Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests
Abstract
1. Introduction
2. Materials and Methods
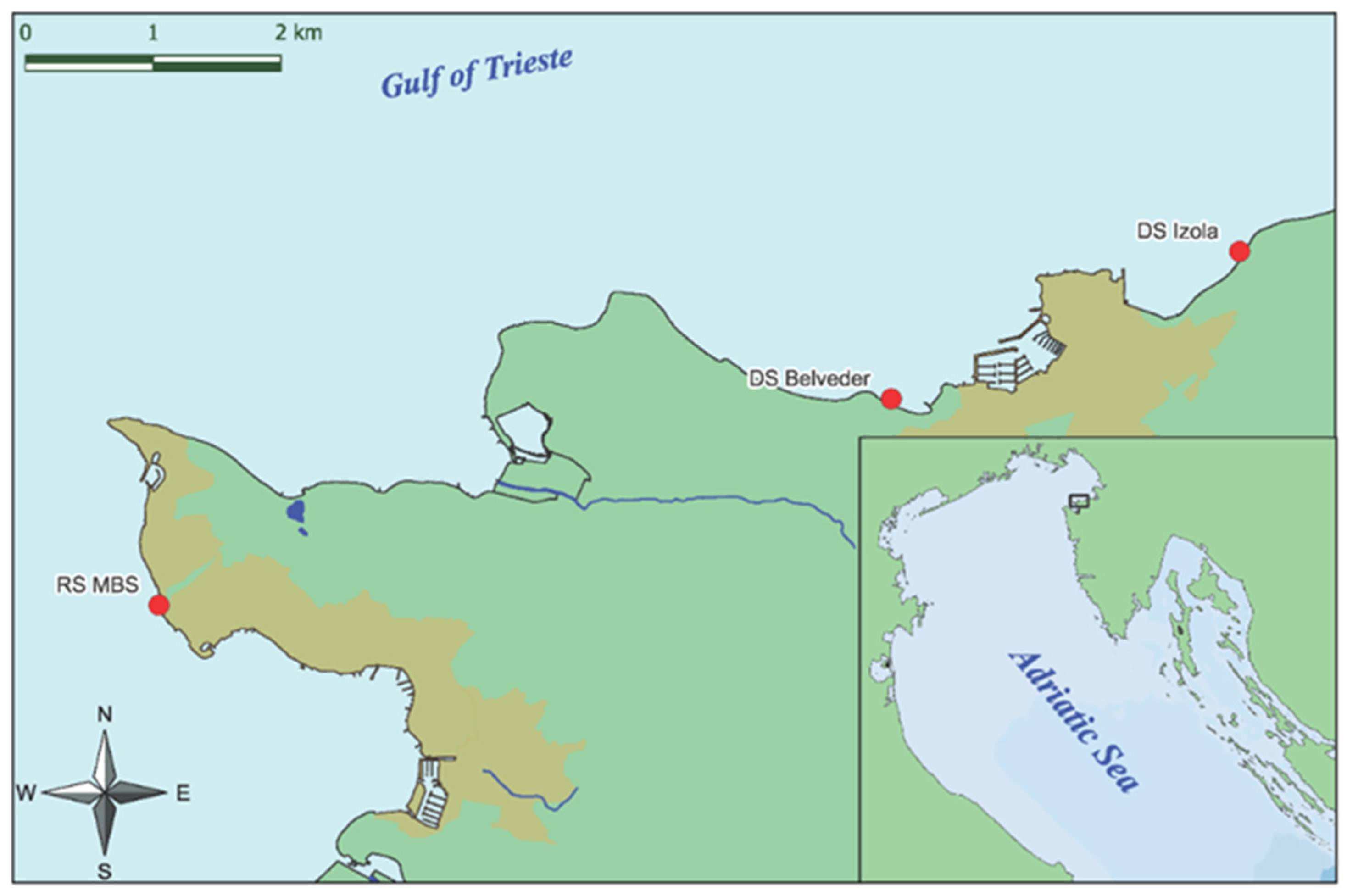
2.1. Study Area and Material Collection
- Izola Merkur, which is characterized by healthy and dense populations of G. barbata and C. compressa; coordinates: 45°32.653, 13°40.554.
- Izola Belvedere, which is characterized by healthy and dense populations of G. barbata; coordinates: 45°31.979, 13°38.488.
