Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth
Abstract
1. Introduction
2. Results
2.1. Physical Properties
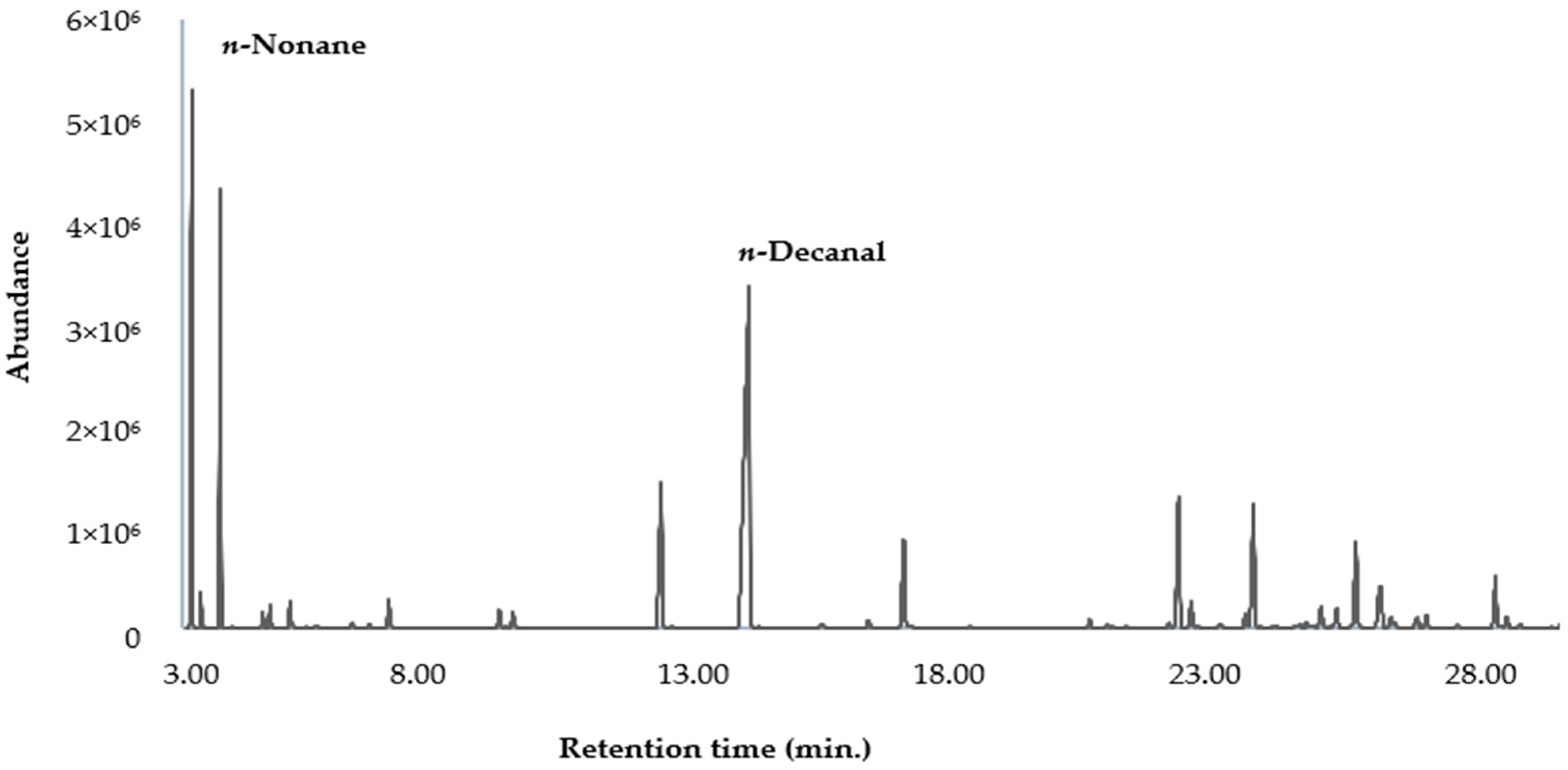
2.2. Chemical Composition
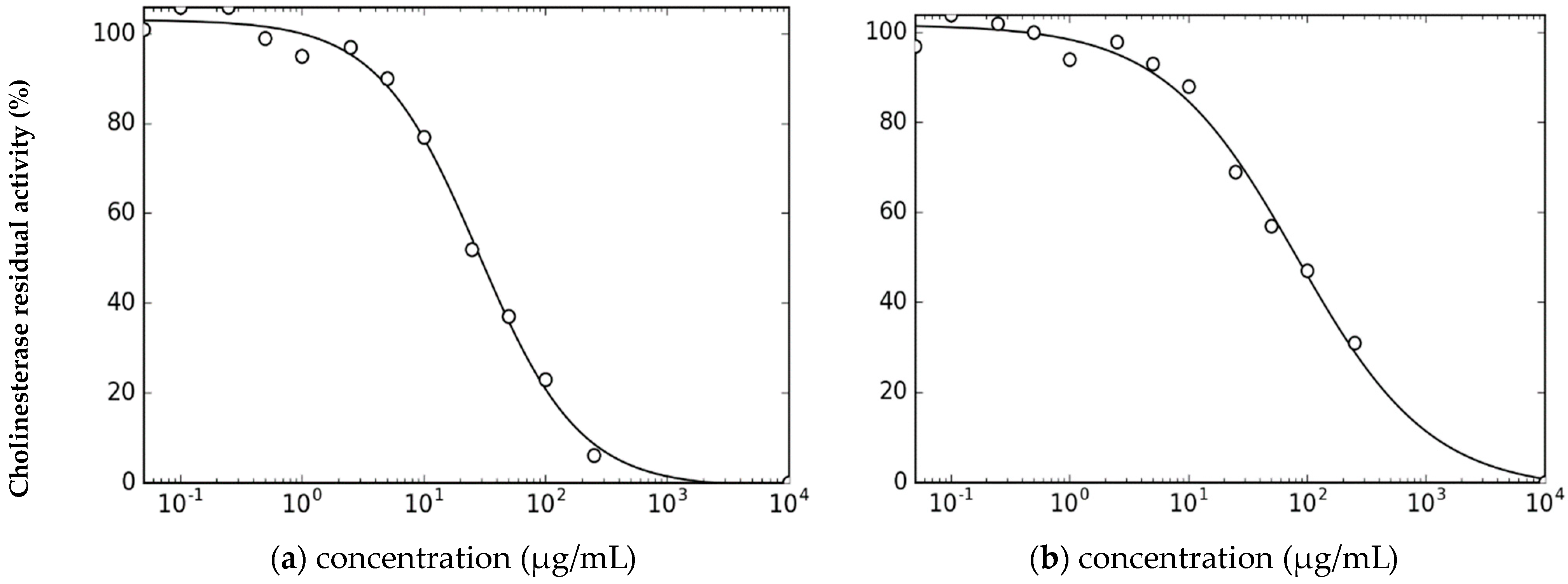
2.3. Inhibition of Cholinesterases
3. Discussion
4. Materials and Methods
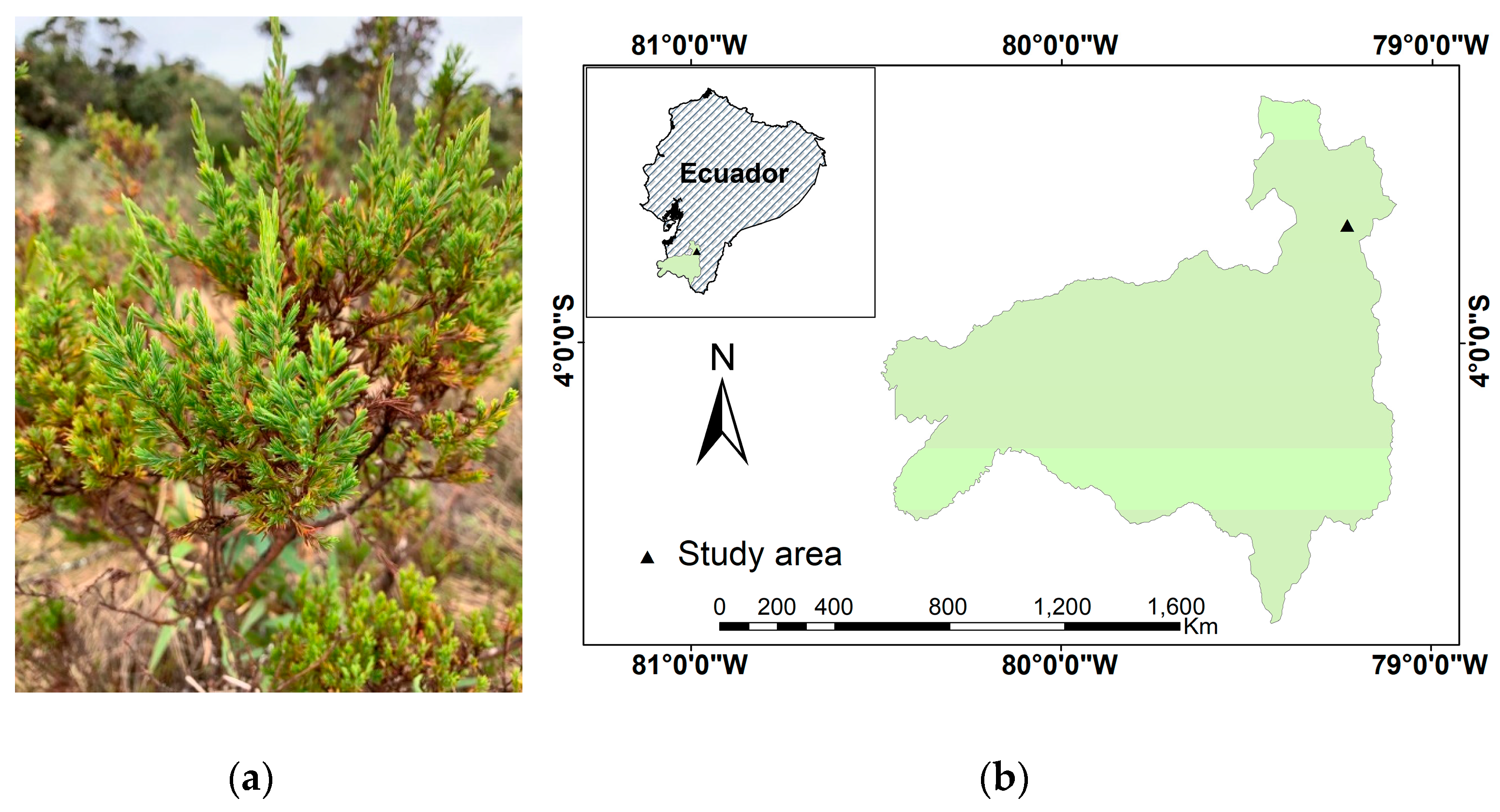
4.1. Plant Material
4.2. Distillation of the Essential Oil
4.3. Physical Properties
4.4. Chemical Composition
4.4.1. GM-MS Analysis
4.4.2. GC-FID Analysis
4.5. Cholinesterase Assays
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P.F. Hypericaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin, Germany, 2007; pp. 194–201. [Google Scholar]
- Albach, D.C.; Martínez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. A new classification of the tribe Veroniceae—Problems and a possible solution. Taxon 2004, 53, 429–452. [Google Scholar] [CrossRef]
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum Genus as a Natural Source for Biologically Active Compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: An Update on the Biological Activities and Metabolic Profiles. Mini-Rev. Med. Chem. 2020, 20, 66–87. [Google Scholar] [CrossRef]
- Avato, P. A Survey on the Hypericum genus: Secondary metabolites and bioactivity. Stud. Nat. Prod. Chem. 2005, 30, 603–634. [Google Scholar]
- Decosterd, L.; Stoeckli-Evans, H.; Msonthi, J.D.; Hostettmann, K. A new antifungal chromene and a related di-chromene from Hypericum revolutum. Planta Med. 1986, 52, 429. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yakamoto, R.; Oku, H. Patulosides A and B, novel xanthones glycosides from cell suspension cultures of Hypericum patulum. J. Nat. Prod. 1999, 62, 113–117. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Feinman, L.; Liebes, L.; Ostrow, N.; Koslowski, V.; Tobia, A.; Cabana, B.E.; Lee, D.; Spritzler, J.; Prince, A.M. Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s wort plant, in patients with chronic hepatitis C virus infection. Antimicrob. Agents Chemother. 2001, 45, 517–524. [Google Scholar] [CrossRef]
- Jayasuriya, H.; McChesney, J.D. Antimicrobial and cytotoxic activity of rottlerin-type compounds from Hypericum drummondii. J. Nat. Prod. 1989, 52, 325–331. [Google Scholar] [CrossRef]
