A Comparison of Different Stomatal Density Phenotypes of Hordeum vulgare under Varied Watering Regimes Reveals Superior Genotypes with Enhanced Drought Tolerance
Abstract
1. Introduction
2. Results
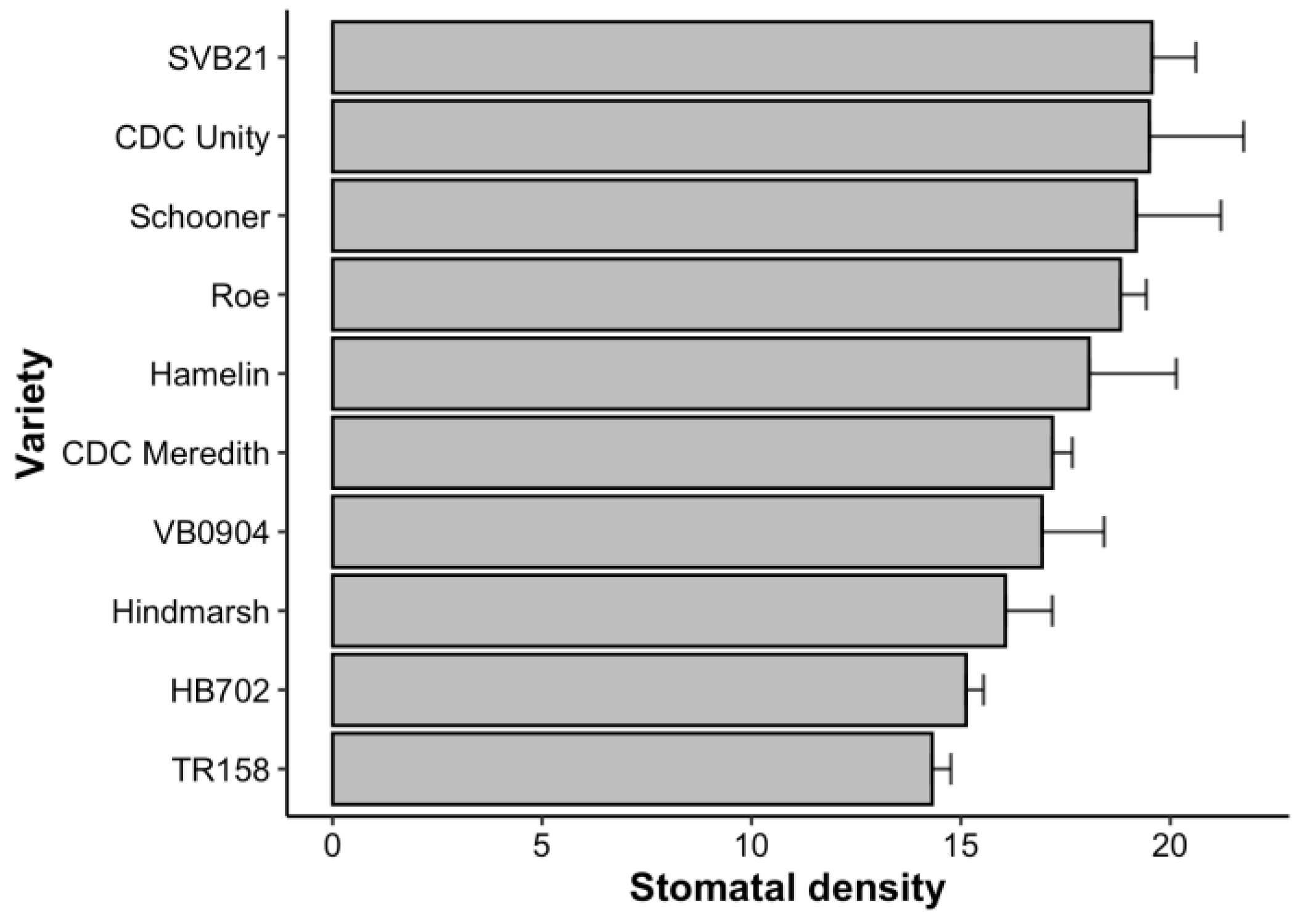
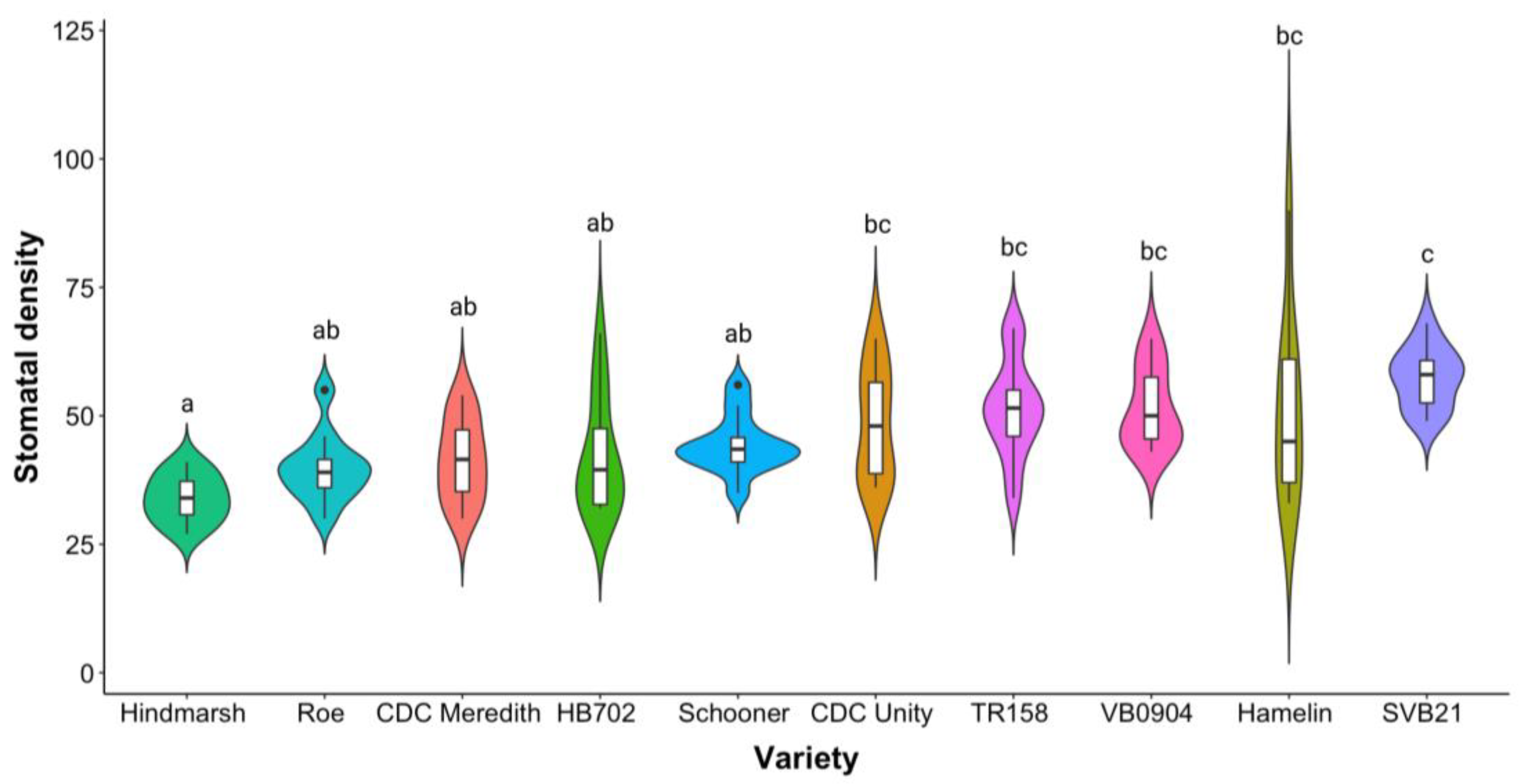
2.1. Distribution of Average Stomatal Density across 10 Barley Varieties
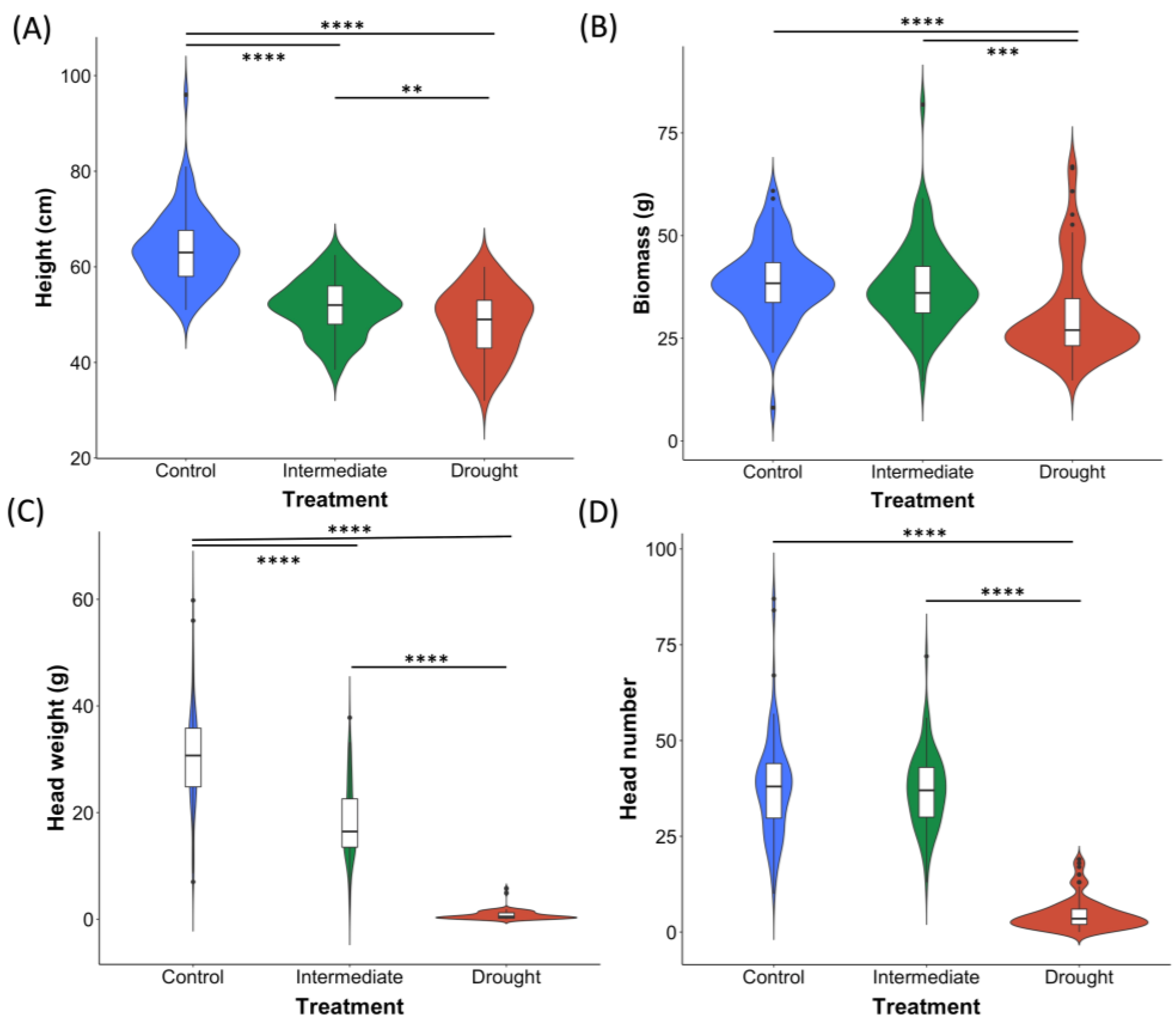
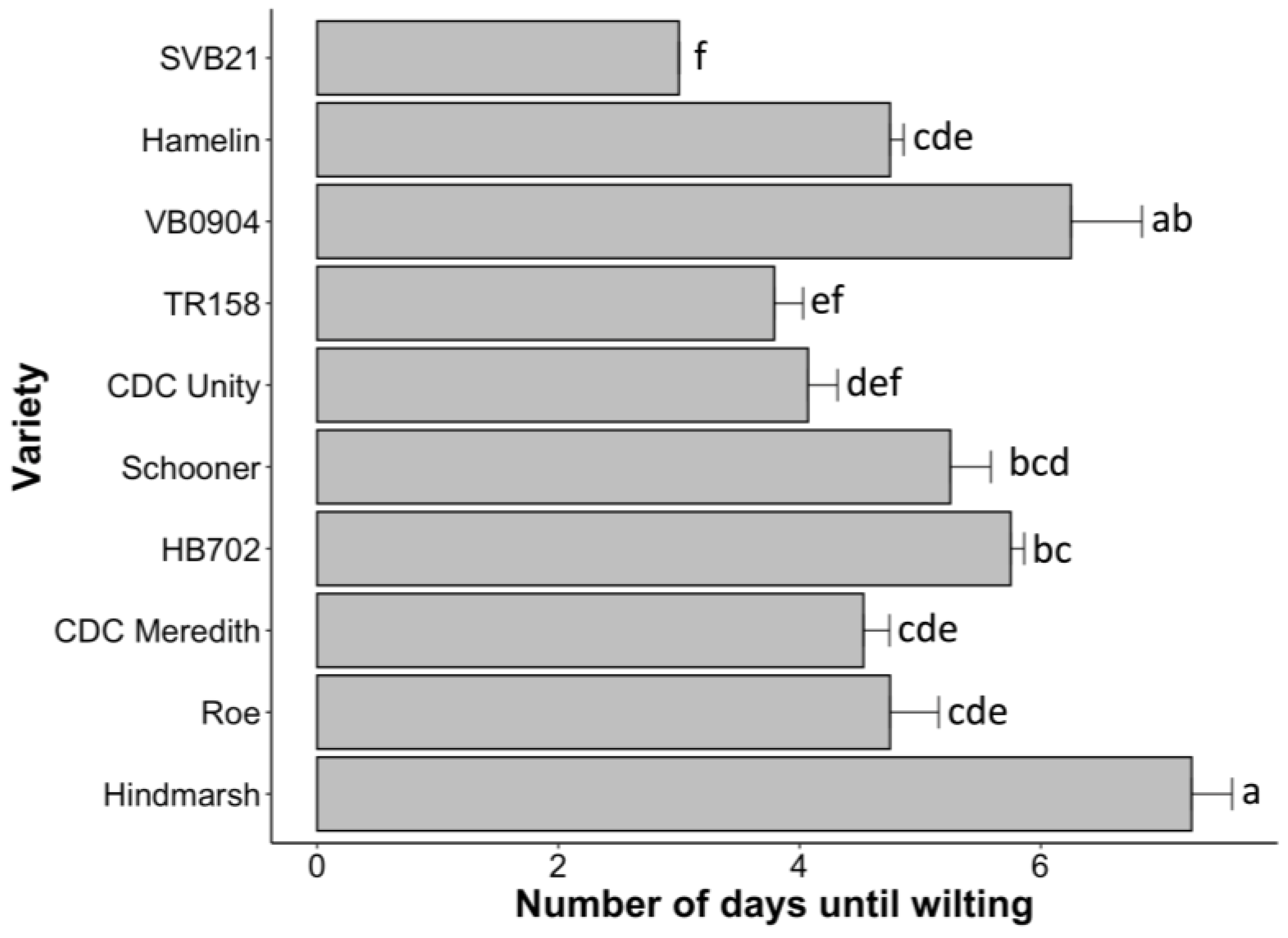
2.2. Hindmarsh Least Impacted by Drought Effects during the Early Stages of Development
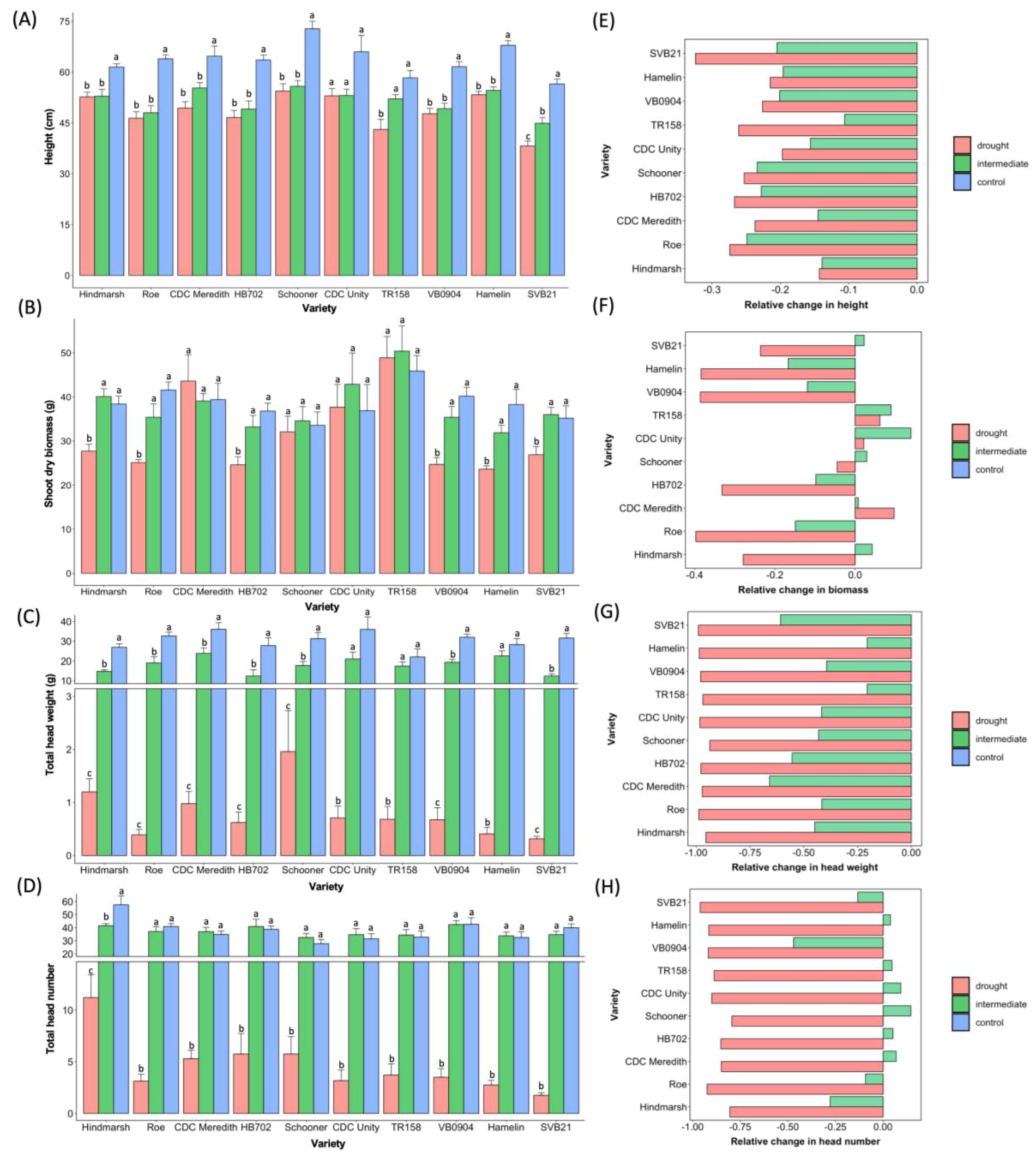
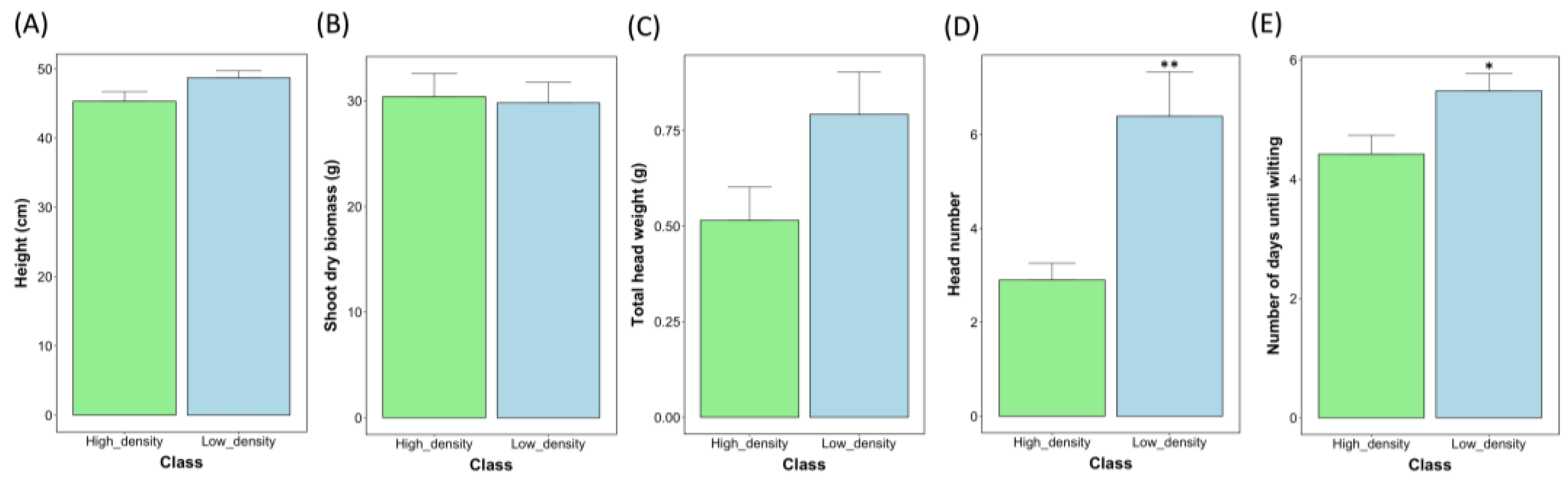
