± 0.09 | 29.08 ± 0.01 | 56.31 ± 0.03 | 69.70 ± 0.06 | 94.95 ± 0.08 | 0.42 ± 0.00 |
| Quercetin | 3.92 ± 0.07 | 6.63 ± 0.28 | 16.03 ± 0.38 | 31.60 ± 1.30 | 56.48 ± 0.83 | 75.59 ± 0.79 | 90.35 ± 0.92 | 0.42 ± 0.01 |
| Sample/ Control | Concentration (mg/mL) | EC50 (μg/mL Final Solution) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.15625 | 0.3125 | 0.625 | 1.25 | 2.5 | 5 | 10 | ||
| Rh-MeOH | 13.67 ± 0.60 | 16.80 ± 0.36 | 19.36 ± 0.29 | 26.12 ± 0.65 | 36.73 ± 0.40 | 100 ± 0.00 | 100 ± 0.00 | 48.18 ± 0.17 |
| Quercetin | 25.33 ± 0.42 | 38.40 ± 0.36 | 53.98 ± 0.46 | 63.08 ± 0.86 | 77.45 ± 1.12 | 100 ± 0.00 | 100 ± 0.00 | 17.45 ± 0.33 |
| Sample/ Control | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 90028 | C. parapsilosis ATCC 22019 | |
| Rh-MeOH | 16.0 ± 0.00 | 10.0 ± 0.00 | 10.0 ± 0.00 | 15.0 ± 0.00 | 17.0 ± 0.00 |
| DMSO | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 28.7 ± 0.06 | 36.5 ± 0.50 | 31.5 ± 0.50 | *NT | *NT |
| Fluconazole | *NT | *NT | *NT | 30.5 ± 0.50 | 21.0 ± 0.00 |
| Nystatin | *NT | *NT | *NT | 23.5 ± 0.50 | 20.0 ± 0.00 |
| Sample/Control | Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| Rh-MeOH | 0.25 | 0.5 | 0.25 | >0.5 |
| Ciprofloxacin | 1 * | 1 * | 1 * | 2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlec, A.F.; Pecio, Ł.; Mircea, C.; Tuchiluș, C.; Corciovă, A.; Danciu, C.; Cioancă, O.; Caba, I.C.; Pecio, S.; Oleszek, W.; et al. Preliminary Phytochemical and Biological Evaluation of Rudbeckia hirta Flowers. Plants 2023, 12, 2871. https://doi.org/10.3390/plants12152871
Burlec AF, Pecio Ł, Mircea C, Tuchiluș C, Corciovă A, Danciu C, Cioancă O, Caba IC, Pecio S, Oleszek W, et al. Preliminary Phytochemical and Biological Evaluation of Rudbeckia hirta Flowers. Plants. 2023; 12(15):2871. https://doi.org/10.3390/plants12152871
Chicago/Turabian StyleBurlec, Ana Flavia, Łukasz Pecio, Cornelia Mircea, Cristina Tuchiluș, Andreia Corciovă, Corina Danciu, Oana Cioancă, Ioana Cezara Caba, Solomiia Pecio, Wiesław Oleszek, and et al. 2023. "Preliminary Phytochemical and Biological Evaluation of Rudbeckia hirta Flowers" Plants 12, no. 15: 2871. https://doi.org/10.3390/plants12152871
APA StyleBurlec, A. F., Pecio, Ł., Mircea, C., Tuchiluș, C., Corciovă, A., Danciu, C., Cioancă, O., Caba, I. C., Pecio, S., Oleszek, W., & Hăncianu, M. (2023). Preliminary Phytochemical and Biological Evaluation of Rudbeckia hirta Flowers. Plants, 12(15), 2871. https://doi.org/10.3390/plants12152871











