Abstract
Gibberellin (GAs) plays an important regulatory role in the development and growth of pineapple (Ananas comosus (L.) Merr.). Bioinformatics was used to confirm the differential expression of GA2 gibberellin oxidase gene AcGA2oxs in the pineapple genome, which laid the foundation for exploring its role in pineapple. In this study, 42 GA2ox genes (AcGA2oxs) were identified in the pineapple genome, named from AcGA2ox1 to AcGA2ox42, and divided into four groups according to phylogenetic analysis. We also analyzed the gene structure, conserved motifs and chromosome localization of AcGA2oxs. AcGA2oxs within the same group had similar gene structure and motifs composition. Collinear analysis and cis-element analysis provided the basis for understanding the evolution and function of GA2ox genes in pineapple. In addition, we selected different tissue parts to analyze the expression profile of AcGA2oxs, and the results show that 41 genes were expressed, except for AcGA2ox18. AcGA2ox18 may not be expressed in these sites or may be pseudogenes. qRT-PCR (real-time fluorescence quantitative PCR) was used to detect the relative expression levels of the GA2ox gene family under different concentrations of GA3 treatment, and it was found that AcGA2ox gene expression was upregulated in different degrees under GA3 treatment. These results provide useful information for further study on the evolution and function of the GA2ox family in pineapple.
1. Introduction
Gibberellins (GAs) is an important hormone in plant growth and development which plays an important role in regulating plant growth and development, including seed germination, stem elongation, root growth, leaf growth, flowering induction and fruit development [1,2,3,4,5]. In recent years, with the in-depth study of gibberellin synthesis and metabolic pathways, almost all gibberellin-related genes have been cloned in large numbers. Although at least 136 kinds of gibberellins with different structures have been isolated and identified from a variety of organisms [6], only a very small number of gibberellins with biological activity have been found through studies (GA1, GA3, GA4, GA5, GA6 and GA7) [7].
The GA2ox genes belongs to the 2OG-Fe(II)oxygenase subfamily, and all members of this subfamily contain a 2OG-Fell_Oxy domain. GA2ox are important genes in the gibberellin metabolic pathway, which is mainly involved in GA inactivation metabolism. The inactivation metabolism of GA is mainly 2β-hydroxylation. The most typical deactivation reaction is 2-β-hydroxylation catalyzed by a class of 2ODDs oxidase (GA2oxs). The initially identified GA2ox was based on C19GA [8,9], including the biologically active GA and its direct precursors GA9 and GA20 [10], which belonged to class I or Class II 2ODDs oxidase according to phylogenetic relationships [10]. Later, a novel GA2ox oxidase that accepts only C20GA as a substrate was reported [11]. These enzymes are classified as Class III 2ODDs oxidase and reduce the amount of biologically active gibberellin by consuming the precursors of synthetic bioactive gibberellin, such as GA12 and GA53 [12].
The GA2ox gene has been identified and cloned in many plants, such as rice (Oryza sativa) [13], Arabidopsis thaliana [14], Phyllostachys heterocycle and tobacco (Nicotiana tobacum) [15,16]. Overexpression of the GA2ox gene can degrade bioactive gibberellin in plants and lead to dwarfing phenotype [17]. For example, in wheat, ectopic expression of the PcGA2ox1 gene in soybean significantly reduces GA content, which led to lower plant height [18]. In rice, overexpression of the OsGA2ox1 gene resulted in a severe reduction in plant height [19], and overexpression of the OsGA2ox6 or OsGA2ox9 gene resulted in a moderate reduction in plant height [20,21].
Pineapple (Ananas comosus (L.) Merr.), widely cultivated in tropical and some subtropical regions, is an important tropical fruit in horticultural production in the world [22]. Pineapple has a high economic value, and pineapple cultivation for local agricultural development is of great significance.
In recent years, the application of plant growth regulators to control the growth and development of pineapple has become a necessary means for the intensive cultivation and development of pineapple. Gibberellin (GA), as an important hormone, has the function of promoting growth. The most obvious biological activity of GA is to promote the elongation of plant cells and make plants grow taller. It can break the dormancy of seeds, tubers and roots and promote their germination. It can stimulate fruit growth, improve seed setting rate or form seedless fruit [23]; promote the early flower bud differentiation of some plants that need low temperature to pass the growth stage and partially compensate the role of sunshine, so that some plants can also bolt and flower under short-day conditions; and induce the formation of α-amylase and accelerate the hydrolysis of stored substances in endosperm cells. As previously reported, we found that exogenous gibberellin can cause browning inside pineapple, and GA2ox can play an important role in reducing GA levels and reducing internal browning (IB) [24]. However, the GA2ox gene family has not been systematically analyzed in pineapple. Therefore, our analysis focused on the identification of the AcGA2ox genes and the characterization of encoding the AcGA2ox family.
In this study, we identified 42 AcGA2ox genes, divided them into four subgroups, and analyzed their gene and protein structures, protein motifs, chromosome distribution and expression profiles. Our results provide a relatively complete profile of the GA2ox gene family in pineapple. This may facilitate further functional analysis of each member and facilitate the improvement of pineapple varieties through gene transfer technology.
2. Results
2.1. Identification and Physicochemical Property Analysis of AcGA2ox Gene Family
The protein sequences of nine Arabidopsis thaliana GA2ox genes were downloaded from Tair, the nine AtGA2ox genes were used as seed sequences and the pineapple whole-genome protein sequence file was used as database, with e value set at 1e-20, and the possible AcGA2ox members were identified by Blast. The 2OG-FeII_Oxy domain HMM matrix of GA2ox was downloaded from the Pfam website, and the AcGA2ox protein with this domain was searched in the database by HMMER3.3.2 software. The protein members identified by Blast and HMM were identified, and incomplete domain sequences were removed by CD-Search tool. Finally, 42 AcGA2ox members were identified, which were named AcGA2ox1-42 according to gene ID sequence. Forty-two members were mapped to nineteen chromosomes (Figure 1), among which chromosomes LG3 and LG4 contained the most GA2ox members (five), and three members were found on LG2, LG6, LG9, LG12 and LG25. No AcGA2ox copies were identified on LG14, LG15, LG16, LG19, LG20 and LG24, and most chromosomes contain one or two members (Table 1). The coding length of the AcGA2ox protein is 150–526 amino acids, the predicted molecular weight is 16.87–118.47 kD and the isoelectric point (pI) is 4.93~8.46. The prediction results show that AcGA2ox41 and AcGA2ox7 exhibit hydrophobicity, and their average hydrophilic values (GRAVY) are 0.136 and 0.146, respectively. Other proteins are hydrophilic, with GRAVY ranges from −0.583 to −0.031 (Table S3).