2.3. A Comparison of Yield Traits at Varied Levels of Water Supplementation at the ZS49 Growth Stage
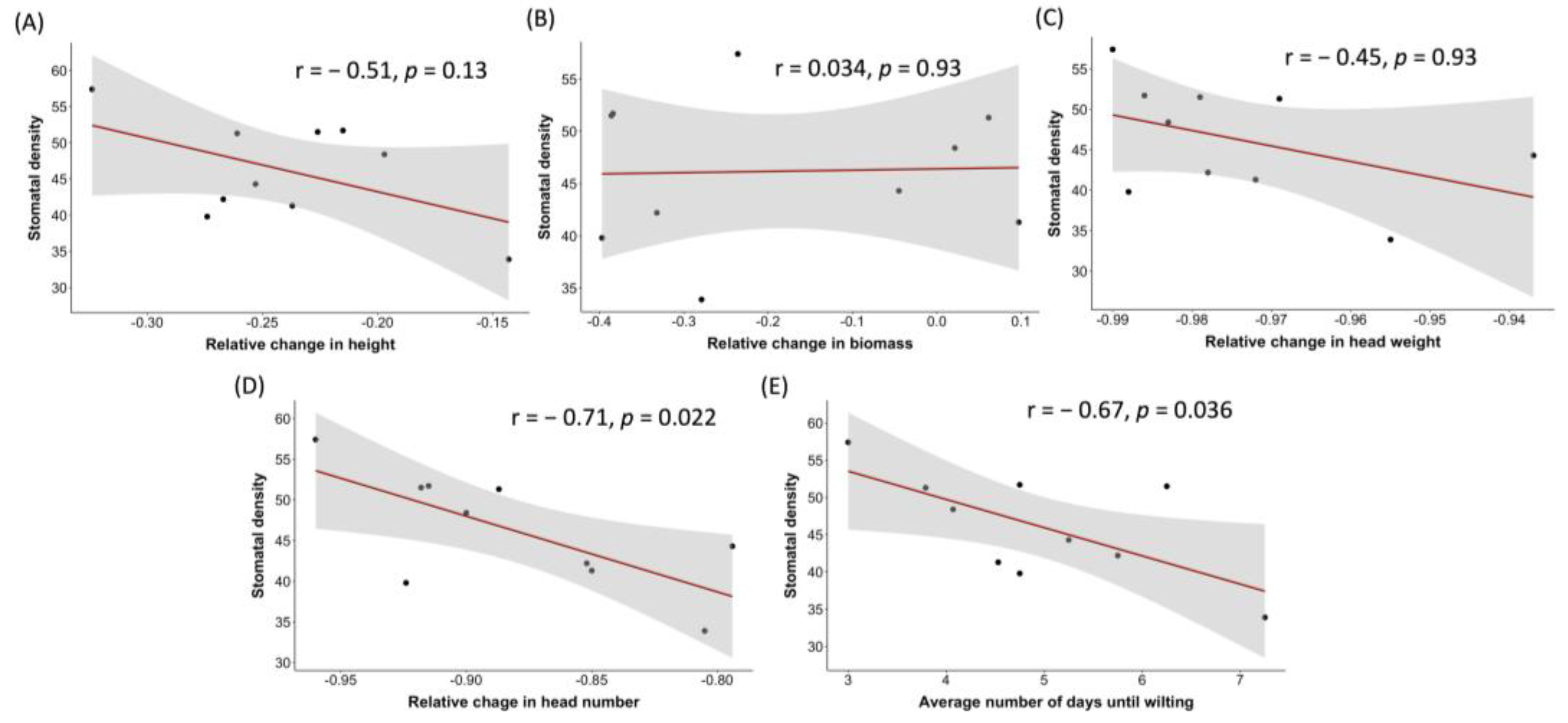
2.4. Association between Stomatal Density and the Traits of Head Number and Wilting Time across Barley Genotypes under Drought Conditions
3. Discussion
3.1. Impacts on Water-Use Efficiency May Reduce with Plant Size for Barley
3.2. Hindmarsh Exhibits Superior Biomass-Production and Water-Retention Traits under Drought Conditions during Early Development
3.3. Barley Height and Head Weight Show Increased Sensitivity to Changes in Water Supplementation during the Reproductive Phase versus Head Number and Shoot Biomass in This Study