- Crockett, S.L. Essential Oil and Volatile Components of the Genus Hypericum (Hypericaceae). Nat. Prod. Commun. 2010, 5, 1934578X1000500. [Google Scholar] [CrossRef]
- Guedes, A.P.; Franklin, G.; Fernandes-Ferreira, M. Hypericum sp.: Essential oil composition and biological activities. Phytochem. Rev. 2012, 11, 127–152. [Google Scholar] [CrossRef]
- Grafakou, M.-E.; Barda, C.; Karikas, G.A.; Skaltsa, H. Hypericum Essential Oils—Composition and Bioactivities: An Update (2012–2022). Molecules 2022, 27, 5246. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D. Plantas Medicinales de los Andes y la Amazonía—La flora Mágica y Medicinal del Norte de Peru, 1st ed.; Centro William, L., Ed.; Brown-Jardin Botánico de Missouri: St. Louis, MO, USA; Oxapampa, Perú, 2015; pp. 160–161. [Google Scholar]
- Abril, O. Estudio Etnobotánico de la Comunidad Shiña, Provincia del Azuay. Bachelor’s Thesis, Universidad del Azuay, Cuenca, Ecuador, 2015. Available online: http://dspace.uazuay.edu.ec/handle/datos/4847 (accessed on 12 May 2023).
- Salamanca, B.; Camargo, G. Protocolo Distrital de Restauración Ecológica: Guía para la restauración de los ecosistemas nativos en las áreas rurales de Santa Fe de Bogotá; DAMA: Bogotá, Colombia, 2000. [Google Scholar]
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H. (Eds.) Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Quito, Ecuador, 2008. [Google Scholar]
- Jørgesen, P.M.; León-Yáñez, S. Catalogue of the Vascular Plants of Ecuador. Available online: http://legacy.tropicos.org/ProjectAdvSearch.aspx?projectid=2 (accessed on 14 May 2023).
- Armijos, C.; Ramiírez, J.; Salinas, M.; Vidari, G.; Suaárez, A. Pharmacology and phytochemistry of Ecuadorian medicinal plants: An update and perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Bec, N.; Calva, J.; Larroque, C.; Vidari, G.; Armijos, C. Constituents, Enantiomeric Content, and ChE Inhibitory Activity of the Essential Oil from Hypericum laricifolium Juss. Aerial Parts Collected in Ecuador. Plants 2022, 11, 2962. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of anti-Alzheimer’s disease activity of selected plant ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.T.; Zargaham, M.K.; Khan, A.U.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with reference of acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Rashed, A.A.; Rahman, A.A.Z.; Rathi, D.N.G. Essential oils as a potential neuroprotective remedy for age-related neurodegenerative diseases: A review. Molecules 2021, 26, 1107. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Ozek, T.; Demirci, B.; Kürkçüoǧlu, M.; Aytaç, Z.; Duman, H. Composition of the essential oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour Fragr. J. 2000, 15, 371–372. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Chahed, T.; Marzouk, B. Essential oil composition of Salvia verbenaca L. growing wild In Tunisia. J. Food Biochem. 2010, 34, 142–151. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, F.-A.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Can Başer, K.H.; Demirci, B.; Tabanca, N.; Özek, T.; Gören, N. Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flavour Fragr. J. 2001, 16, 195–200. [Google Scholar] [CrossRef]
- Demirci, B.; Can-Başer, K.H.; Yıldız, B.; Bahçecioǧlu, Z. Composition of the essential oils of six endemic Salvia spp. from Turkey. Flavour Fragr. J. 2003, 18, 116–121. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allure Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- De La Cruz, L.G.; Caballero-Caballero, S.; Zamudio, S.; Duarte-Lisci, G.; Navarrete, A. Essential oil composition of aerial parts of Hypericum silenoides Juss. and Hypericum philonotis Cham. & Schlecht. growing in central Mexico. J. Essent. Oil Bear. Plants 2013, 16, 456–460. [Google Scholar]
- Zorzetto, C.; Sánchez-Mateo, C.; Rabanal, R.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F.; et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Wu, W.; Yao, H.; Li, Y.; Zhang, C. Chemical composition and antioxidant activity of the essential oil of Hypericum patulum (Family: Clusiaceae). Int. J. Agric. Biol. 2018, 20, 6. [Google Scholar]