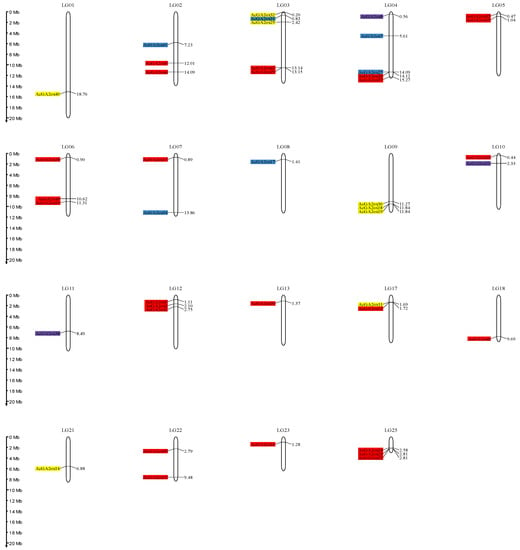
Figure 1.
Genomic distribution of AcGA2ox genes on pineapple chromosomes. Note: Only the chromosomes bearing AcGA2ox genes are represented. Red: Clade I. Yellow: Clade II-A. Purple: Clade II-B. Blue: Clade II-C.

Table 1.
Distribution of AcGA2ox genes on chromosomes.
2.2. Construction and Analysis of Phylogenetic Tree of AcGA2ox Members
In order to elucidate the evolutionary relationship between the pineapple GA2ox protein and the model plant Arabidopsis thaliana, a phylogenetic tree was constructed by NJ method. According to sequence similarity, 9 AtGA2ox members and 42 AcGA2ox members were grouped into two clades, namely Clade I and Clade II. The Clade II clades can be further classified into three subclasses: Clade II-A, Clade II-B and Clade II-C (Figure 2). Notably, Clade I and Clade II-A did not cluster to the Arabidopsis GA2ox protein, and Clade I accounted for a relatively large proportion in pineapple, containing 25 members. Clade II-A contains eight members. Clade II-B has three members and Clade II-C has six members (Table 2).
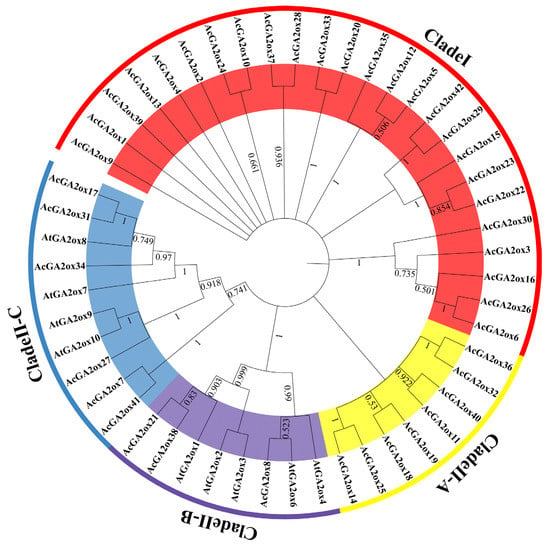
Figure 2.
Phylogenetic analysis of GA2ox proteins from pineapple and Arabidopsis: The pineapple GA2ox proteins are classified into four groups: Clade I, Clade II-A, B and C.

Table 2.
Number distribution of GA2ox proteins.
According to the phylogenetic tree, among the nine GA2ox members in Arabidopsis, AtGA2ox1 is related to AtGA2ox2, AtGA2ox3, AtGA2ox4 and AtGA2ox6. AtGA2ox7 is closely related to AtGA2ox8, AtGA2ox9 and AtGA2ox10 (Figure 3). Interestingly, 33 members of GA2ox in pineapple have no direct homologous genes in Arabidopsis, which may be a new subclass formed in pineapple. These 33 AcGA2ox were clustered into Clade II-A and Clade II-A, suggesting that some Clade II-A may have similar biological functions with Clade II-B and Clade II-C, while Clade I may not. These results suggest that the GA2ox gene in pineapple may have a similar function to that in Arabidopsis and may also derive some new biological functions.
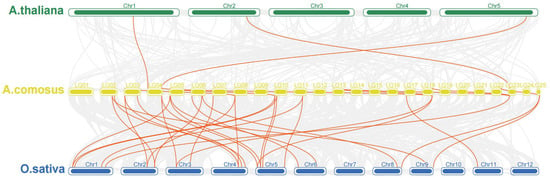
Figure 3.
Collinearity analysis of Ga2ox family genes between A. thaliana, A. comosus and O. Sativa.
2.3. Collinearity Analysis of AcGA2ox Genes
The collinearity analysis of GA2ox genes among the 3 model plants of pineapple, Arabidopsis and rice showed that there were 3 pairs of collinearity genes between pineapple and Arabidopsis, and 19 pairs of collinearity genes between pineapple and rice (Figure 3). The intraspecies collinearity analysis showed that 11 pairs of GA2ox existed in the pineapple genome (Figure 4). The difference in the number of homologous gene pairs between pineapple and the two model plants may be due to the small Arabidopsis genome. There are few collinear gene pairs, which indicates that the retention rate of GA2ox gene is not high during the evolution of pineapple species.
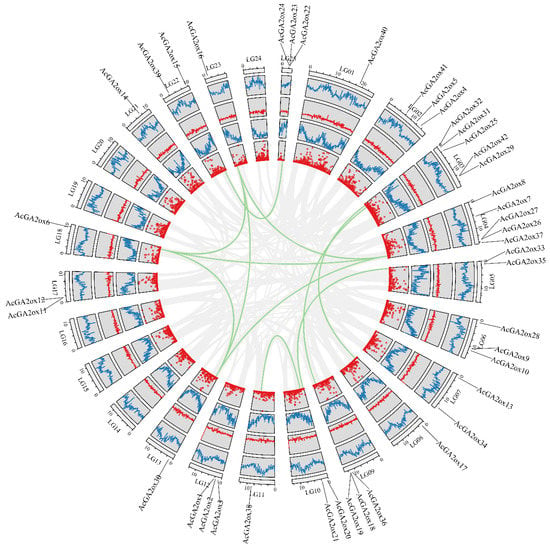
Figure 4.