3.4. Low-Stomatal-Density Variety Hindmarsh Outcompetes High-Stomatal-Density Variety SVB21 across Multiple Yield-Associated Traits under Both Low and High Water Supplementation
3.5. Low Stomatal Density May Have a Positive Impact on Head Development and Wilting Resistance in Barley under Water-Limiting Conditions
4. Materials and Methods
4.1. Glasshouse Experiment for the Investigation of Barley-Yield Response under Various Watering Regimes
4.2. Experimental Setup for Accessions Grown to the Tillering Stage
4.3. Experimental Setup for Accessions Grown to the ZS49 Stage
4.4. Phenotyping of Stomatal Density
4.5. Phenotyping of Yield-Associated Traits
4.6. Statistical Analysis of Trait Data Collected from Both Glasshouse Experiments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Food Programme. Climate Crisis and Malnutrition: A Case for Acting Now; World Food Programme: Rome, Italy, 2021; p. 1. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Habib-ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Gouldson, A.; Colenbrander, S.; Sudmant, A.; Papargyropoulou, E.; Kerr, N.; McAnulla, F.; Hall, S. Cities and climate change mitigation: Economic opportunities and governance challenges in Asia. Cities 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Frederiksen, J.S.; Osbrough, S.L. Tipping points and changes in Australian climate and extremes. Climate 2022, 10, 73. [Google Scholar] [CrossRef]
- Hughes, N.; Soh, W.Y.; Boult, C.; Lawson, K.; Donoghoe, M.; Valle, H.; Chancellor, W. Farmpredict: A Micro-Simulation Model of Australian Farms; The Australian Bureau of Agricultural and Resource Economics and Sciences: Canberra, Australia, 2019; pp. 1–44.
- Climate Council of Australia Limited. Compound Costs: How Climate Change Is Damaging Australia’s Economy; Climate Council of Australia Limited: Melbourne, Australia, 2019; p. 2. [Google Scholar]
- Sudmeyer, R.; Edward, A.; Foster, I.; Fazakerley, V.; Simpkin, L. Climate Change: Impacts and Adaptation for Agriculture in Western Australia; Department of Agriculture and Food: Perth, Australia, 2016; pp. 7–120.
- Watson, J.; Zheng, B.; Chapman, S.; Chenu, K. Projected impact of future climate on water-stress patterns across the Australian wheatbelt. J. Exp. Bot. 2017, 68, 5907–5921. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Agricultural Commodities, Australia; Australian Bureau of Statistics: Canberra, Australia, 2021.
