Characterization and Valorization of Maize Landraces from Aosta Valley
Abstract
1. Introduction
2. Results and Discussion
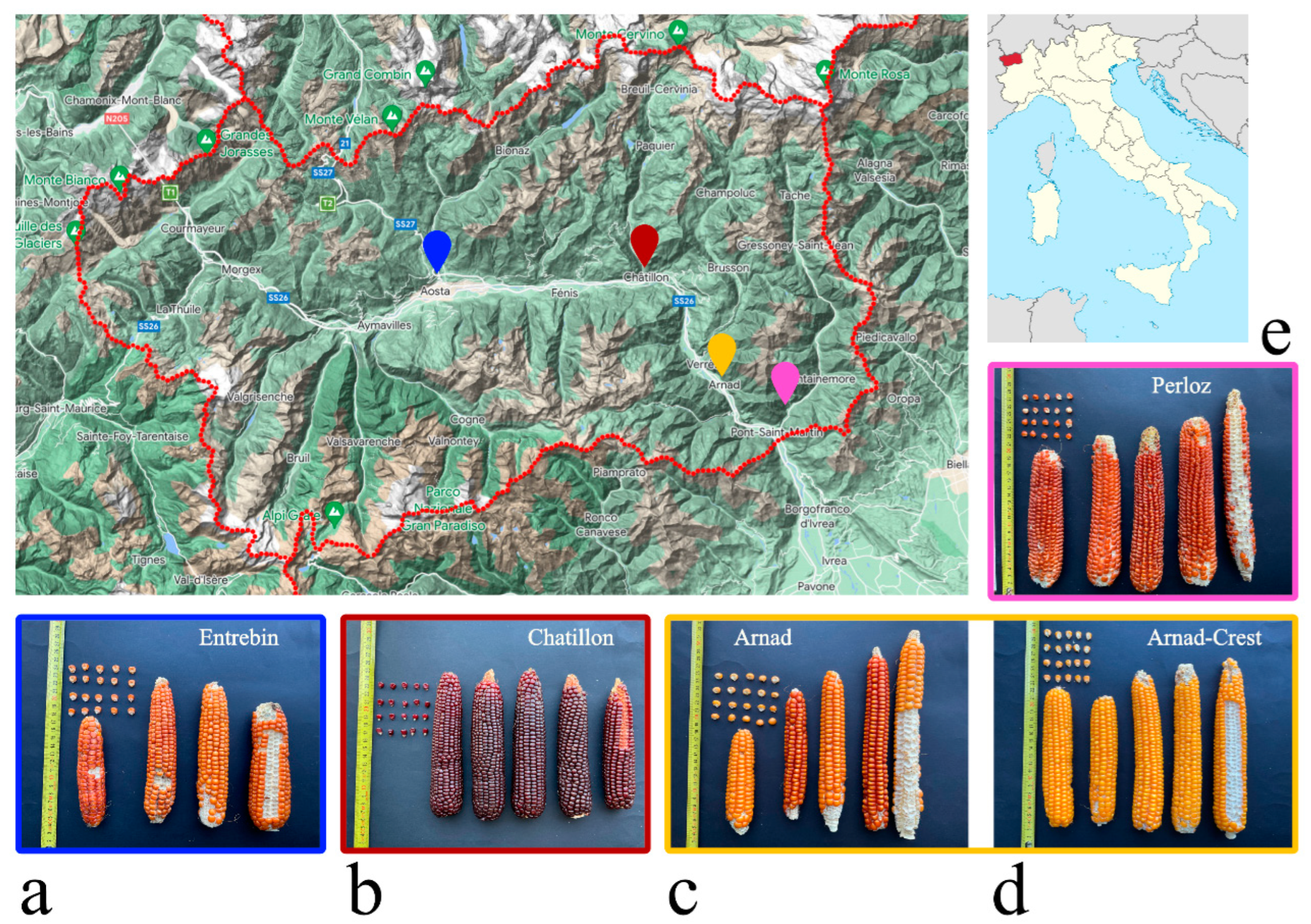
2.1. Historical and Phenotypic Characterization of Maize Landraces
2.2. Genetic Characterization of the Accessions
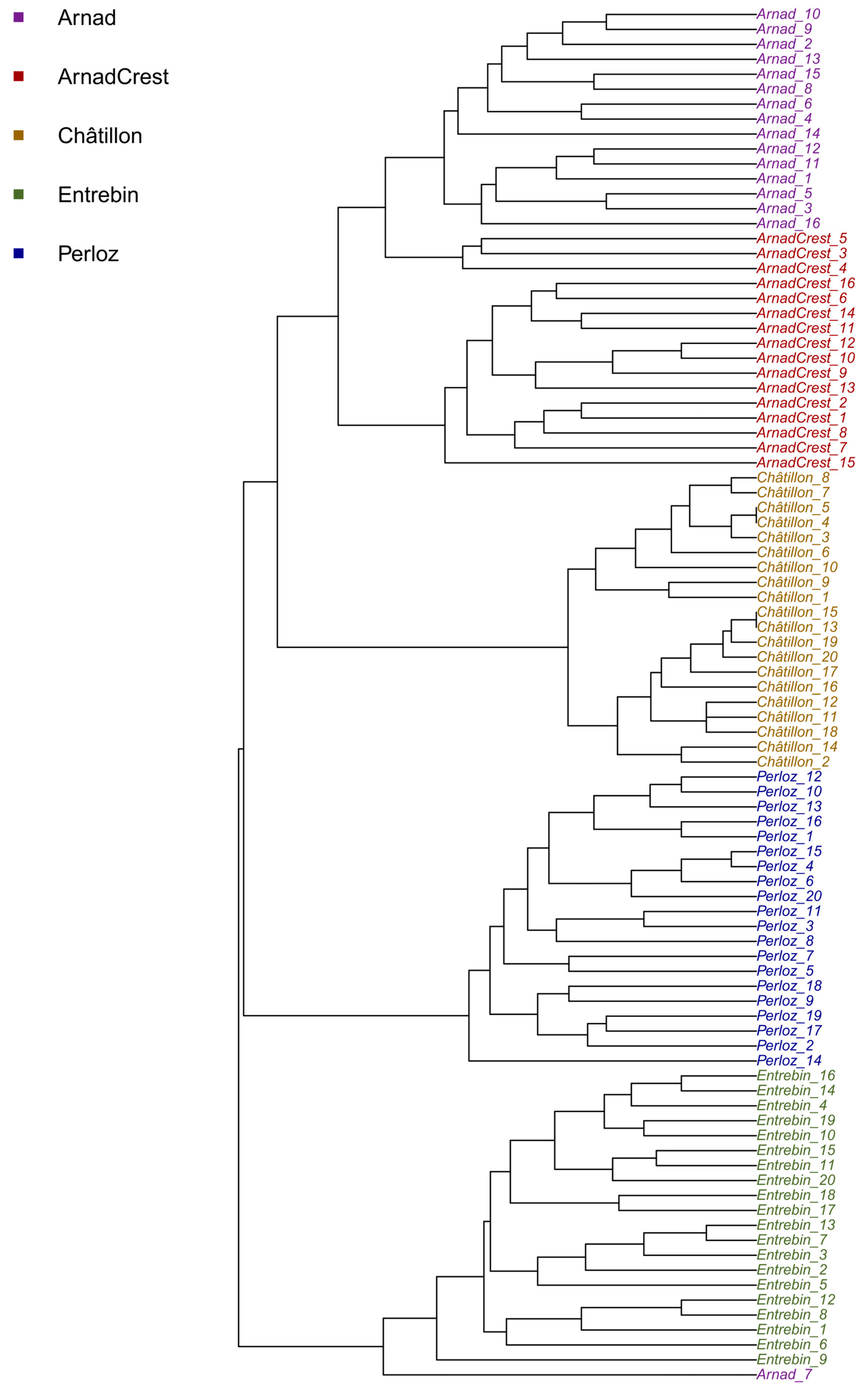
2.3. Cluster Analysis and Phylogenetic Tree
2.4. Population Structure
3. Materials and Methods
3.1. Germplasm Acquisition and Historical Characterization
3.2. Experimental Fields and Phenotypic Characterization
3.3. DNA Extraction and PCR Amplification
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istat Coltivazioni Agricole. Annata Agraria 2019–2020 e Previsioni 2020–2021. Available online: https://www.istat.it/it/files//2021/04/Previsioni-coltivazioni-agricole.pdf (accessed on 30 May 2023).