2.2. Laboratory Work
2.2.1. Preliminary Testing of the Effectiveness of UV Sterilizers
- Test for bacterial growth on agar plates. Cultures from unsterilized, 1× UVC-sterilized, and 5× UVC-sterilized water (water flowed through sterilizer 5 times at water flow 150 L/h) were inoculated onto growth media plates by (a) adding 100 μL of each water sample to separate agar plates, (b) filtering 50 mL of each water sample through a 0.45 μm filter that was then put on separate agar plates. The agar plates were monitored, and CFU (=colony forming units) were counted daily for 3 days.
- Test for bacterial and microalgal growth on the clay tiles in the growth chamber. Three 60 cm × 30 cm × 30 cm aquaria were prepared, each containing three clay tiles. Unfiltered seawater (control) was added to the first aquarium, 1× UVC-sterilized water to the second, and 5× UVC-sterilized water to the third. Under established culture conditions (T = 17 °C, photoperiod 15:9 h light:dark cycle, light intensity = 125 μmol photons m−2 s−1), the clay tiles were monitored daily for 23 days.
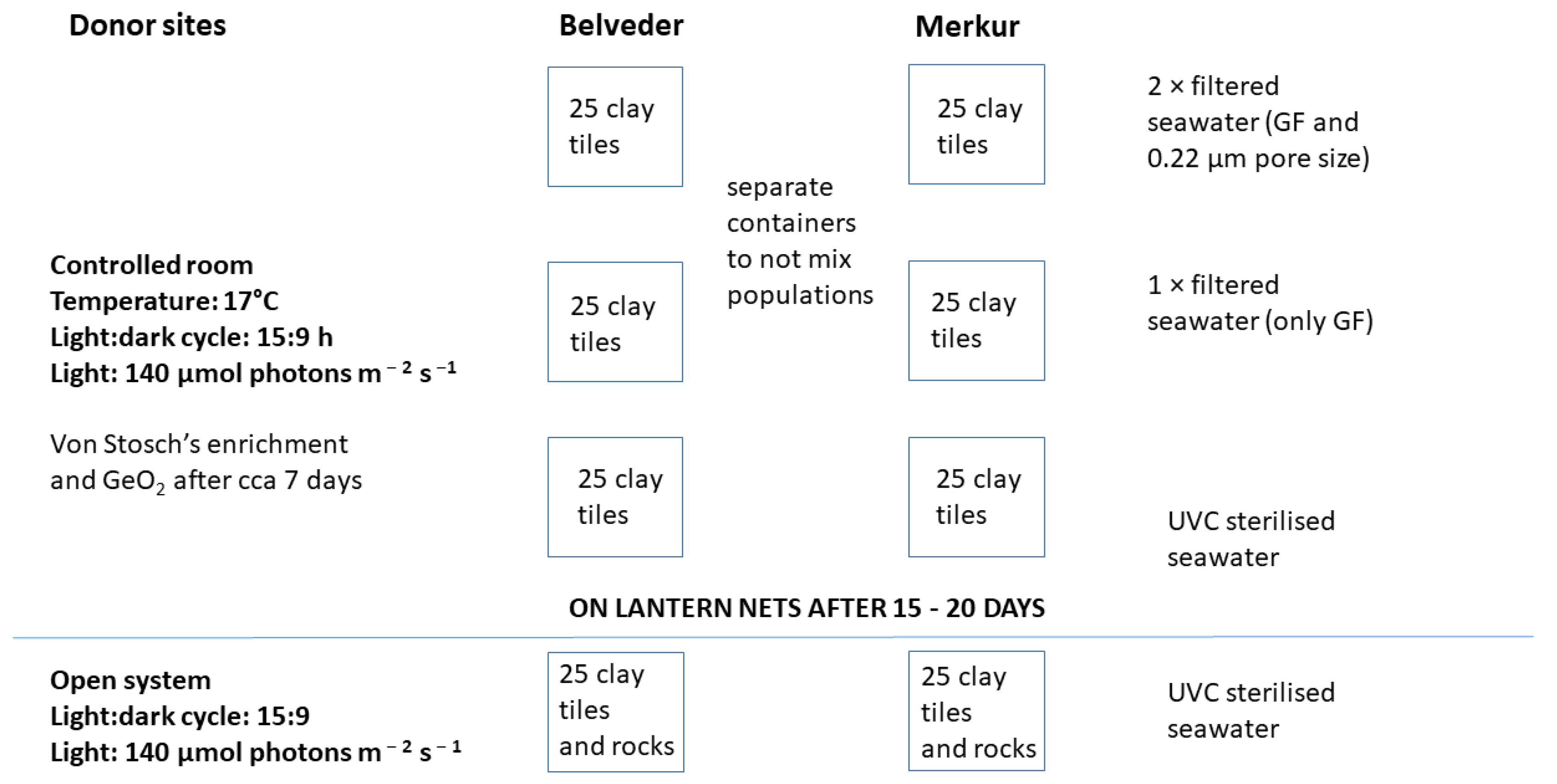
2.2.2. Ex situ Cultivation in the Mesocosm
2.2.3. Ex Situ Cultivation in Open System
2.3. Out-Planting in the Marine Environment
2.4. Photo-Processing and Statistical Analyses
3. Results
3.1. Preliminary Test of the Efficiency of UV Sterilizers
3.1.1. Evaluation of Bacterial Growth on Agar Plates