- Dorđević, A.; Lazarević, J.; Smelcerović, A.; Stojanović, G. The case of Hypericum rochelii Griseb. & Schenk and Hypericum umbellatum A. Kern. essential oils: Chemical composition and antimicrobial activity. J. Pharm. Biomed. Anal. 2013, 77, 145–148. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Chanotiya, C.S.; Yadav, A. Chemical composition of the aliphatic compounds rich essential oil of Hypericum japonicum Thunb. ex Murray from India. J. Essent. Oil Res. 2012, 24, 501–505. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araujo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Ozkan, E.E.; Ozden, T.Y.; Ozsoy, N.; Mat, A. Evaluation of chemical composition, antioxidant and anti-acetylcholinesterase activities of Hypericum neurocalycinum and Hypericum malatyanum. S. Afr. J. Bot. 2018, 114, 104–110. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14((Suppl. 1)), 77–91. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2015, 2, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Peitzika, S.-C.; Pontiki, E. A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease. Molecules 2023, 28, 1084. [Google Scholar] [CrossRef]
- Zahlsen, K.; Nilsen, A.M.; Eide, I.; Nilsen, O.G. Accumulation and distribution of aliphatic (n-nonane), aromatic (1, 2, 4-trimethylbenzene) and naphthenic (1, 2, 4-trimethylcyclohexane) hydrocarbons in the rat after repeated inhalation. Pharmacol. Toxicol. 1990, 67, 436–440. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase Inhibitory Activity and Chemical Composition Of Commercial Essential Oils. J. Agric. Food Chem. 2009, 10, 4313–4318. [Google Scholar] [CrossRef]
- Ferreira, O.O.; Cruz, J.N.; de Moraes, Â.A.B.; de Jesús Pereira Franco, C.; Lima, R.R.; Anjos, T.O.d.; Siqueira, G.M.; Nascimento, L.D.d.; Cascaes, M.M.; de Oliveira, M.S.; et al. Aceite Esencial de las Plantas que Crecen en la Amazonía Brasileña: Composición Química, Antioxidantes y Aplicaciones Biológicas. Moléculas 2022, 27, 4373. [Google Scholar] [CrossRef]
