Cytotoxicity of Nine Medicinal Plants from San Basilio de Palenque (Colombia) on HepG2 Cells
Abstract
1. Introduction
2. Results
2.1. Total Phenolic, Flavonoid, Tannin Contents, and DPPH Free Radical Scavenging Activity
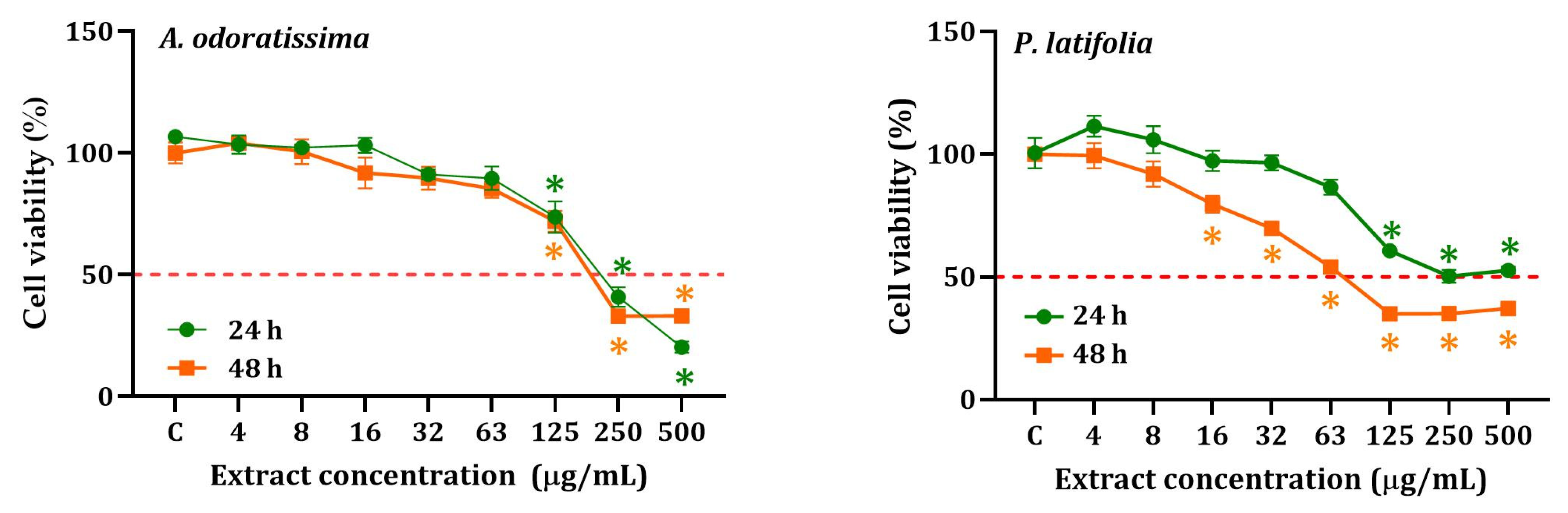
2.2. Screening of Cytotoxic Activity of Hydroethanolic Extracts
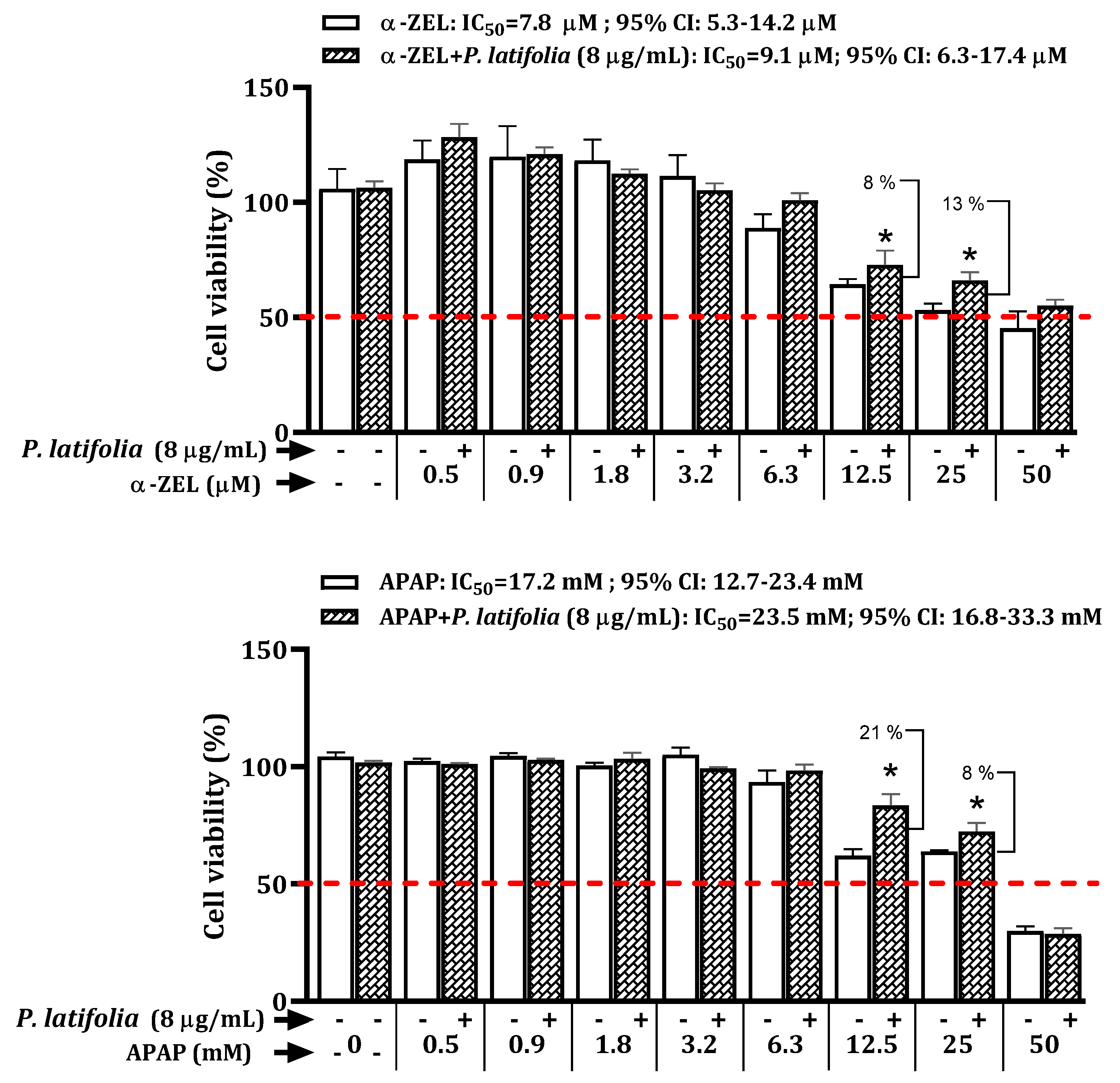
2.3. Cytoprotective Effects of P. latifolia against α-ZEL and APAP
2.4. ALT and AST Activities
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Total Phenol Content
4.4. Total Flavonoid Content
4.5. Total Condensed Tannins
4.6. DPPH• Free Radical Scavenging Assay
4.7. Cell Line
4.8. MTT Assay
4.9. Cytoprotective Effects of P. latifolia and A. odoratissima Extracts against ZEN Metabolites
4.10. Biochemical Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.S.; Ahmad, M.S. Worldwide importance of medicinal plants: Current and historical perspectives. J. Recent Adv. Biol. Med. 2016, 2, 909. [Google Scholar] [CrossRef]
- Shafi, A.; Hassan, F.; Zahoor, I.; Majeed, U.; Khanday, F.A. Biodiversity, Management and Sustainable Use of Medicinal and Aromatic Plant Resources. Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 85–111. [Google Scholar]
- Beyene, B.; Beyene, B.; Deribe, H. Review on application and management of medicinal plants for the livelihood of the local community. J. Resour. Dev 2016, 22, 33–39. [Google Scholar]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. J. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.C.; Andrea, J. Caracterización Fitoquímica, Actividad Antibacteriana y Antioxidante de Extractos de Plantas Medicinales Utilizadas en Pereira y Santa Rosa de Cabal (Risaralda); Universidad Tecnológica de Pereira, Facultad de Tecnologías; Tecnología Química: Pereira, Colombia, 2011. [Google Scholar]
- Gardi, C.; Angelini, M.; Barceló, S. Atlas de Suelos de America Latina y el Caribe. Comisión Europea; Oficina de Publicaciones de la Unión Europea: Luxembourg, 2013. [Google Scholar]