3.1.2. Evaluation of Bacterial and Microalgal Growth on Clay Tiles in the Growth Chamber
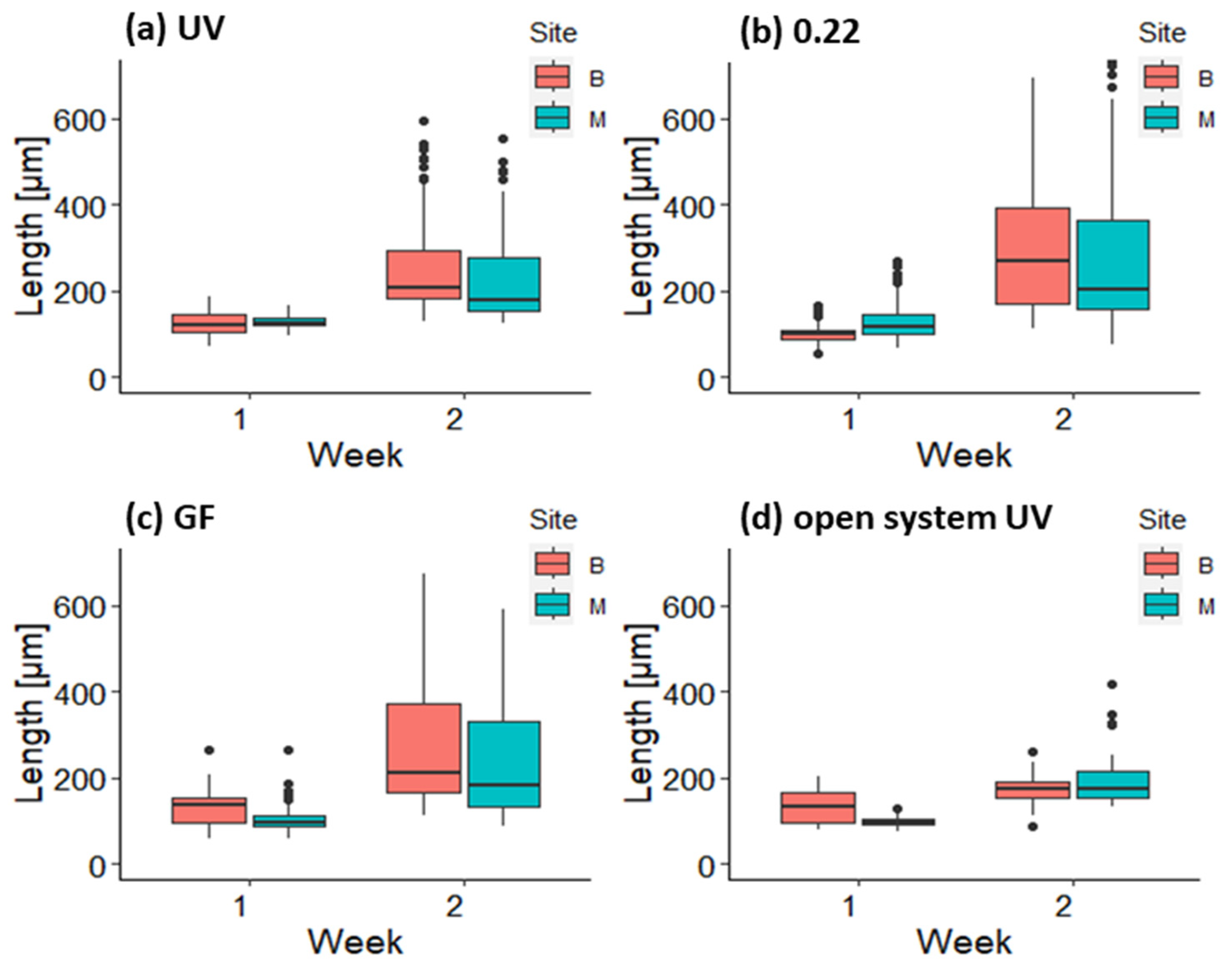
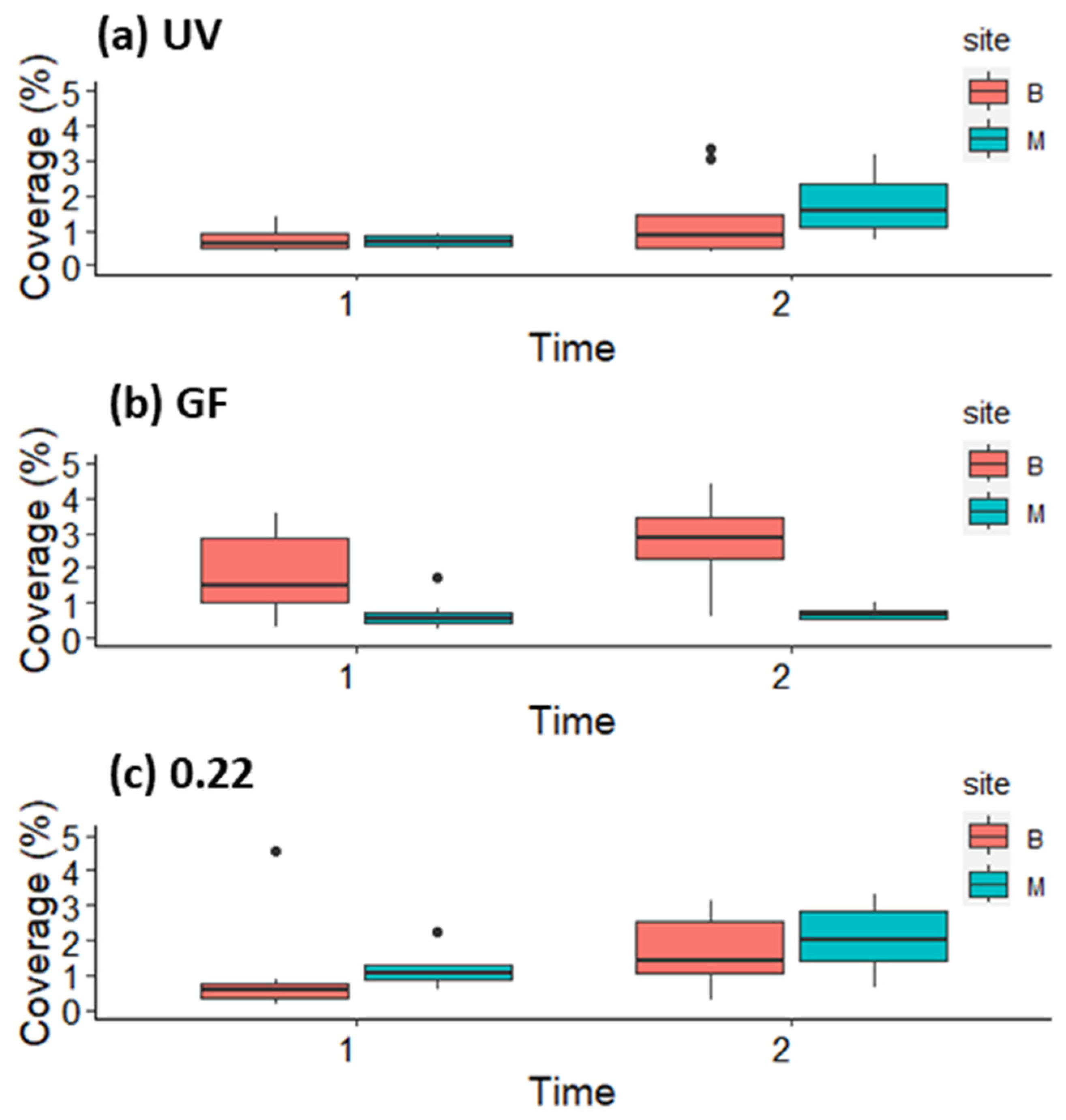
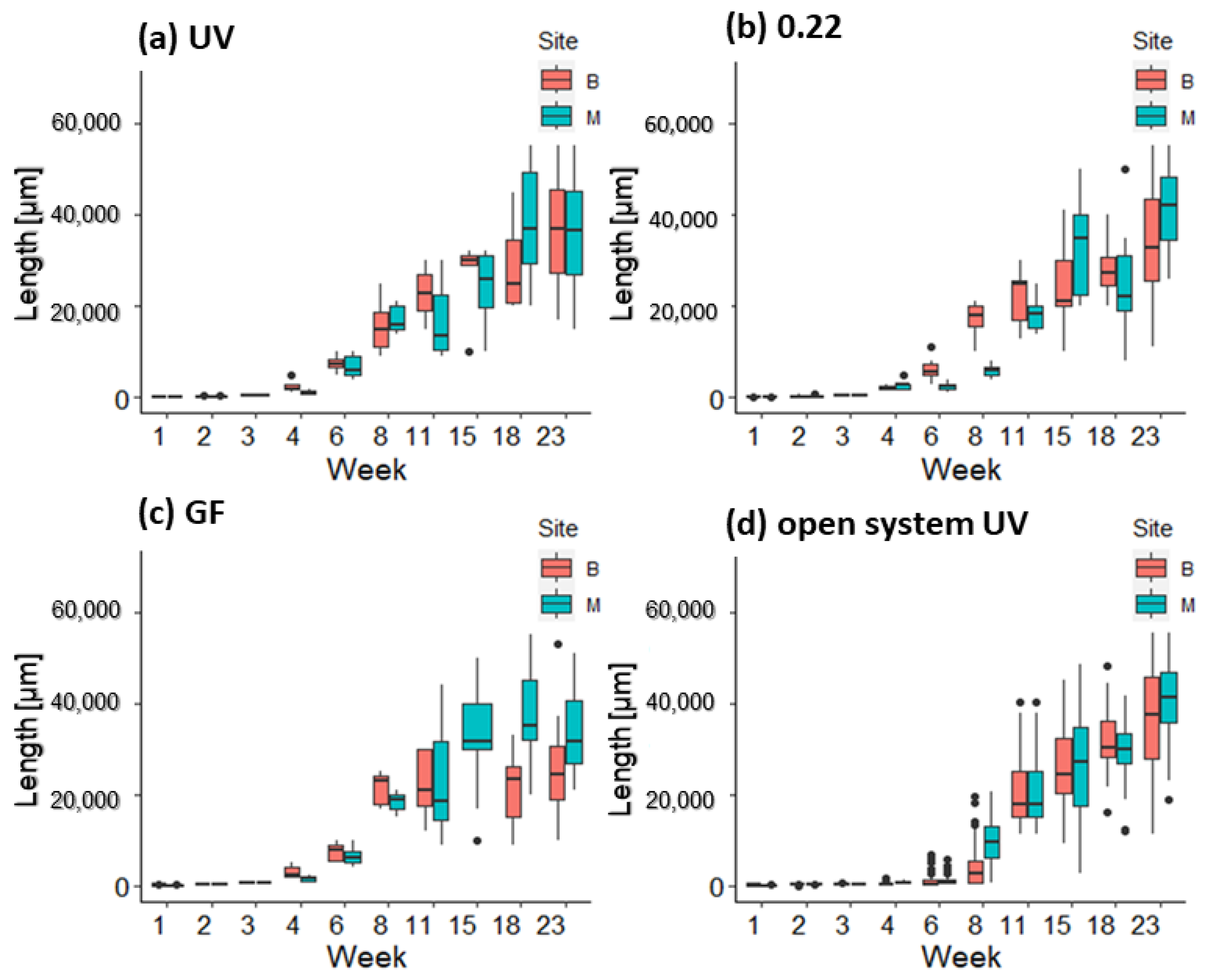
3.2. Growth and Survival of Germlings in the First and Second Week in Mesocosm and Open System
3.3. Growth of Germlings in the Lantern Net Baskets
3.4. Growth of Germlings in the Open System
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assis, J.; Fragkopoulou, E.; Frade, D.; Neiva, J.; Oliveira, A.; Abecasis, D.; Faugeron, S.; Serrão, E.A. A fine-tuned global distribution dataset of marine forests. Sci. Data 2020, 7, 119. [Google Scholar] [CrossRef]
- Bringloe, T.T.; Starko, S.; Wade, R.M.; Vieira, C.; Kawai, H.; De Clerck, O.; Cock, J.M.; Coelho, S.M.; Destombe, C.; Valero, M.; et al. Phylogeny and Evolution of the Brown Algae. Crit. Rev. Plant Sci. 2020, 39, 281–321. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso-Carrillo, J. Guía de las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Ediciones Omega S.A: Barcelona, Spain, 2013; p. 656. [Google Scholar]
- Orfanidis, S.; Rindi, F.; Cebrian, E.; Fraschetti, S.; Nasto, I.; Taskin, E.; Bianchelli, S.; Papathanasiou, V.; Kosmidou, M.; Caragnano, A.; et al. Effects of Natural and Anthropogenic Stressors on Fucalean Brown Seaweeds Across Different Spatial Scales in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 658417. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Susini, M.-L.; Meinesz, A.; Cattaneo-Vietti, R.; Thibaut, T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. J. Exp. Mar. Biol. Ecol. 2012, 412, 72–80. [Google Scholar] [CrossRef]
- Novoa, E.A.M.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Eur. J. Phycol. 2019, 54, 456. [Google Scholar]
- CLAYTON, M.N. The adaptive significance of life history characters in selectedorders of marine brown maeroalgae. Aust. J. Ecol. 1990, 15, 439–452. [Google Scholar] [CrossRef]
- Ballesteros, E. Flora Phycologica Iberica; Servicio de Publicationes; Universidad Murcia: Murcia, Spain, 2002; Volume 1, p. 192. [Google Scholar]
- Ballesteros, E. Production of seaweeds in Northwestern Mediterranean marine communities: Its relation with environmental factors. Sci. Mar. 1989, 53, 357–364. [Google Scholar]
- De La Fuente, G.; Chiantore, M.; Asnaghi, V.; Kaleb, S.; Falace, A. First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 2019, 7, e7290. [Google Scholar] [CrossRef]
- Ballesteros, E.; Garrabou, J.; Hereu, B.; Zabala, M.; Cebrian, E.; Sala, E. Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: Insights into assemblage structure and population dynamics. Estuar. Coast. Shelf Sci. 2009, 82, 477–484. [Google Scholar] [CrossRef]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Popović, A.; Lipej, L. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (Northern Adriatic Sea). Mediterr. Mar. Sci. 2014, 15, 225–238. [Google Scholar] [CrossRef]