Collinearity analysis between AcGA2ox genes: The outer circle shows the distribution of the N ratio, the density of the GC skew gene and the change in the GC ratio of pineapple genome from inside to outside, respectively.
2.4. Gene Structure and Cis-Acting Element Analysis of Conserved Motif of AcGA2ox Gene Family
The 42 AcGA2ox proteins contained 10 conserved moTIFs (Motif1~10, Figure S1), and Motif1 existed in all Clades. Motifs 2, 3, 5 and 7 were widely present in Clade II, while some members of Clade I were not. Motifs 4 and 6 were widely present in Clade II-B and Clade II-C, while some members of Clade I and Clade II-A were absent. Motif 9 was widely present in Clade II-B but not in Clade I, Clade II-A or Clade II-C. Motifs 8 and 10 were present in some members of Clade I and Clade II-A but not in Clade II-B and Clade II-C (Figure 5A). All 42 AcGA2ox proteins contain the 2OG-FeII_Oxy domain, AcGA2ox19 contains 3 of this domain, AcGA2ox37, AcGA2ox12, AcGA2ox32 and AcGA2ox14 contain 2 of this domain, and the other proteins contain only 1 of this domain (Figure 5B). TBTools software was used to predict the cy-acting elements in 42 AcGA2ox gene promoter regions (Figure 5C), and the number of cy-acting elements related to hormones, meristem, stress and circadian rhythm was analyzed to further explore the function of AcGA2ox. In this study, our results show that stress-related action elements are prevalent and relatively abundant on the promoter of AcGA2ox. Hormone-related action elements such as methyl jasmonate, gibberellin and abscisic acid were compared. Overall, the amount associated with abscisic acid was relatively small, with only the AcGA2ox10 and AcGA2ox30 elements associated with wound response, and only the AcGA2ox28 and AcGA2ox35 genes were associated with cell cycle response. Nearly half of the genes did not contain gibberellin-responsive elements. Many genes contain abiotic stress elements, such as low temperature, drought and other stress response components. Among the 42 AcGA2ox genes, the number of CDS (Coding sequence) ranged from 2 to 11 (Figure 5D). AcGA2ox1, AcGA2ox18, AcGA2ox20, AcGA2ox29, AcGA2ox33, AcGA2ox39 and AcGA2ox40 had only 2 CDS, while AcGA2ox4 had the largest number of CDS, reaching 11. Some closely related members showed consistency in gene structure, but some showed abundant variation, and no significant difference was found in gene structure among different subclasses, which indicated that the selection pressure of the gene structure was relatively relaxed and the variation was abundant.
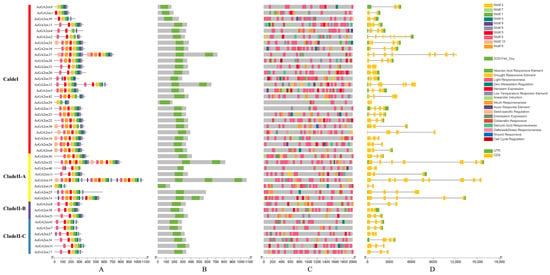
Figure 5.
Gene structure and cis-acting element analysis of conserved motifs of the AcGA2ox gene family: (A) Motif composition of pineapple GA2ox proteins pattern of numbers 1–10 is displayed in boxes of different colors. Sequence information for each motif is provided in attached Figure S1. The length of protein can be estimated by following the scale. (B) The 2OG-Fell_Oxy domain is highlighted with a green box. (C) The promoter sequences of 42 AcGA2ox genes were analyzed with TBTools software (−2000 bp). According to the scale, the upstream length to the translation starting point can be inferred. (D) Exon–intron structure of the AcGA2ox gene green box indicates exon; black line indicates intron; and yellow box indicates the upstream/downstream region of AcGA2ox gene.
2.5. The AcGA2ox Gene Family Showed a Tissue-Specific Expression Pattern
To further analyze the biological function of AcGA2oxs, we collected biological samples of various tissues of pineapple (ovule, sepal, stamen, petal, root, flower, leaf and fruit). The expression of 42 AcGA2ox genes in these tissues was obtained by RNA-Seq technique, and the expression data heat map was constructed (Figure 6). The expression of AcGA2ox was found to be low in half of these tissues. However, it is worth noting that AcGA2ox1, AcGA2ox13, AcGA2ox2, AcGA2ox10, AcGA2ox23, AcGA2ox22, AcGA2ox41 and AcGA2ox7 had high expression levels in the above tissues. The expression of AcGA2ox9 was low only in ovule but high in other tissues. The expression of AcGA2ox39 was lower in petal and fruit but higher in other tissues. The expression of AcGA2ox35 was low in flower and leaf but high in other tissues. AcGA2ox4, AcGA2ox24, AcGA2ox37, AcGA2ox20, AcGA2ox15, AcGA2ox3, AcGA2ox6, AcGA2ox32, AcGA2ox40, AcGA2ox25, AcGA2ox14, AcGA2ox38 and AcGA2ox17 were highly expressed in some tissues. AcGA2ox18 was not expressed in any of the tested samples, possibly because it is a pseudogene or has a special spatiotemporal expression pattern not detected in our library. Therefore, it is speculated that AcGA2ox may play an important role in various growth and development stages of pineapple through the specific expression of some members.
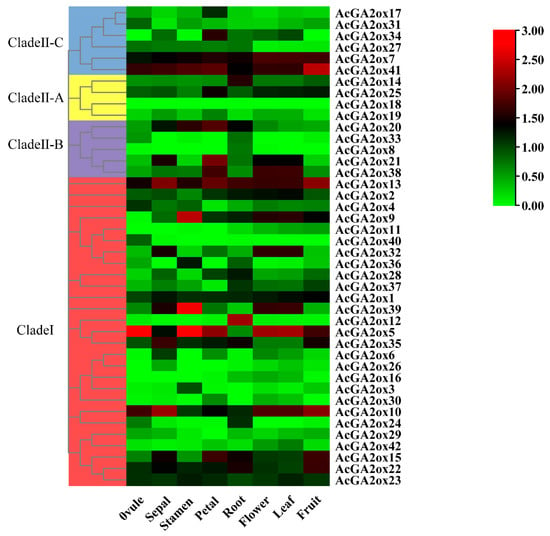
Figure 6.