- Nunes, A.T.; Albuquerque, U.P. South American Biodiversity and Its Potential in Medicinal and Aromatic Plants. In Medicinal and Aromatic Plants of South America; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–15. [Google Scholar]
- García-Martínez, S.; Mercado-Gómez, J.D. Diversidad de briófitos en fragmentos de bosque seco tropical, Montes de María, Sucre, Colombia. Rev. Mex. Biodivers. 2017, 88, 824–831. [Google Scholar] [CrossRef]
- Sampedro, A.; Gómez, H.; Ballut, G. Estado de la vegetación en localidades abandonadas por “desplazamiento”, en los Montes de María Sucre, Colombia. Rev. Colombiana Cienc. Anim. RECIA 2014, 6, 184–193. [Google Scholar] [CrossRef]
- Soto, D.; Balanzó, A.; Herrera, B.; Ordóñez, G.; Vargas, J.; Marrugo, L.; Pérez, M. San Basilio de Palenque, Colombia: Cultura presente, territorio ausente. In El Valor del Patrimonio Cultural: Territorios Rurales, Experiencias y Proyecciones Latinoamericanas; RIMISP: Lima, Peru, 2008; pp. 141–174. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Mariyammal, V.; Sathiageetha, V.; Amalraj, S.; Gurav, S.S.; Amiri-Ardekani, E.; Jeeva, S.; Ayyanar, M. Chemical profiling of Aristolochia tagala Cham. leaf extracts by GC-MS analysis and evaluation of its antibacterial activity. J. Indian Chem. Soc. 2023, 100, 100807. [Google Scholar] [CrossRef]
- Michl, J.; Kite, G.C.; Wanke, S.; Zierau, O.; Vollmer, G.; Neinhuis, C.; Simmonds, M.S.; Heinrich, M. LC-MS-and 1H NMR-based metabolomic analysis and in vitro toxicological assessment of 43 Aristolochia species. J. Nat. Prod 2016, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Usubillaga, A.; Khouri, N.; Rojas, L. Essential Oil from the Leaves of Aristolochia odoratissima L. J. Essent. Oil Res. 2001, 13, 128–129. [Google Scholar] [CrossRef]
- Montiel-Ruiz, R.M.; Córdova-de la Cruz, M.; González-Cortázar, M.; Zamilpa, A.; Gómez-Rivera, A.; López-Rodríguez, R.; Lobato-García, C.E.; Blé-González, E.A. Antinociceptive effect of hinokinin and kaurenoic acid isolated from Aristolochia odoratissima L. Molecules 2020, 25, 1454. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical re-evaluation of DPPH assay: Presence of pigments affects the results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Májeková, M.; Košt’álová, D.; Štefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef]