- Calva, J.; Bec, N.; Gilardoni, G.; Larroque, C.; Cartuche, L.; Bicchi, C.; Montesinos, J.V. Acorenone B: AChE and BChE inhibitor as a major compound of the essential oil distilled from the Ecuadorian species Niphogeton dissecta (Benth.) J.F. Macbr. Pharmaceuticals 2017, 10, 84. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Silva, S.G.; Cruz, J.N.; Santana de Oliveira, M.; Oliveira, J.; de Moraes, A.A.B.; da Costa, F.A.M.; da Costa, K.S.; Diniz do Nascimento, L.; Helena de Aguiar Andrade, E. First report on the Annona exsucca DC. Essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021, 36, 4009–4012. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, D.; Calva, J.; Bec, N.; Larroque, C.; Vidari, G.; Armijos, C. Chemical Characterization and Biological Activity of the Essential Oil from Araucaria brasiliensis Collected in Ecuador. Molecules 2022, 27, 3793. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Armijos, C.; Matailo, A.; Bec, N.; Salinas, M.; Aguilar, G.; Solano, N.; Calva, J.; Ludeña, C.; Larroque, C.; Vidari, G. Chemical composition and selective BuChE inhibitory activity of the essential oils from aromatic plants used to prepare the traditional Ecuadorian beverage horchata lojana. J. Ethnopharmacol. 2020, 263, 113–162. [Google Scholar] [CrossRef] [PubMed]

| DB-5ms | HP-INNOWax | |||||||
|---|---|---|---|---|---|---|---|---|
| N° | Compound | LRI a | LRI b | % ± SD | LRI a | LRI c | Ref. | % ± SD |
| 1 | n-Octane | 800 | 800 | 0.20 ± 0.01 | - | - | - | - |
| 2 | 2-Methyl-octane | 855 | 858 | 1.68 ± 0.02 | 860 | 858 | [26] | 3.26 ± 0.18 |
| 3 | n-Nonane | 900 | 900 | 28.71 ±0.61 | 904 | 900 | [27] | 16.36 ± 1.18 |
| 4 | α-Pinene | 930 | 932 | 2.05 ± 0.03 | 1046 | 1055 | [25] | 3.38 ± 0.38 |
| 5 | Isoamyl propionate | 968 | 960 | 0.29 ± 0.01 | - | - | - | - |
| 6 | β-Pinene | 975 | 974 | 0.17 ± 0.00 | 1109 | 1108 | [25] | 0.25 ± 0.02 |
| 7 | Myrcene | 988 | 988 | 0.69 ± 0.02 | 1161 | 1163 | [25] | 0.90 ± 0.07 |
| 8 | n-Decane | 1000 | 1000 | 0.02 ± 0.03 | - | - | - | - |
| 9 | Limonene | 1027 | 1024 | 0.10 ± 0.01 | - | - | - | - |
| 10 | (Z)-β-Ocimene | 1035 | 1032 | 0.15 ± 0.01 | 1236 | 1236 | [25] | 0.36 ± 0.02 |
| 11 | (E)-β-Ocimene | 1045 | 1044 | 1.93 ± 0.07 | 1254 | 1253 | [25] | 3.09 ± 0.26 |
| 12 | n-Undecane | 1100 | 1100 | 1.01 ± 0.00 | 1097 | 1100 | [27] | 1.35 ± 0.10 |
| 13 | n-Nonanal | 1105 | 1100 | 0.52 ± 0.03 | 1401 | 1395 | [28] | 0.36 ± 0.06 |
| 14 | (2E)-Nonenol | 1170 | 1163 | 4.02 ± 0.11 | 1457 | - | - | 3.85 ± 0.56 |