Expression profile of GA2ox genes in eight pineapple tissues (ovule, sepal, stamen, petal, root, flower, leaf and fruit).
The reliability of the transcriptome data was further validated by an RT-PCR assay, which validated 7 representative samples of 10 selected AcGA2ox genes (Figure 7). Some genes were highly expressed in the detected tissues, including three genes (AcGA2ox7, AcGA2ox9 and AcGA2ox10) in stamens. The expression levels of three genes (AcGA2ox7, AcGA2ox10 and AcGA2ox13) in sepals and one gene (AcGA2ox15) in petals were higher. Some genes are only expressed in certain locations, for example, AcGA2ox5 and AcGA2ox9 are only detected in pistil, stamen and flower.
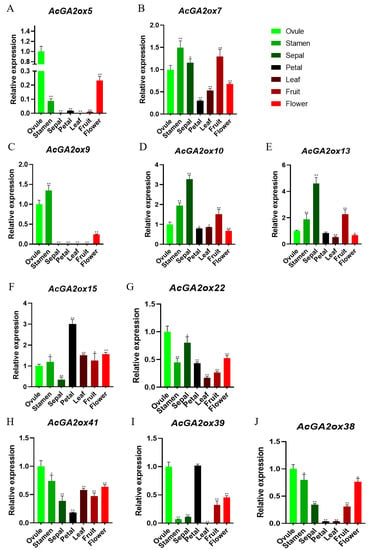
Figure 7.
The expression of 10 AcGA2ox genes in 7 tissues was analyzed by qRT-PCR: Vertical bar indicates standard deviation. The asterisk indicates that the corresponding genes are significantly upregulated or downregulated compared with the untreated control group (* p < 0.05, ** p < 0.01 and Student’s t-test).
2.6. Analysis of GA Hormone-Induced Expression Pattern of AcGA2ox Gene Family
In order to explore the potential role of AcGA2ox in the interaction with hormones, we analyzed the gene expression of some members of the AcGA2ox family by q-PCR. According to previous studies, some GA2ox genes are regulated by exogenous hormones, such as those of medicago truncatula [25] and corn [26]. In order to study the effect of exogenous gibberellin on the expression of the AcGA2ox gene, 10 AcGA2ox genes were selected from the leaves to study their response to exogenous GA3. Three gradients of gibberellin concentration were used to study the effect of exogenous gibberellin on the expression the of AcGA2ox gene. The expression profiles of GA3 at the concentrations of 25 mg/L, 50 mg/L and 100 mg/L were analyzed by RT-PCR (Figure 8). Generally speaking, we found that the expression of these genes was upregulated to different degrees. The expression of AcGA2ox15, AcGA2ox39 and AcGA2ox41 increased first and then decreased; AcGA2ox5 decreased first and then increased; and AcGA2ox38 showed a fluctuation between decline, rise and fall. Interestingly, different concentrations of GA3 also had different effects on the expression of the same gene. For example, AcGA2ox9, AcGA2ox22 and AcGA2ox10 were upregulated and then downregulated at 25 mg/L, and AcGA2ox9 returned to its original expression level after 12 h. These genes were upregulated at 100 mg/L, which indicated that some genes may need a longer expression time to eliminate the effect of higher concentrations of GA3. The highest expression level of AcGA2ox39 was 114.8 times that of the control. The expression of AcGA2ox5 was the least affected by exogenous GA3.
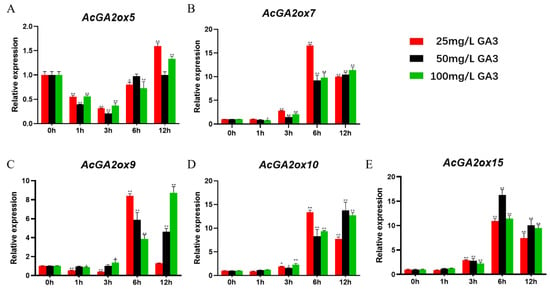
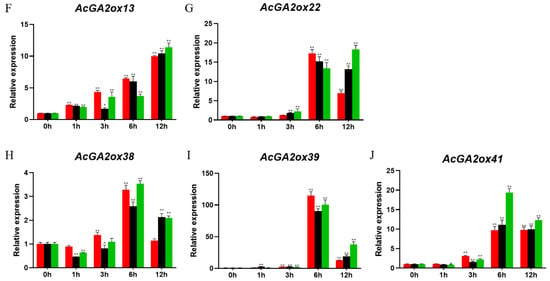
Figure 8.
Expression levels of AcGA2ox in grape leaves after 12 h under different treatments with CK (contrast check): The expression level of the 10 AcGA2ox were genes acquired by qRT-PCR. Error bars indicate the standard deviation. Asterisks on top of the bars indicate statistically significant differences between the stress and counterpart controls (* p < 0.05, ** p < 0.01 and Student’s t-test).
2.7. Subcellular Localization of AcGA2ox Proteins
Several representative proteins (AcGA2ox5, AcGA2ox7, AcGA2ox10, AcGA2ox13, AcGA2ox9, AcGA2ox38 and AcGA2ox22) from each subgroup were selected for subcellular localization studies to explore the expression location of AcGA2ox protein in cells. The Ubi:AcGA2ox:GFP carrier and the control Ubi:GFP were injected into the epidermal cells of Ben’s tobacco leaves (Figure 9). Subcellular localization analysis showed that AcGA2ox5, AcGA2ox7, AcGA2ox10 and AcGA2ox13 were located in the nucleus and cell membrane of tobacco epidermal cells. AcGA2ox9, AcGA2ox38 and AcGA2ox22 were localized only in the cell membrane. This is consistent with previous results [27].
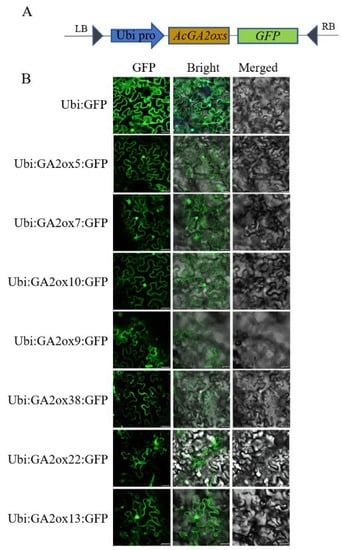
Figure 9.