- Mei, X.; Zhong, X.; Vincent, V.; Liu, X. Improving water-use efficiency of wheat crop varieties in the north china plain: Review and analysis. J. Integr. Agric. 2013, 12, 1243–1250. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Kubásek, J.; Hájek, T.; Duckett, J.; Pressel, S.; Šantrůček, J. Moss stomata do not respond to light and CO2 concentration but facilitate carbon uptake by sporophytes: A gas exchange, stomatal aperture, and 13 C-labelling study. New Phytol. 2021, 230, 1815–1828. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Meselmani, M.A.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef]
- Vráblová, M.; Vrábl, D.; Hronková, M.; Kubásek, J.; Šantrůček, J. Stomatal function, density and pattern, and CO2 assimilation in Arabidopsis thaliana tmm1 and sdd1-1 mutants. Plant Biol. 2017, 19, 689–701. [Google Scholar] [CrossRef]
- Franks, P.J.W.; Doheny-Adams, T.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.J.; Harrison, C.J.; Hetherington, A.M.; Williams, T.A. Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Curr. Biol. 2020, 30, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Morales-Navarro, S.; Pérez-Díaz, R.; Ortega, A.; de Marcos, A.; Mena, M.; Fenoll, C.; González-Villanueva, E.; Ruiz-Lara, S. Overexpression of a SDD1-like gene from wild tomato decreases stomatal density and enhances dehydration avoidance in Arabidopsis and cultivated Tomato. Front. Plant Sci. 2018, 9, 940. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Liu, L.; Wang, Z.; Li, Y.; Guo, L.; Li, Y.; Zhang, X.; Ren, S.; Zhao, B.; et al. sltlfp8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 2020, 43, 2666–2679. [Google Scholar] [CrossRef]
- Capasso, G.; Santini, G.; Petraretti, M.; Esposito, S.; Landi, S. Wild and traditional barley genomic resources as a tool for abiotic stress tolerance and biotic relations. Agriculture 2021, 11, 1102. [Google Scholar] [CrossRef]
- Australian Export Grains Innovation Centre. Australian Barley: Quality, Safety and Reliability; Department of Primary Industries and Regional Development: Perth, Australia, 2018; pp. 1–4.
- Ferrante, A.; Cullis, B.R.; Smith, A.B.; Able, J.A. A multi-environment trial analysis of frost susceptibility in wheat and barley under Australian frost-prone field conditions. Front. Plant Sci. 2021, 12, 722637. [Google Scholar] [CrossRef]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, T.J.; Al-Abdallat, A.; Thomas, W.T.B.; Bull, H.J.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef]
- Xiong, X.; James, V.A.; Zhang, H.; Altpeter, F. Constitutive expression of the barley HvWRKY38 transcription factor enhances drought tolerance in turf and forage grass (Paspalum notatum Flugge). Mol. Breed. 2009, 25, 419–432. [Google Scholar] [CrossRef]
- Gürel, F.; Öztürk, Z.N.; Uçarlı, C.; Rosellini, D. Barley genes as tools to confer abiotic stress tolerance in crops. Front. Plant Sci. 2016, 7, 1137. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Anderson, G.C.; Hüberli, D. Crop sequences in Western Australia: What are they and are they sustainable? Findings of a four-year survey. Crop Pasture Sci. 2015, 66, 634. [Google Scholar] [CrossRef][Green Version]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at the early growth and maturity stages. Bull. Natl. Res. Cent. 2021, 45, 117. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef] [PubMed]
- Forster, B.P.; Ellis, R.P.; Moir, J.; Talame, V.; Sanguineti, M.C.; Tuberosa, R.; This, D.; Teulat-Merah, B.; Ahmed, I.; Mariy, S.A.; et al. Genotype and phenotype associations with drought tolerance in barley tested in North Africa. Ann. Appl. Biol. 2004, 144, 157–168. [Google Scholar] [CrossRef]
- Ginzburg, D.N.; Bossi, F.; Rhee, S.Y. Uncoupling differential water usage from drought resistance in a dwarf Arabidopsis mutant. Plant Physiol. 2022, 190, 2115–2121. [Google Scholar] [CrossRef]
- Langridge, J. The aseptic culture of Arabidopsis thaliana (L.) heynh. Aust. J. Biol. Sci. 1957, 10, 243. [Google Scholar] [CrossRef][Green Version]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Blum, A.; Sullivan, C.Y.; Nguyen, H.T. The effect of plant size on wheat response to agents of drought stress. II. water deficit, heat and Aba. Funct. Plant Biol. 1997, 24, 43. [Google Scholar] [CrossRef]
- O’Donnell, A.J.; McCaw, W.L.; Cook, E.R.; Grierson, P.F. Megadroughts and Pluvials in Southwest Australia: 1350–2017 CE. Clim. Dyn. 2021, 57, 1817–1831. [Google Scholar] [CrossRef]