- Berthi, W.; González, A.; Rios, A.; Blair, S.; Cogollo, Á.; Pabón, A. Anti-plasmodial effect of plant extracts from Picrolemma huberi and Picramnia latifolia. Malar J. 2018, 17, 151. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Souza, T.; Menezes, Y.A.; Cabral, B.; Câmara, R.B.; Silva-Junior, A.A.; Rocha, H.A.; Rebecchi, I.M.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Aqueous leaf extract of Jatropha gossypiifolia L. (Euphorbiaceae) inhibits enzymatic and biological actions of Bothrops jararaca snake venom. PLoS ONE 2014, 9, e104952. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. Jatropha gossypiifolia L. and its biologically active metabolites: A mini review. J. Ethnopharmacol. 2019, 234, 197–203. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pinto, A.C.; Chaves, F.C.M.; dos Santos, P.A.; Nunez, C.V.; Tadei, W.P.; Pohlit, A.M. Piper peltatum: Biomass and 4-nerolidylcatechol production. Planta Med. 2010, 76, 1473–1476. [Google Scholar] [CrossRef]
- Rocha e Silva, L.F.; da Silva Pinto, A.C.; Pohlit, A.M.; Quignard, E.L.J.; Vieira, P.P.R.; Tadei, W.P.; Chaves, F.C.M.; Samonek, J.F.; Lima, C.A.J.; Costa, M.R.F. In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytother. Res. 2011, 25, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Ceballos, L.; Mercado-Camargo, J.; del Olmo-Fernández, E.; Serrano-García, M.L.; Robledo, S.M.; Gómez-Estrada, H. Antileishmanial activity and in silico molecular docking studies of Malachra alceifolia Jacq. fractions against Leishmania mexicana Amastigotes. Trop. Med. Infect. Dis. 2023, 8, 115. [Google Scholar] [CrossRef]
- Gómez-Estrada, H.; Díaz-Castillo, F.; Franco-Ospina, L.; Mercado-Camargo, J.; Guzmán-Ledezma, J.; Medina, J.D.; Gaitán-Ibarra, R. Folk medicine in the northern coast of Colombia: An overview. J. Ethnobiol. Ethnomed. 2011, 7, 27. [Google Scholar] [CrossRef]
- Powers, C.N.; Mayo, J.A.; Moriarity, D.M.; Vogler, B.; Setzer, W.N.; McFeeters, R.L. Identification of anticryptococcal bornyl compounds from Verbesina turbacensis and their structure-activity relationships. Planta Med. 2022, 88, 1341–1347. [Google Scholar] [CrossRef]