- Brandolini, A.; Brandolini, A. Maize Introduction, Evolution and Diffusion in Italy. Maydica 2009, 54, 233. [Google Scholar]
- Stagnati, L.; Soffritti, G.; Desiderio, F.; Lanubile, A.; Zambianchi, S.; Marocco, A.; Rossi, G.; Busconi, M. The Rediscovery of Traditional Maize Agrobiodiversity: A Study Case from Northern Italy. Sustainability 2022, 14, 12110. [Google Scholar] [CrossRef]
- Sangiorgio, S.; Colombo, F.; Ghidoli, M.; Giupponi, L.; Ferro, G.; Ferro, C.G.; Cassani, E.; Landoni, M.; Pilu, R. The Ancient Varieties of Mountain Maize: The Inheritance of the Pointed Character and Its Effect on the Natural Drying Process. Agronomy 2021, 11, 2295. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Colombo, F.; Cassani, E.; Hejna, M.; Rossi, L.; Pilu, R. Characterization of “Mais Delle Fiorine” (Zea mays L.) and Nutritional, Morphometric and Genetic Comparison with Other Maize Landraces of Lombardy Region (Northern Italy). Genet. Resour. Crop Evol. 2021, 68, 2075–2091. [Google Scholar] [CrossRef]
- Bertolini, M.; Verderio, A.; Motto, M.; Berardo, N.; Brugna, E.; Balduini, C. Mais in Lombardia: Varietà Tradizionali. In Quaderni Della Ricerca Regione Lombardia; Stamperia Stefanoni: Bergamo, Italy, 2002. [Google Scholar]
- Zapparoli, T.V. Il Granturco; Ramo Editoriale degli Agricoltori: Roma, Italy, 1939. [Google Scholar]
- Argentier, L. Leçons Sur l’agriculture Valdôtaine—1887, 1st ed.; Le Château: Aosta, Italy, 2004. [Google Scholar]
- Perrin, J.C. Le Commerce à La Fin Du XVIIIe Siècle. Bull. Société Académique Relig. Sci. l’Ancien Duché D’aoste—Nouv. Série 1991, 3, 117–118. [Google Scholar]
- Nicco, R. Donnas e Vert Nel Corso Del Secolo XVIII. In Sources et Documents d’Histoire Valdôtaine—Tome Second; Archives Historiques Régionales: Aosta, Italy, 1982; pp. 324–325. [Google Scholar]
- Vescoz, P.L. Origine Du Riz et Du Maïs et Leur Usage Dans La Vallée d’Aoste. Bull. Flore Valdôtaine 1911, 7, 98–100. [Google Scholar]
- Beétemps, A.; Philippot, L. Autour de La Table de Meizoón. In Conserver le Souvenir… Se Souvenir Pour Conserver; Tipografia Duc: Saint-Christophe, Italy, 2005; pp. 14–51. [Google Scholar]
- Zapparoli, T.V. Il Granturco; Biblioteca Agricola “Paravia,” G. B. Paravia & C.: Torino, Italy, 1930. [Google Scholar]
- Anonymous. Jacques Bonhomme: Organe Des Paysans. J. Pop. Polit. Adm. Économique Vallée d’Aoste 1904, 38, 1. [Google Scholar]
- Newton, A.C.; Akar, T.; Baresel, J.P.; Bebeli, P.J.; Bettencourt, E.; Bladenopoulos, K.V.; Czembor, J.H.; Fasoula, D.A.; Katsiotis, A.; Koutis, K.; et al. Cereal Landraces for Sustainable Agriculture. A review. Agron. Sustain. Dev. 2010, 30, 237–269. [Google Scholar] [CrossRef]
- Giupponi, L.; Pedrali, D.; Leoni, V.; Rodari, A.; Giorgi, A. The Analysis of Italian Plant Agrobiodiversity Databases Reveals That Hilly and Sub-Mountain Areas Are Hotspots of Herbaceous Landraces. Diversity 2021, 13, 70. [Google Scholar] [CrossRef]
- Canella, M.; Ardenghi, N.M.G.; Müller, J.V.; Rossi, G.; Guzzon, F. An Updated Checklist of Plant Agrobiodiversity of Northern Italy. Genet. Resour. Crop Evol. 2022, 69, 2159–2178. [Google Scholar] [CrossRef]