| 15 | n-Decanal | 1207 | 1201 | 20.68 ± 0.3 | 1504 | 1498 | [28] | 23.12 ± 2.73 |
| 16 | Linalool | - | - | - | 1566 | 1554 | [25] | 0.50 ± 0.02 |
| 17 | Citronellol | 1228 | 1223 | 0.53 ± 0.31 | 1783 | 1772 | [25] | 1.04 ± 0.06 |
| 18 | 1-Nonanol | - | - | - | 1676 | 1664 | [29] | 0.48 ± 0.02 |
| 19 | (E)-Ocimenone | 1237 | 1235 | 3.30 ±0.06 | - | - | - | - |
| 20 | ϒ-Selinene | - | - | - | 1698 | 1690 | [29] | 0.71 ± 0.02 |
| 21 | Linalyl acetate | 1251 | 1254 | 1.35 ± 0.06 | - | - | - | - |
| 22 | (2E)-Decenal | 1268 | 1260 | 0.13 ± 0.09 | - | - | - | - |
| 23 | Geranial | 1268 | 1264 | 4.53 ± 0.06 | 1740 | 1732 | [25] | 5.32 ± 0.15 |
| 24 | 1-Decanol | 1274 | 1266 | 1.05 ± 0.04 | 1778 | 1766 | [30] | 2.80 ± 0.19 |
| 25 | Pearlate | 1278 | 1287 | 3.23 ± 0.09 | - | - | - | - |
| 26 | 2-Undecanone | 1292 | 1293 | 0.07 ± 0.05 | 1603 | 1604 | [30] | 0.22 ± 0.02 |
| 27 | (2E)-Undecenal | 1364 | 1357 | 0.09 ± 0.07 | - | - | - | - |
| 28 | Cyclosativene | 1372 | 1369 | 0.06 ± 0.09 | - | - | - | - |
| 29 | Linalyl isobutanoate | 1378 | 1373 | 0.49 ± 0.02 | - | - | - | - |
| 30 | β-Longipinene | 1399 | 1400 | 0.07 ± 0.00 | - | - | - | - |
| 31 | (E)-β-Caryophyllene | 1414 | 1417 | 4.59 ± 0.06 | 1580 | 1593 | [28] | 5.76 ± 0.22 |
| 32 | Dodecanal | 1409 | 1408 | 0.26 ± 0.01 | 1713 | 1715 | [28] | 0.34 ± 0.09 |
| 33 | α-Guaiene | 1437 | 1437 | 0.10 ± 0.02 | - | - | - | - |
| 34 | α-Humulene | 1450 | 1452 | 4.92 ± 0.04 | 1653 | 1658 | [25] | 6.62 ± 0.27 |
| 35 | 9-epi-(E)-Caryophyllene | 1469 | 1464 | 0.36 ± 0.36 | - | - | - | - |
| 36 | γ-Himachalene | 1479 | 1481 | 0.01 ± 0.07 | - | - | - | - |
| 37 | β-Selinene | 1483 | 1489 | 0.81 ± 0.09 | 1704 | 1708 | [28] | 0.99 ± 0.04 |
| 38 | α-Selinene | - | - | - | 1734 | 1744 | [26] | 1.75 ± 0.06 |
| 39 | α-Zingiberene | 1490 | 1493 | 3.79 ± 0.05 | - | - | - | - |
| 40 | 2-Tridecanone | 1494 | 1495 | 0.23 ± 0.00 | - | - | - | - |
| 41 | (E,E)-α-Farnesene | 1503 | 1505 | 2.47 ± 0.02 | 1751 | 1751 | [25] | 4.47 ± 0.16 |
| 42 | (Z)-γ-Bisabolene | 1507 | 1514 | 0.01 ± 0.00 | 1760 | 1741 | [25] | 0.92 ± 0.02 |
| 43 | Geranyl acetate | - | - | - | 1766 | 1761 | [25] | 0.73 ± 0.01 |
| 44 | Nerol | - | - | - | 1816 | 1805 | [25] | 0.39 ± 0.02 |