- Ogungbe, I.V.; Crouch, R.A.; Haber, W.A.; Setzer, W.N. Phytochemical investigation of Verbesina turbacensis Kunth: Trypanosome cysteine protease inhibition by (–)-bornyl esters. Nat. Prod. Commun. 2010, 5, 1934578X1000500801. [Google Scholar] [CrossRef]
- Elkousy, R.H.; Said, Z.N.; Abd El-Baseer, M.A. Antiviral activity of castor oil plant (Ricinus communis) leaf extracts. J. Ethnopharmacol. 2021, 271, 113878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. A review on phytochemical constituents and pharmacological activities of Ricinus communis L. Plant. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 466–472. [Google Scholar] [CrossRef]
- Hooper, A.; Caulfield, J.; Hao, B.; Pickett, J.; Midega, C.; Khan, Z. Isolation and identification of Desmodium root exudates from drought tolerant species used as intercrops against Striga hermonthica. Phytochemistry 2015, 117, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.M.F.; Guidobono, R.R. Genotoxicity of plant extracts. Mem. Inst. Oswaldo Cruz. 1991, 86, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Brondani, J.C.; Reginato, F.Z.; da Silva Brum, E.; de Souza Vencato, M.; Lhamas, C.L.; Viana, C.; da Rocha, M.I.U.M.; de Freitas Bauermann, L.; Manfron, M.P. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis-cati L. leaves in rats. J. Ethnopharmacol. 2017, 202, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Chai, H.-B.; Mi, Q.; Su, B.-N.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M. Anthrone and oxanthrone C-glycosides from Picramnia latifolia collected in Peru. J. Nat. Prod. 2004, 67, 352–356. [Google Scholar] [CrossRef]
- Duran-Izquierdo, M.; Taboada-Alquerque, M.; Sierra-Marquez, L.; Alvarez-Ortega, N.; Stashenko, E.; Olivero-Verbel, J. Hydroalcoholic extract of Haematoxylum brasiletto protects Caenorhabditis elegans from cadmium-induced toxicity. BMC Complement. Med. Ther. 2022, 22, 184. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, J.A.; Moreno-Lorenzana, D.; Álvarez-Bernal, D.; Rodríguez-Campos, J.; Medina-Medrano, J.R. Phenolic profile, antioxidant and anti-proliferative activities of methanolic extracts from Asclepias linaria Cav. Leaves. Molecules 2019, 25, 54. [Google Scholar] [CrossRef]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Taboada-Alquerque, M.; Franco, J.; Stashenko, E.E.; Juan, C.; Juan-García, A.; Olivero-Verbel, J. Protective effects of the hydroethanolic extract of Fridericia chica on undifferentiated human neuroblastoma cells exposed to α-Zearalenol (α-ZEL) and β-Zearalenol (β-ZEL). Toxins 2021, 13, 748. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Stashenko, E.E.; Olivero-Verbel, J. Photoprotective agents obtained from aromatic plants grown in Colombia: Total phenolic content, antioxidant activity, and assessment of cytotoxic potential in cancer cell lines of Cymbopogon flexuosus L. and Tagetes lucida Cav. essential oils. Plants 2022, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Low blood lead levels impair intellectual and hematological function in children from Cartagena, Caribbean coast of Colombia. J. Trace Elem. Med. Biol. 2017, 44, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Toxicological effects in children exposed to lead: A cross-sectional study at the Colombian Caribbean coast. Environ. Int. 2019, 130, 104809. [Google Scholar] [CrossRef] [PubMed]
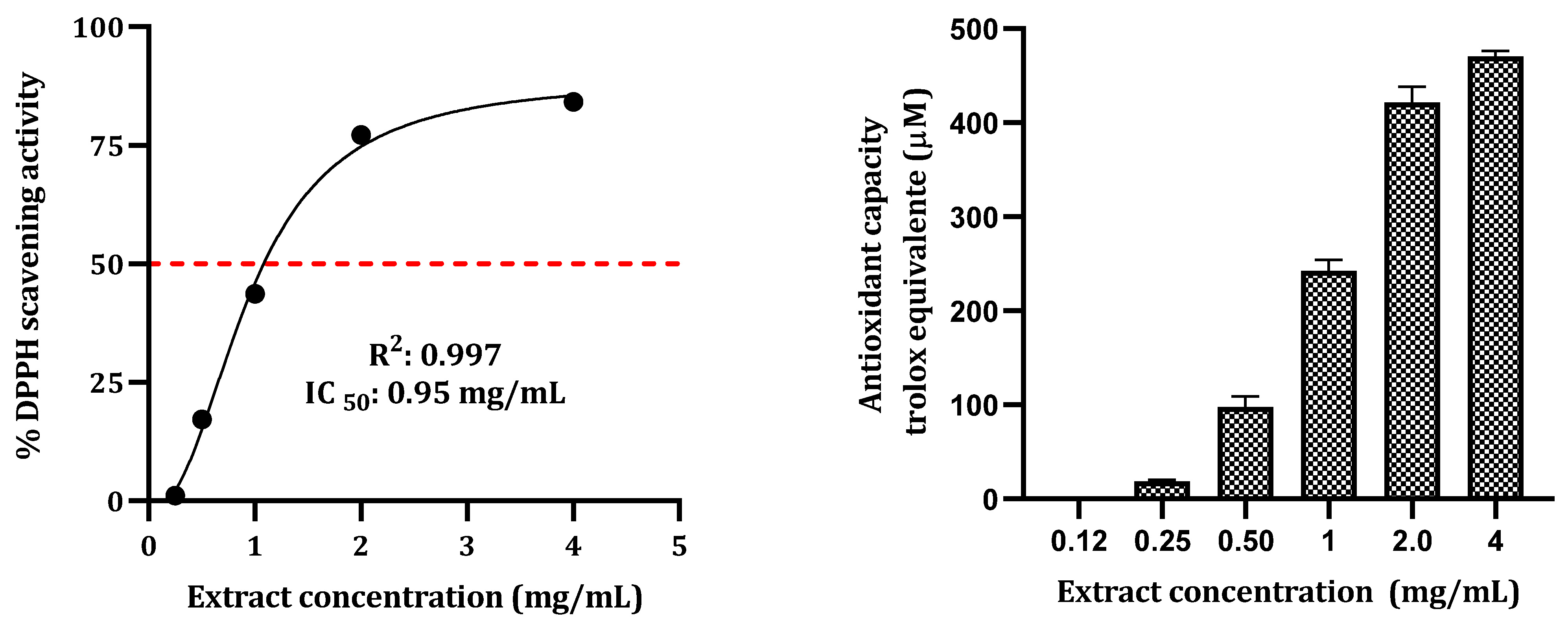



| Hydroethanolic Extract | Total Phenolic Content mg GAE/g DT | Total Flavonoid Content mg RE/g DT | Tannin Content mg CE/g DT | DPPH IC50 (mg/mL) |
|---|---|---|---|---|
| P. latifolia | 145.5 ± 1.7 | 95.1 ± 0.5 | 73.5 ± 0.6 | 0.9 |
| J. gossypiifolia L. | 156.7 ± 1.4 | 17.7 ± 0.1 | 37.7 ± 0.7 | 1.2 |
| M. alceitoflia Jacq | 109.4 ± 0.6 | 7.1 ± 0.1 | 25.5 ± 0.5 | 2.2 |
| V. turbacensis Kunth | 96.7 ± 1.0 | 19.9 ± 0.1 | 30.5 ± 0.3 | 2.5 |
| P. peltatum L. | 157.9 ± 2.3 | 6.9 ± 0.5 | 32.8 ± 0.4 | 5.4 |
| R. communis L. | 131.5± 1.0 | 14.6 ± 0.8 | 19.3 ± 0.5 | 5.8 |
| D. incanum (Sw.) | 162.7 ± 4.9 | 65.1 ± 1.0 | 28.7 ± 0.5 | 0.04 |
| D. unguis-cati (L.) L.G. Lohmann | 151.3 ± 2.0 | 21.9 ± 0.4 | 22.0 ± 0.7 | 0.9 |