- Cassani, E.; Puglisi, D.; Cantaluppi, E.; Landoni, M.; Giupponi, L.; Giorgi, A.; Pilu, R. Genetic Studies Regarding the Control of Seed Pigmentation of an Ancient European Pointed Maize (Zea mays L.) Rich in Phlobaphenes: The “Nero Spinoso” from the Camonica Valley. Genet. Resour. Crop Evol. 2017, 64, 761–773. [Google Scholar] [CrossRef]
- Ardenghi, N.M.G.; Rossi, G.; Guzzon, F. Back to Beaked: Zea Mays Subsp. Mays Rostrata Group in Northern Italy, Refugia and Revival of Open-Pollinated Maize Landraces in an Intensive Cropping System. PeerJ 2018, 6, e5123. [Google Scholar] [CrossRef] [PubMed]
- Lanzanova, C.; Arrigoni, A.; Valoti, P.; Alfieri, M.; Locatelli, S. Agronomic Performance, Chemical Composition and Fusarium Verticillioides Resistance of Italian White Maize Varieties. Qual. Assur. Saf. Crop Foods 2019, 11, 549–559. [Google Scholar] [CrossRef]
- Oppong, A.; Bedoya, C.A.; Ewool, M.B.; Asante, M.D.; Thompson, R.N.; Adu-Dapaah, H.; Lamptey, J.N.; Ofori, K.; Offei, S.K.; Warburton, M.L. Open Access Bulk Genetic Characterization of Ghanaian Maize Landraces Using Microsatellite Markers. Maydica 2013, 59, 1–8. [Google Scholar]
- Ignjatović-Micić, D.; Mladenović Drinić, S.; Nikolić, A.; Lazić-Jančić, V. SSR Analysis for Genetic Structure and Diversity Determination of Maize Local Populations from Former Yugoslavia Territories. Russ. J. Genet. 2008, 44, 1317–1324. [Google Scholar] [CrossRef]
- Cömertpay, G.; Baloch, F.S.; Kilian, B.; Ülger, A.C.; Özkan, H. Diversity Assessment of Turkish Maize Landraces Based on Fluorescent Labelled SSR Markers. Plant Mol. Biol. Rep. 2011, 30, 261–274. [Google Scholar] [CrossRef]
- Palumbo, F.; Galla, G.; Martínez-Bello, L.; Barcaccia, G. Venetian Local Corn (Zea mays L.) Germplasm: Disclosing the Genetic Anatomy of Old Landraces Suited for Typical Cornmeal Mush Production. Diversity 2017, 9, 32. [Google Scholar] [CrossRef]
- Stagnati, L.; Martino, M.; Soffritti, G.; Lanubile, A.; Ravasio, A.; Marocco, A.; Rossi, G.; Busconi, M. Microsatellite and Morphological Characterization of Three Rostrato Di Val Chiavenna (Sondrio, Italy) Maize (Zea mays L.) Accessions. Genet. Resour. Crop Evol. 2021, 68, 3025–3038. [Google Scholar] [CrossRef]
- Stagnati, L.; Soffritti, G.; Martino, M.; Lanubile, A.; Desiderio, F.; Ravasio, A.; Marocco, A.; Rossi, G.; Busconi, M. Morphological and Genetic Characterization of Local Maize Accessions from Emilia Romagna Region, Italy. Sustainability 2021, 14, 91. [Google Scholar] [CrossRef]
- Brandolini, A.; Brandolini, A. Il Mais in Italia: Storia Naturale e Agricola, 2nd ed.; CRF Press: Bergamo, Italy, 2006. [Google Scholar]
- Gage, J.L.; White, M.R.; Edwards, J.W.; Kaeppler, S.; de Leon, N. Selection Signatures Underlying Dramatic Male Inflorescence Transformation during Modern Hybrid Maize Breeding. Genetics 2018, 210, 1125–1138. [Google Scholar] [CrossRef]
- Pepper, G.E. The Effect of Leaf Orientation and Plant Density on the Yield of Maize (Zea mays L.). Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1974. [Google Scholar]