| 45 | Geraniol | - | - | - | 1867 | 1854 | [25] | 2.67 ± 0.15 |
| 46 | Caryophyllene oxide | - | - | - | 1971 | 1967 | [25] | 0.40 ± 0.01 |
| 47 | Methyleugenol | - | - | - | 2034 | 2028 | [27] | 0.44 ± 0.03 |
| 48 | (E)-Nerolidol | 1560 | 1561 | 2.89 ± 0.03 | 2060 | 2050 | [19] | 5.04 ± 0.38 |
| 49 | trans-β-Elemenone | 1595 | 1602 | 0.10 ± 0.09 | - | - | - | - |
| 50 | Khusimone | 1604 | 1604 | 0.26 ± 0.18 | - | - | - | - |
| 51 | γ-Eudesmol | - | - | - | 2175 | 2185 | [27] | 0.14 ± 0.06 |
| 52 | Bisaboladien-4-ol | 1618 | 1618 | 0.10 ± 0.07 | - | - | - | - |
| 53 | Decanoic acid | - | - | - | 2280 | 2284 | [25] | 0.42 ± 0.07 |
| Aliphatics | ||||||||
| Alkanes (%) | 31.62 | 20.97 | ||||||
| Alcohols (%) | 5.07 | 7.13 | ||||||
| Aldehydes (%) | 21.68 | 23.82 | ||||||
| Ketones (%) | 0.30 | 0.22 | ||||||
| Fatty acids (%) | -- | 0.42 | ||||||
| Esters (%) | 3.52 | -- | ||||||
| Terpenes | ||||||||
| Monoterpene hydrocarbons (%) | 5.09 | 7.98 | ||||||
| Oxygenated monoterpenoids (%) | 10.2 | 10.65 | ||||||
| Sesquiterpene hydrocarbons (%) | 17.19 | 21.22 | ||||||
| Oxygenated sesquiterpenes (%) | 3.35 | 5.44 | ||||||
| Diterpenes (%) | - | 0.14 | ||||||
| Others (%) | - | 0.44 | ||||||
| TOTAL identified (%) | 98.02 | 98.43 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calva, J.; Ludeña, C.; Bec, N.; Larroque, C.; Salinas, M.; Vidari, G.; Armijos, C. Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants 2023, 12, 2621. https://doi.org/10.3390/plants12142621
Calva J, Ludeña C, Bec N, Larroque C, Salinas M, Vidari G, Armijos C. Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants. 2023; 12(14):2621. https://doi.org/10.3390/plants12142621
Chicago/Turabian StyleCalva, James, Carlos Ludeña, Nicole Bec, Christian Larroque, Melissa Salinas, Giovanni Vidari, and Chabaco Armijos. 2023. "Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth" Plants 12, no. 14: 2621. https://doi.org/10.3390/plants12142621
APA StyleCalva, J., Ludeña, C., Bec, N., Larroque, C., Salinas, M., Vidari, G., & Armijos, C. (2023). Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants, 12(14), 2621. https://doi.org/10.3390/plants12142621