| A. odoratissima | 167.6 ± 1.3 | 31.4 ± 0.5 | 23.5 ± 0.8 | 2.6 |
| Hydroethanolic Extract | Exposure Time (h) | IC50, µg/mL (95% CI) | SI | |
|---|---|---|---|---|
| HepG2 | HEKn | |||
| P. latifolia | 24 | 128.9 (110.3–135.3) | 160.0 (142.9–187.2) | 0.4 |
| 48 | 17.2 (12.5–19.9) | 38.9 (31.5–40.5) | 2.3 | |
| A. odoratissima | 24 | 95.7 (110.3–135.3) | 133.8(105.9–166.9) | 1.4 |
| 48 | 54.2 (48.9–60.2) | 111.9 (88–164.5) | 2.1 | |
| Species | Current Uses | Reported Secondary Metabolites | References |
|---|---|---|---|
| J. gossypiifolia | Anti-inflammatory, anti-hemorrhagic, edematogenic, and antimicrobial. Latex used to treat wounds and bites of snakes. | Jatrophone, jatrophenone, jatrophatrione, Jatrophenone, curcusone A, japogadrol, multifolone. | [19,20] |
| P. peltatum | Anti-inflammatory, antimalarial, antiplasmodial, and antioxidant. | Nerolidylcatechol. | [21,22] |
| M. alceifolia | Anti-inflammatory, antileishmanial. | Episwertenol, α-amiryn, methyl commate. | [23,24] |
| V. turbacensis | Anticryptococcal. | Alpha-pinene (Bark oil); germacrene-D, delta-elemene (leaf oil), bornyl hydroxycinnamic esters (bornyl caffeate and bornyl ferulate). | [25,26] |
| R. communis | Anti-inflammatory properties. It is also used to treat liver infections, stomach ache, flatulence, constipation, colic, enteritis, fever, and headache, among others. | Kaempferol-3-O-β-D-xylopyranoside, kaempferol-3-O-β-D-glucopyranoside, kaempferol-3-O-β-rutinoside, quercetin-3-O-rutinoside, quercetin-3-O-β-D-xylopyranoside, quercetin, and rutin, among others. | [27,28] |
| D. incanum | Diuretic and anti-inflammatory. | 6-C-galactosyl-8-C-glucosylapigenin, vicenin-2, 6-C-galactosyl-8-C-glucosylapigenin, 6-C-galactosyl-8-C-arabinosylapigenin, isoschaftoside, 6-C-arabinosyl-8-C-galactosylapigenin. | [29,30] |
| D. unguis-cati | Antipyretic, anti-inflammatory, and anti-tumoral. Potential hypocholesterolemic activity. | Chlorogenic acid, caffeic acid, ferulic acid, vanillinic acid, p-coumaric acid, rosmarinic acid, trans-cinnamic acid, luteolin, apigenin, quercitrin and quercetin. | [31] |