- Giardini, A. La Coltivazione del Mais: Moderni Aspetti della Tecnica Colturale; Dakalb Italiana S.p.A.: Mestre, Italy, 1976. [Google Scholar]
- Bianchi, A.; Lorenzoni, C.; Salamini, F. Mais. In Genetica dei Cereali; Edizioni Agricole della Calderini s.r.l: Bologna, Italy, 1989. [Google Scholar]
- Arteaga, M.C.; Moreno-Letelier, A.; Mastretta-Yanes, A.; Vázquez-Lobo, A.; Breña-Ochoa, A.; Moreno-Estrada, A.; Eguiarte, L.E.; Piñero, D. Genomic Variation in Recently Collected Maize Landraces from Mexico. Genom. Data 2016, 7, 38–45. [Google Scholar] [CrossRef]
- Hartings, H.; Berardo, N.; Mazzinelli, G.F.; Valoti, P.; Verderio, A.; Motto, M. Assessment of Genetic Diversity and Relationships among Maize (Zea mays L.) Italian Landraces by Morphological Traits and AFLP Profiling. Theor. Appl. Genet. 2008, 117, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; do Santos Rabaiolli, S.M.; Stefanel, C.M. Determining the Polymorphism Information Content of a Molecular Marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Liu, K.; Goodman, M.; Muse, S.; Smith, J.S.; Buckler, E.; Doebley, J. Genetic Structure and Diversity Among Maize Inbred Lines as Inferred From DNA Microsatellites. Genetics 2003, 165, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.P. Phangorn: Phylogenetic Analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef]
- Clark, L.V.; Jasieniuk, M. Polysat: An R Package for Polyploid Microsatellite Analysis. Mol. Ecol. Resour. 2011, 11, 562–566. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype Data: Linked Loci and Correlated Allele Frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]




| Alleles | Na | Ne | I | Ho | uHe | PIC | F | FIS | FIT | FST | Nm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phi076 | 10 | 3.40 | 2.21 | 0.76 | 0.18 | 0.41 | 0.72 | 0.65 | 0.57 | 0.74 | 0.39 | 0.40 |
| umc1401 | 7 | 3.40 | 1.97 | 0.84 | 0.55 | 0.50 | 0.67 | −0.11 | −0.09 | 0.19 | 0.26 | 0.72 |
| phi127 | 4 | 2.20 | 1.39 | 0.40 | 0.32 | 0.25 | 0.48 | −0.11 | −0.28 | 0.53 | 0.63 | 0.15 |
| phi031 | 10 | 3.40 | 2.27 | 0.82 | 0.61 | 0.50 | 0.74 | −0.28 | −0.23 | 0.10 | 0.27 | 0.69 |
| umc1075 | 5 | 3.40 | 2.49 | 1.00 | 0.42 | 0.61 | 0.68 | 0.30 | 0.31 | 0.38 | 0.10 | 2.13 |
| umc1327 | 5 | 3.20 | 2.17 | 0.82 | 0.20 | 0.47 | 0.69 | 0.39 | 0.57 | 0.70 | 0.31 | 0.56 |
| phi084 | 6 | 3.40 | 2.34 | 0.85 | 0.51 | 0.50 | 0.52 | −0.01 | −0.02 | 0.24 | 0.26 | 0.71 |
| umc1786 | 8 | 3.00 | 1.93 | 0.69 | 0.49 | 0.41 | 0.74 | −0.23 | −0.20 | 0.28 | 0.40 | 0.37 |
| p-bnlg176 | 4 | 3.00 | 2.09 | 0.81 | 0.30 | 0.50 | 0.48 | 0.35 | 0.40 | 0.56 | 0.26 | 0.71 |
| umc1941 | 8 | 3.40 | 2.24 | 0.85 | 0.41 | 0.47 | 0.57 | 0.10 | 0.13 | 0.39 | 0.30 | 0.58 |
| Mean | 6.70 | 3.18 | 2.11 | 0.78 | 0.40 | 0.46 | 0.63 | 0.10 | 0.12 | 0.41 | 0.32 | 0.70 |
| St. dev. | 2.263 | 0.38 | 0.30 | 0.16 | 0.15 | 0.09 | 0.11 | 0.31 | 0.33 | 0.22 | 0.14 | 0.54 |
| Entrebin | 29 | 2.90 | 2.05 | 0.77 | 0.41 | 0.49 | 0.08 | 0.16 | 0.39 | 0.28 | 0.64 | |
| Perloz | 33 | 3.30 | 2.13 | 0.84 | 0.51 | 0.50 | −0.02 | −0.01 | 0.24 | 0.26 | 0.73 | |
| Châtillon | 24 | 2.40 | 1.51 | 0.43 | 0.16 | 0.25 | 0.21 | 0.35 | 0.76 | 0.64 | 0.14 | |