Subcellular localization analysis: (A) Construction pattern diagram of expression vector. (B) The fusion proteins Ubi:GFP and Ubi:AcGA2ox:GFP were transiently expressed in Nicotiana benthamiana leaf cells and observed with a laser scanning confocal microscope. Scale bar = 25 µm.
3. Discussion
The GA2ox family plays an important role in the synthesis and metabolism of gibberellin in plants and belongs to the gibberellin oxidase gene family, which also includes GA3ox and GA20ox [28]. It has been reported that members of the GA2ox family play a crucial role in the growth and development of various plants [29,30,31,32]. Previous studies have identified 12 GA2ox family members in maize [26], 11 GA2ox family members in grape and 10 GA2ox family members in soybean [33,34]. Eleven and nine members of the GA2ox family have been identified in rice and Arabidopsis, but the pineapple GA2ox family has not been identified. Pineapple is a tropical fruit with great economic value and research value, so it is necessary to study the role of the GA2ox family in pineapple.
In this study, bioinformatics was used to search for gene members with similar 2OG-Fell_Oxy domain in the pineapple genome. A total of 42 AcGA2ox genes were identified, some of which may be pseudogenes or duplicate events. The degree of genetic differentiation is still much higher than that of maize, grape, soybean, rice and Arabidopsis, and the diversity of gene structural patterns means that the process of generating new parallel sequences is more complex. Members of the pineapple GA2 oxidase gene with longer introns appear, such as AcGA2ox14, AcGA2ox19, AcGA2ox11, AcGA2ox32 and AcGA2ox37. Long introns can arise from unequal crossings, while random insertion of a retrotransposon can produce a new gene. In order to further reveal the phylogeny of the pineapple GA2ox family, a phylogenetic tree of the pineapple GA2ox family was constructed, and 42 members of the pineapple GA2ox family were divided into 4 groups (Figure 2).
Analysis of promoter cis-acting elements showed that AcGA2ox contained some cis-acting elements related to hormones, meristem, stress and cell cycle regulation. These genes are also affected by GA regulation. Interestingly, a considerable number of gene promoters do not contain gibberellin-related elements, suggesting that their function may be to regulate changes in endogenous GA content through other factors on the GA signaling pathway, thereby regulating GA levels in plants.
Gene expression patterns can preliminarily predict gene function. Transcriptome results showed that only a small part of the AcGA2ox gene was highly expressed in plants, because GA2ox, as an enzyme for the digestion of gibberellin activity, can only increase the expression level when gibberellin is overpresent in plants. qRT-PCR results show that three genes (AcGA2ox10, AcGA2ox13 and AcGA2ox15) showed high expression levels in selected pineapple tissues, suggesting that GA2ox may play a role in regulating gibberellin concentration during the development of pineapple. Some AcGA2ox genes are highly expressed in pistils, stamens and sepals, such as AcGA2ox7, AcGA2ox10, AcGA2ox13, etc., indicating that these genes play an important role in flower formation and the reproductive stage of plants. In addition, some AcGA2ox genes are expressed in different tissues or at different stages, indicating that these genes may be more stable than genes expressed only at a certain stage in specific tissues or organs [35]. The tissue expression pattern indicated that AcGA2oxs play essential roles in pineapple reproductive development, as shown in (Figure 10). It also provides an important foundation for understanding the roles of GA2ox genes in regulating the leaf development and root growth in pineapple [8,36].
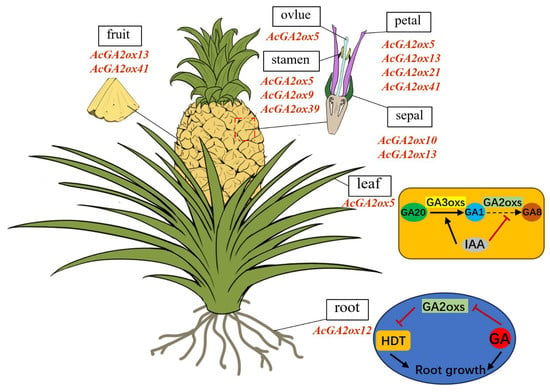
Figure 10.
The potential function of GA2ox genes in pineapple: Schematic model of AcGA2ox gene expression patterns during leaf and root development. The AcGA2ox genes listed were highly expressed genes in corresponding tissues.
The relationship between GA2oxs and GA in pineapple was studied by qRT-PCR method after different concentrations of gibberellin were treated. The results show that the expression of these genes was upregulated after GA3 treatment for a period of time, because GA2 oxidase is a key enzyme in the degradation process of GA, which can inactivate bioactive GAs, its precursors and other intermediates in plants, thus maintaining the balance between biologically active GAs and intermediates in plants [37,38]. Generally, the response time of pineapple GA2oxs to gibberellin at different concentrations was different, and the overall trend was that the expression of GA3 at low concentration reached the peak earlier than that at high concentration and then began to decline, indicating that the deactivation of exogenous GA at higher concentration required more expression of GA2oxs in pineapple.
4. Materials and Methods
4.1. Identification of GA2ox Gene Family Members and Analysis of Physical and Chemical Properties of Pineapple Protein
Using Tair (https://www.arabidopsis.org/ accessed on 22 July 2022) to retrieve the Arabidopsis (Arabidopsis thaliana) GA2ox genes, its gene ID and protein sequences were reported. The protein sequence, genomic DNA sequence, gene CDS sequence and GFF annotation information of Arabidopsis thaliana and pineapple (Ananas comosus (L.) Merr.) were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov/ accessed on 22 July 2022). The obtained AtGA2ox protein sequence was used as the seed sequence, and the whole protein sequence of pineapple was used to build a library. BLAST was carried out in the library, the E value was set to 1e-20 and the copies were deleted. From the PFAM database (https://www.ebi.ac.uk/interpro, accessed on 22 July 2022), the matrix of the 2OG-FeII_Oxy domain was downloaded from (http://pfam.xfam.org/, accessed on 22 July 2022), and the AcGA2ox gene was retrieved by HMMER 3.3.2 software [39]. Combining the results obtained from BLAST and HMMER, AcGA2ox members were identified. Using the CD-Search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi/, accessed on 22 July 2022) to verify the integrity and reliability of the structural domain (E value < 0.01), the incomplete structure domain sequence was removed. Finally, the number of AcGA2ox genes in the family was determined. The ExPASy ProtParam tool was used on the server (https://web.expasy.org/protparam/, accessed on 22 July 2022) to predict GA2ox proteins’ isoelectric point (pI), molecular weight (MW) and other physical and chemical properties.