| A. odoratissima | Analgesic, antidote against snake venom. | Kaurenoic acid, hinokinin. | [15] |
| P. latifolia | Anti-malarial activity. | Picramniosides G and H, mayosides D and E, 6,8-dihydroxy-10-methyl-7H-benz[de]anthracen-7-one, 6,8-dihydroxy-4-methyl-7H-benz[de]anthracen-7-one, nataloe-emodin, chrysophanein, chrysophanol, 1,5-dihydroxy-7-methoxy-3-methylanthraquinone, pulmatin, 7-hydroxycoumarin, 7-hydroxy-6-methoxycoumarin, β-sitosterol. | [18,32] |
| Code | Plant Name | Family Name | Plant Part Used | Voucher Number |
|---|---|---|---|---|
| 001 | Aristolochia odoratissima L. | Aristolochiaceae | Stem | 617813 |
| 003 | Jatropha gossypiifolia L. | Euphorbiaceae | Stem | 617821 |
| 004 | Piper peltatum L. | Piperaceae | Stem | 617811 |
| 005 | Malachra alceifolia Jacq. | Malvaceae | Stem | 617817 |
| 006 | Picramnia latifolia Tul. | Picramniaceae | Stem | 617812 |
| 007 | Verbesina turbacensis Kunth | Asteraceae | Stem | 617818 |
| 008 | Ricinus communis L. | Euphorbiaceae | Stem | 617814 |
| 010 | Desmodium incanum (Sw.) DC. | Fabaceae | Stem, leaves | 617819 |
| 012 | Dolichandra unguis-cati (L.) L.G. Lohmann | Bignoniaceae | Stem | 617820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-Gallardo, K.; Alvarez-Ortega, N.; Olivero-Verbel, J. Cytotoxicity of Nine Medicinal Plants from San Basilio de Palenque (Colombia) on HepG2 Cells. Plants 2023, 12, 2686. https://doi.org/10.3390/plants12142686
Caballero-Gallardo K, Alvarez-Ortega N, Olivero-Verbel J. Cytotoxicity of Nine Medicinal Plants from San Basilio de Palenque (Colombia) on HepG2 Cells. Plants. 2023; 12(14):2686. https://doi.org/10.3390/plants12142686
Chicago/Turabian StyleCaballero-Gallardo, Karina, Neda Alvarez-Ortega, and Jesus Olivero-Verbel. 2023. "Cytotoxicity of Nine Medicinal Plants from San Basilio de Palenque (Colombia) on HepG2 Cells" Plants 12, no. 14: 2686. https://doi.org/10.3390/plants12142686
APA StyleCaballero-Gallardo, K., Alvarez-Ortega, N., & Olivero-Verbel, J. (2023). Cytotoxicity of Nine Medicinal Plants from San Basilio de Palenque (Colombia) on HepG2 Cells. Plants, 12(14), 2686. https://doi.org/10.3390/plants12142686