| Arnad | 35 | 3.50 | 2.39 | 0.91 | 0.44 | 0.53 | 0.13 | 0.17 | 0.35 | 0.22 | 0.87 | |
| Arnad-Crest | 38 | 3.80 | 2.47 | 0.96 | 0.47 | 0.55 | 0.13 | 0.14 | 0.31 | 0.19 | 1.05 | |
| Mean | 31.8 | 3.18 | 2.11 | 0.78 | 0.40 | 0.46 | 0.10 | 0.16 | 0.41 | 0.32 | 0.69 | |
| St. dev. | 5.45 | 0.54 | 0.38 | 0.21 | 0.14 | 0.12 | 0.08 | 0.13 | 0.20 | 0.18 | 0.34 |
| Landrace | Locus | Allele | Frequency |
|---|---|---|---|
| Entrebin | phi031 | 196 | 0.475 |
| Entrebin | umc1786 | 124 | 0.050 |
| Entrebin | umc1786 | 144 | 0.075 |
| Entrebin | umc1941 | 103 | 0.050 |
| Perloz | phi127 | 111 | 0.500 |
| Perloz | phi031 | 192 | 0.425 |
| Perloz | phi084 | 149 | 0.050 |
| Perloz | umc1786 | 130 | 0.375 |
| Perloz | umc1941 | 96 | 0.125 |
| Perloz | umc1941 | 108 | 0.050 |
| Châtillon | phi076 | 142 | 0.125 |
| Châtillon | umc1401 | 116 | 0.300 |
| Châtillon | phi031 | 205 | 0.025 |
| Châtillon | umc1075 | 142 | 0.300 |
| Arnad | umc1401 | 138 | 0.031 |
| Arnad | phi031 | 172 | 0.031 |
| Arnad | phi031 | 209 | 0.031 |
| Arnad | phi031 | 224 | 0.219 |
| Arnad-Crest | phi076 | 133 | 0.031 |
| Arnad-Crest | phi076 | 136 | 0.125 |
| Arnad-Crest | phi076 | 144 | 0.094 |
| Arnad-Crest | phi076 | 146 | 0.031 |
| Arnad-Crest | phi076 | 163 | 0.094 |
| Arnad-Crest | umc1401 | 143 | 0.125 |
| Arnad-Crest | phi127 | 100 | 0.094 |
| Arnad-Crest | phi031 | 189 | 0.063 |
| Entrebin | Perloz | Châtillon | Arnad | Arnad-Crest | |
|---|---|---|---|---|---|
| 0.000 | Entrebin | ||||
| 0.711 | 0.000 | Perloz | |||
| 0.855 | 1.063 | 0.000 | Châtillon | ||
| 0.657 | 0.666 | 0.707 | 0.000 | Arnad | |
| 0.900 | 0.613 | 0.663 | 0.353 | 0.000 | Arnad-Crest |
| Entrebin | Perloz | Châtillon | Arnad | Arnad-Crest | |
|---|---|---|---|---|---|
| 0.000 | Entrebin | ||||
| 0.197 | 0.000 | Perloz | |||
| 0.326 | 0.343 | 0.000 | Châtillon | ||
| 0.197 | 0.194 | 0.287 | 0.000 | Arnad | |
| 0.229 | 0.181 | 0.269 | 0.117 | 0.000 | Arnad-Crest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lezzi, A.; Stagnati, L.; Madormo, F.; Chabloz, D.; Lanubile, A.; Letey, M.; Marocco, A.; Bassignana, M.; Busconi, M. Characterization and Valorization of Maize Landraces from Aosta Valley. Plants 2023, 12, 2674. https://doi.org/10.3390/plants12142674
Lezzi A, Stagnati L, Madormo F, Chabloz D, Lanubile A, Letey M, Marocco A, Bassignana M, Busconi M. Characterization and Valorization of Maize Landraces from Aosta Valley. Plants. 2023; 12(14):2674. https://doi.org/10.3390/plants12142674
Chicago/Turabian StyleLezzi, Alessandra, Lorenzo Stagnati, Francesca Madormo, Denise Chabloz, Alessandra Lanubile, Marilisa Letey, Adriano Marocco, Mauro Bassignana, and Matteo Busconi. 2023. "Characterization and Valorization of Maize Landraces from Aosta Valley" Plants 12, no. 14: 2674. https://doi.org/10.3390/plants12142674
APA StyleLezzi, A., Stagnati, L., Madormo, F., Chabloz, D., Lanubile, A., Letey, M., Marocco, A., Bassignana, M., & Busconi, M. (2023). Characterization and Valorization of Maize Landraces from Aosta Valley. Plants, 12(14), 2674. https://doi.org/10.3390/plants12142674