4.2. Construction of Phylogenetic Tree of GA2ox Gene Family
Multiple sequence alignment and phylogenetic analysis were used to detect the evolutionary relationship between Arabidopsis and pineapple GA2ox protein. In MEGAX 7.0 software [40], the neighbor join (NJ) method was used to generate the system tree, and multiple sequences were compared with default parameters. The bootstrap number was set to 1000 to estimate the branch length. The tree is visualized using iTOL (https://itol.embl.de/, accessed on 22 July 2022).
4.3. Prediction of Gene Structure and Cis-Acting Element of Protein Conserved Domain
The MEME website (http://meme-suite.org/tools/meme, accessed on 22 July 2022) was used to predict the conserved Motif of the AcGA2ox protein, and the number of motifs was set to 10. The gene structure of AcGA2ox was analyzed by TBTools [41]. The upstream 2 kb of the start codon of the AcGA2ox gene family was extracted as the promoter sequence, and the composition of its cis-acting elements was analyzed by TBTools to understand its abiotic stress response specificity. The above analysis results were visualized using TBTools.
4.4. Chromosome Location and Collinearity Analysis
Using the downloaded GFF annotation information, the GA2ox genes present on the definite chromosomes of pineapple were located on their respective chromosomal locations and visualized using the MG2C website [42]. MCScanX was used to analyze the collinearity between pineapple genome and Arabidopsis and rice [43], and TBTools was used for visualization.
4.5. Expression Analysis of GA2ox Gene Family in Different Tissues of Pineapple
By studying the transcription levels of various tissues during the development of pineapple, we hope to find the important role of AcGA2ox in plant growth. Transcriptome data came from the previous study of our research group [35,38,44]. Transcripts with consistent sequences were obtained by local BLAST comparison, then corresponding FPKM values were calculated and expression heat maps were drawn by TBTools.
4.6. Plant Material and Sample Preparation
The pineapple variety is “MD2”, provided by Qin Yuan’s research group (Genomics and Biotechnology Center, Fujian Agriculture and Forestry University, Fuzhou, China). It is grown in a greenhouse at 25 °C, with 16 h light and 8 h dark treatment and relative humidity at 70%, lasting for one month. When the plants reached the flowering stage, different floral organs (i.e., ovule, sepal, stamen, petal, flower and fruit) were collected, including root and leaf. Gibberellin treatment group treated in the following ways: 25 mg/L GA3, 50 mg/L GA3 and 100 mg/L GA3. Pineapple leaf samples were collected 1, 3, 6 and 12 h after treatment. One-month-old pineapple plants without any treatment were used as controls. All samples were harvested, snap-frozen using liquid nitrogen and kept at −80 °C until RNA extraction. Three biological replicates of each sample were collected for analysis.
4.7. RNA Isolation and qRT-PCR Analysis
RNA kit (OMEGA, Shanghai, China) extracted total RNA. All samples were reverse-transcribed into cDNA with 1 μg RNA by a reverse transcription reagent (Vazyme HiScript III All-in-One RT SuperMix Perfect for qPCR). For the differences between primer design and respective AcGA2ox sequences, all primers were synthesized by Shenggong Biological Company (Fuzhou, China), and the primer sequences are shown in (Table S1). The qRT-PCR reaction system was designed to be 20 μL, including 10 μL mix (Taq Pro Universal SYBR qPCR Master Mix, Shanghai, China), 6 μL water, 1 μL F-terminal primer, 1 μL R-terminal primer and 2 μL template. The RT-PCR reactions program was completed with the following conditions: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 34 s; and 95 °C for 15 s [45,46]. The analyses were confirmed in triplicate. The relative expression level of each AcGA2ox gene was calculated based on the comparison threshold period (2−ΔΔCt) method [47]. Pineapple actin-101 gene was used as the internal reference gene. Relative quantitative analysis was performed on the data obtained, and statistical analysis was performed using EXCEL 2021 and Graph Pad Prism 8.0 software.
4.8. Subcellular Localization of GA2ox Gene Family in Pineapple
Seven gene design primers were selected from the four clades of AcGA2ox, the cDNA of pineapple was cloned and the bands of correct size (TIANGENDP219-03) were recovered using a kit, and the primer sequences are shown in (Table S2). The vector was enzymatized with SmaI infusionDNA, ligase was used to connect to the Ubi-GFP (Qin-Lab, Fuzhou, China) carrier and the obtained ligate was transformed into DH5α receptor cells. After PCR amplification and sequencing, positive clones were screened, and the plasmid was extracted to obtain the fusion expression vector Ubi:AcGA2ox:GFP and the target gene. The constructed vector plasmid was transferred to Agrobacterium GV3101 by electrotransformation method and cultured at 30 °C for 2d; Agrobacterium was suspended from solid to liquid medium of 10 mL LB, the suspensions were suspended and the activated bacterial solution OD600 ranged from 0.6–1.0. The tobacco infective liquid (MES 10 mM, MgCl2 10 mM and AS 0.2 mM) was injected into the lower epidermis of the tobacco leaf, cultured in low light for 2d, prepared into the slide, observed and photographed by Leica-SP8 laser confocal microscope.
5. Conclusions
In this study, 42 GA2ox gibberellin oxidase genes were identified in pineapple, which were divided into 4 subfamilies. GA2ox is related to plant response to exogenous hormone expression and abiotic stress expression, and the expression level of GA2ox is different in different tissues. The main function of GA2ox is to inactivate the active bioactive gas GA. The results of this study provide a basis for further study on the evolution and function of the AcGA2ox gene family in pineapple plants.
Supplementary Materials
The following support information can be downloaded from https://www.mdpi.com/article/10.3390/plants12142673/s1. Figure S1. AcGA2ox protein contains ten conserved motifs. Table S1. The primers used for quantification. Table S2. Primers used for subcellular localization. Table S3. Physical and chemical properties of AcGA2ox protein.