- Teng, Z.; Chen, Y.; Meng, S.; Duan, M.; Zhang, J.; Ye, N. Environmental stimuli: A major challenge during grain filling in cereals. Int. J. Mol. Sci. 2023, 24, 2255. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Randin, C.; Körner, C. Water availability predicts forest canopy height at the global scale. Ecol. Lett. 2015, 18, 1311–1320. [Google Scholar] [CrossRef]
- Foxx, A.J.; Fort, F. Root and shoot competition lead to contrasting competitive outcomes under water stress: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220674. [Google Scholar] [CrossRef]
- Wang, X.; Singh, D.; Marla, S.; Morris, G.; Poland, J. Field-based high-throughput phenotyping of plant height in sorghum using different sensing technologies. Plant Methods 2018, 14, 53. [Google Scholar] [CrossRef]
- Casebow, R.; Hadley, C.; Uppal, R.; Addisu, M.; Loddo, S.; Kowalski, A.; Griffiths, S.; Gooding, M. Reduced height (RHT) alleles affect wheat grain quality. PLoS ONE 2016, 11, e0156056. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Cao, L.; Liu, M.; Ma, F.; Yan, P.; Zhang, X.; Hu, J.; Niu, F.; He, S.; Cui, J.; et al. The identification and characterization of a plant height and grain length related gene HFR131 in Rice. Front. Plant Sci. 2023, 14, 1152196. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z.-Y. Brassinosteroid regulates stomatal development byGSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef]
- Mahalingam, R. Phenotypic, physiological and malt quality analyses of us barley varieties subjected to short periods of heat and drought stress. J. Cereal Sci. 2017, 76, 199–205. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Arcand, M.M.; Knight, J.D.; Farrell, R.E. Temporal Dynamics of nitrogen rhizodeposition in field pea as determined by 15N labelling. Can. J. Plant Sci. 2013, 93, 941–950. [Google Scholar] [CrossRef]
- Santosh Kumar, V.V.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Senthil Kumar, T.; Chinnusamy, V. The abscisic acid receptor OSPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2020, 72, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Li, Y.; Meng, Z.; Yan, R.; Zhu, T.; Wang, Y.; Kang, S.; Ali Abid, M.; Malik, W.; et al. Activation of ABA receptors gene GHPYL9-11A is positively correlated with cotton drought tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1453. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Heitholt, J.J.; Vaughn, K.C. Gas exchange differences and comparative anatomy among cotton leaf-type isolines. Crop Sci. 1993, 33, 1295–1299. [Google Scholar] [CrossRef]
- Jiang, C.; Wright, R.J.; Woo, S.S.; DelMonte, T.A.; Paterson, A.H. QTL analysis of leaf morphology in tetraploid Gossypium (cotton). Theor. Appl. Genet. 2000, 100, 409–418. [Google Scholar] [CrossRef]
- Wei, H.; Lu, C. Farmland change and its implications in the Three River region of Tibet during recent 20 years. PLoS ONE 2022, 17, e0265939. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, M.-M.; Qiu, C.-W.; Cao, F.; Chen, Z.-H.; Zhang, G.; Wu, F. Response of Tibetan wild barley genotypes to drought stress and identification of quantitative trait loci by genome-wide association analysis. Int. J. Mol. Sci. 2019, 20, 791. [Google Scholar] [CrossRef]
- Scheepens, J.F.; Deng, Y.; Bossdorf, O. Phenotypic plasticity in response to temperature fluctuations is genetically variable, and relates to climatic variability of origin, in Arabidopsis thaliana. AoB PLANTS 2018, 10, ply043. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Wickham, H. GGPLOT2: Elegant Graphics for Data Analysis; Springer Science and Business Media, LLC: Berlin/Heidelberg, Germany, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, B.C.; Han, Y.; Li, C. A Comparison of Different Stomatal Density Phenotypes of Hordeum vulgare under Varied Watering Regimes Reveals Superior Genotypes with Enhanced Drought Tolerance. Plants 2023, 12, 2840. https://doi.org/10.3390/plants12152840
Robertson BC, Han Y, Li C. A Comparison of Different Stomatal Density Phenotypes of Hordeum vulgare under Varied Watering Regimes Reveals Superior Genotypes with Enhanced Drought Tolerance. Plants. 2023; 12(15):2840. https://doi.org/10.3390/plants12152840
Chicago/Turabian StyleRobertson, Brittany Clare, Yong Han, and Chengdao Li. 2023. "A Comparison of Different Stomatal Density Phenotypes of Hordeum vulgare under Varied Watering Regimes Reveals Superior Genotypes with Enhanced Drought Tolerance" Plants 12, no. 15: 2840. https://doi.org/10.3390/plants12152840
APA StyleRobertson, B. C., Han, Y., & Li, C. (2023). A Comparison of Different Stomatal Density Phenotypes of Hordeum vulgare under Varied Watering Regimes Reveals Superior Genotypes with Enhanced Drought Tolerance. Plants, 12(15), 2840. https://doi.org/10.3390/plants12152840