Author Contributions
Data curation, J.Q. and J.C.; formal analysis, S.M., K.L., H.S., M.C., Y.H., X.X. and Z.C.; writing—original draft, W.Z.; writing—review and editing, Y.Q. and H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the funding from the National Natural Science Foundation of China (32170352, 31970333), the Excellent Youth Foundation of Fujian Province to H.C. (2022J06014), the Excellent Youth Foundation of Fujian Agriculture and Forestry University to H.C. (xjq202108) and the Science and technology innovation project of Pingtan Science and Technology Research Institute (PT2021007, PT2021003).
Data Availability Statement
All data analyzed during this study are included in this article and its additional files.
Acknowledgments
We would like to thank the reviewers for their helpful comments on the original manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Zhang, L.L.; Ni, S.J.; Zhang, Z.; Zhao, Y.Z.; Li, X.; Mao, T.; Liu, Y.; Liu, F.C. Sustained release effects of exogenous GA3 on germination and growth of rice seedling under salt stress. China Rice 2018, 24, 42–46. [Google Scholar]
- Ouellette, L.; Tuan, P.A.; Toora, P.K.; Yamaguchi, S.; Ayele, B.T. Heterologous functional analysis and expression patterns of gibberellin 2-oxidase genes of barley (Hordeum vulgare L.). Gene 2023, 861, 147255. [Google Scholar] [PubMed]
- Wang, F.; Wang, M.; Jiang, X.J.; Bai, C.Q.; Lin, J. Effects of Gibberellin Acid (GA3) of Leaf Characters and Panicle Characters at Different Stages of Lu18S. Hunan Agric. Sci. 2018, 4, 27–30. [Google Scholar]
- Sun, X.; Wang, K.C.; Xue, Q.; Mao, X.M.; Zeng, J.L.; Chen, Q.F. Effects of gibberellin soaking on nutrient metabolism and anthesis of dormant saffron. Chin. J. Soil Sci. 2018, 49, 355–361. [Google Scholar]
- Tang, D.; Wen, T.J.; Long, L.U.; Chang, X.; Jian-Fang, H.U. Effects of gibberellin treatment on flowering-time of ‘Fenghou’ grapevin and its molecular mechanisms. J. China Agric. Univ. 2015, 20, 92–98. [Google Scholar]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez-y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar]
- Zhang, X.R. Auxin and Gibberellins Involved in the Changes of Maize Root Morphology during Phosphorous Starvation. Master’s Thesis, School of Life Science, Shandong University, Jinan, China, 2011. [Google Scholar]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [PubMed]
- Li, Q.; Wu, J.M.; Liang, H.; Huang, X.; Qiu, L.H. Gibberellins biosynthesis and signaling transduction pathway in higher plant. Biotechnol. Bull. 2014, 10, 16–22. [Google Scholar]
- Thomas, S.G.; Phillips, A.L.; Hedden, P. Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 1999, 96, 4698–4703. [Google Scholar]
- Lee, D.J.; Zeevaart, J.A.D. Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol. 2005, 138, 243–254. [Google Scholar]
- Varbanova, M.; Yamaguchi, S.; Yang, Y.; McKelvey, K.; Hanada, A.; Borochov, R.; Yu, F.; Jikumara, Y.; Ross, J.; Cortes, D.; et al. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell 2007, 19, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Kobayashi, M.; Itoh, H.; Tagiri, A.; Kayano, T.; Tanaka, H.; Iwahori, S.; Matsuoka, M. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 2001, 125, 1508–1516. [Google Scholar] [PubMed]
- Ribeiro, D.M.; Araújo, W.L.; Fernie, A.R.; Schippers, J.H.M.; Mueller-Roeber, B. Translatome metabolome effects triggered by gibberellins during rosette growth in Arabidopsis. J. Exp. Bot. 2012, 63, 2769–2786. [Google Scholar] [PubMed]
- Yang, Y.H.; Wang, S.N.; Xu, H.; Sun, H.Y.; Zhao, H.S.; Chen, D.F.; Gao, Z.M. Genome-wide identification of the enzyme genes involved in gibberellin biosynthesis and their expression analysis in moso bamboo. Genom. Appl. Biol. 2018, 37, 3966–3977. [Google Scholar]
- Jiang, S.; Dai, C.B.; Sun, Y.H. Cloning, expression and subcellular localization of gibberellin 2-oxidase gene NtGA2ox1 from Nicotinana tobacum. Genom. Appl. Biol. 2020, 39, 132–137. [Google Scholar]
- Giacomelli, L.; Rota-Stabelli, O.; Masuero, D.; Acheampong, A.K.; Moretto, M.; Caputi, L.; Vrhovsek, U.; Moser, C. Gibberellin metabolism in Vitis vinifera L. during bloom fruit-set: Functional characterization evolution of grapevine gibberellin oxidases. J. Exp. Bot. 2013, 64, 4403–4419. [Google Scholar]
- Appleford, N.E.J.; Wilkinson, M.D.; Ma, Q.; Evans, D.J.; Stone, M.C.; Pearce, S.P.; Powers, S.J.; Thomas, S.G.; Jones, H.D.; Phillips, A.L.; et al. Decreased shoot stature grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J. Exp. Bot. 2007, 58, 3213–3226. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Mei, Z.; Duan, J.; Chen, H.; Feng, H.; Cai, W. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS ONE 2014, 9, e87110. [Google Scholar]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.; Chen, L.-J.; Yu, S.-M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar]
- Huang, J.; Tang, D.; Shen, Y.; Qin, B.; Hong, L.; You, A.; Li, M.; Wang, X.; Yu, H.; Gu, M.; et al. Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). J. Genet. Genom. 2010, 37, 23–36. [Google Scholar] [CrossRef]
- Moyle, R.; Fairbairn, D.J.; Ripi, J.; Crowe, M.; Botella, J.R. Developing pineapple fruit has a small transcriptome dominated by metallothionein. J. Exp. Bot. 2005, 56, 101–112. [Google Scholar] [PubMed]
- Chen, P.; Li, Y.; Zhao, L.; Hou, Z.; Yan, M.; Hu, B.; Liu, Y.; Azam, S.M.; Zhang, Z.; Rahman, Z.U.; et al. Genome-Wide Identification and Expression Profiling of ATP-Binding Cassette (ABC) Transporter Gene Family in Pineapple (Ananas comosus (L.) Merr.) Reveal the Role of AcABCG38 in Pollen Development. Front. Plant Sci. 2017, 8, 2150. [Google Scholar] [PubMed]
- Zhang, Q.; Rao, X.; Zhang, L.; He, C.; Yang, F.; Zhu, S. Mechanism of internal browning of pineapple: The role of gibberellins catabolism gene (AcGA2ox) and GAs. Sci. Rep. 2016, 6, 33344–33359. [Google Scholar]
- Kim, G.B.; Son, S.U.; Yu, H.J.; Mun, J.H. MtGA2ox10 encoding C20-GA2-oxidase regulates rhizobial infection and nodule development in Medicago truncatula. Sci. Rep. 2019, 9, 5952–5966. [Google Scholar]
- Li, Y.; Shan, X.; Jiang, Z.; Zhao, L.; Jin, F. Genome-wide identification and expression analysis of the GA2ox gene family in maize (Zea mays L.) under various abiotic stress conditions. Plant Physiol. Biochem. 2021, 166, 621–633. [Google Scholar]
- Zhang, J.Q.; Hu, H.K.; Xu, C.M.; Hu, Y.Y.; Huang, Y.J.; Xia, G.H.; Huang, J.Q.; Chang, Y.Y.; Ye, L.; Lou, H.Q.; et al. Cloning, Subcellular Localization and Function Verification of Gibberellin 2-Oxidase Gene in Walnut (Juglans regia). Sci. Silvae Sin. 2019, 55, 50–60. [Google Scholar]
- Xie, Y.; Chen, L. Epigenetic Regulation of Gibberellin Metabolism and Signaling. Plant Cell Physiol. 2020, 61, 1912–1918. [Google Scholar]
- Yang, Y.; Wassie, M.; Liu, N.-F.; Deng, H.; Zeng, Y.-B.; Xu, Q.; Hu, L.-X. Genotypic-specific hormonal reprogramming and crosstalk are crucial for root growth and salt tolerance in bermudagrass (Cynodon dactylon). Front. Plant Sci. 2022, 13, 95–107. [Google Scholar]
- Liu, X.; Wang, J.; Sabir, I.A.; Sun, W.; Wang, L.; Xu, Y.; Zhang, N.; Liu, H.; Jiu, S.; Liu, L.; et al. PavGA2ox-2L inhibits the plant growth and development interacting with PavDWARF in sweet cherry (Prunus avium L.). Plant Physiol. Biochem. 2022, 186, 299–309. [Google Scholar]
- Wang, Y.; Du, F.; Wang, J.; Li, Y.; Zhang, Y.; Zhao, X.; Zheng, T.; Li, Z.; Xu, J.; Wang, W.; et al. Molecular Dissection of the Gene OsGA2ox8 Conferring Osmotic Stress Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 9107. [Google Scholar] [CrossRef]
- Albertos, P.; Wlk, T.; Griffiths, J.; Pimenta Lange, M.J.; Unterholzner, S.J.; Rozhon, W.; Lange, T.; Jones, A.M.; Poppenberger, B. Brassinosteroid-regulated bHLH transcription factor CESTA induces the gibberellin 2-oxidase GA2ox7. Plant Physiol. 2022, 188, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liang, G.; Lu, S.; Wang, P.; Liu, T.; Ma, Z.; Zuo, C.; Sun, X.; Chen, B.; Mao, J. Genome-Wide Identification and Expression Analysis of GA2ox, GA3ox, and GA20ox Are Related to Gibberellin Oxidase Genes in Grape (Vitis vinifera L.). Genes 2019, 10, 680–691. [Google Scholar] [PubMed]
- Han, F.; Zhu, B. Evolutionary analysis of three gibberellin oxidase genes in rice, Arabidopsis, and soybean. Genes 2011, 473, 23–35. [Google Scholar]
- Huang, Y.; Liu, Y.; Zhang, M.; Chai, M.; He, Q.; Jakanda, B.H.; Chen, F.; Chen, H.; Jin, X.; Cai, H.; et al. Genome-wide identification and expression analysis of the ERF transcription factor family in pineapple (Ananas comosus (L.) Merr.). PeerJ 2020, 8, 15–26. [Google Scholar] [CrossRef]
- Wuddineh, W.A.; Mazarei, M.; Zhang, J.; Poovaiah, C.R.; Mann, D.G.J.; Ziebell, A.; Sykes, R.W.; Davis, M.F.; Udvardi, M.K.; Stewart, C.N. Identification and overexpression of gibberellin 2-oxidase (GA2ox) in switchgrass (Panicum virgatum L.) for improved plant architecture and reduced biomass recalcitrance. Plant Biotechnol. J. 2015, 13, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.O.; Nunes-Nesi, A.; Araújo, W.L.; Fernie, A.R. To Bring Flowers or Do a Runner: Gibberellins Make the Decision. Mol. Plant 2018, 11, 4–6. [Google Scholar] [CrossRef]
- Chen, J.; Wan, H.; Zhu, W.; Dai, X.; Yu, Y.; Zeng, C. Identification and Expression Analysis of the Isopentenyl Transferase (IPT) Gene Family under Lack of Nitrogen Stress in Oilseed (Brassica napus L.). Plants 2023, 12, 2166–2186. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; López, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, 200–204. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, L.; Li, W.; Zhao, L.; Huang, X.; Azam, S.M.; Qin, Y. Genome-Wide Identification of Auxin Response Factor (ARF) Genes Family and its Tissue-Specific Prominent Expression in Pineapple (Ananas comosus (L.) Merr.). Trop. Plant Biol. 2017, 10, 86–96. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Shi, H.; Guo, M.; Chai, M.; He, Q.; Yan, M.; Cao, D.; Zhao, L.; Cai, H. Evolutionary and expression analyses of soybean basic Leucine zipper transcription factor family. BMC Genom. 2018, 19, 159. [Google Scholar] [CrossRef]
- Chai, M.; Cheng, H.; Yan, M.; Priyadarshani, S.; Zhang, M.; He, Q.; Huang, Y.; Chen, F.; Liu, L.; Huang, X.; et al. Identification and expression analysis of the DREB transcription factor family in pineapple (Ananas comosus (L.) Merr.). PeerJ 2020, 8, 9006–9033. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, M.; Chai, M.; He, Q.; Huang, X.; Zhao, L.; Qin, Y. Epigenetic regulation of anthocyanin biosynthesis by an antagonistic interaction between H2A.Z and H3K4me3. N. Phytol. 2019, 221, 295–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).