- Bianchelli, S.; Pusceddu, A.; Buschi, E.; Danovaro, R. Trophic status and meiofauna biodiversity in the Northern Adriatic Sea: Insights for the assessment of good environmental status. Mar. Environ. Res. 2016, 113, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Orlando-Bonaca, M.; Trkov, D.; Klun, K.; Pitacco, V. Diversity of Molluscan Assemblage in Relation to Biotic and Abiotic Variables in Brown Algal Forests. Plants 2022, 11, 2131. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzi, L.; Bevilacqua, S.; Alongi, G.; Asnaghi, V.; Chiantore, M.; Pagnotta, A.; Paoli, C.; Rigo, I.; Vassallo, P.; Falace, A. Assessing the Effect of Full Protection on the Biomass of Ericaria amentacea and Understory Assemblages: Evidence from Two Mediterranean Marine Protected Areas. Diversity 2023, 15, 89. [Google Scholar] [CrossRef]
- Bonaca, M.O.; Lipej, L. Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Mar. Ecol. 2005, 26, 42–53. [Google Scholar] [CrossRef]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.-M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 2020, 10, 12341. [Google Scholar] [CrossRef]
- Peleg, O.; Guy-Haim, T.; Yeruham, E.; Silverman, J.; Rilov, G. Tropicalization may invert trophic state and carbon budget of shallow temperate rocky reefs. J. Ecol. 2020, 108, 844–854. [Google Scholar] [CrossRef]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfì, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea var. stricta. Mar. Drugs 2021, 19, 2. [Google Scholar] [CrossRef]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- UNEP, United Nations Environment Programme. Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and Its Protocols. Mediterranean Action Plan—Barcelona Convention Secretariat; United Nations Environment Programme: Nairobi, Kenya, 2019; p. 153. [Google Scholar]
- European Union. European Commission: Common Implementation Strategy for the Water Framework Directive; Policy Paper. Climate Change and Water; European Union: Brussels, Belgium, 2008. [Google Scholar]
- European Parliament, Council of the European Union. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 26, 19–40. [Google Scholar]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Airoldi, L.; Balata, D.; Beck, M.W. The gray zone: Relationships between habitat loss and marine diversity and their applications in conservation. J. Exp. Mar. Biol. Ecol. 2008, 366, 8–15. [Google Scholar] [CrossRef]
- Arévalo, R.; Pinedo, S.; Ballesteros, E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 2007, 55, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Perkol-Finkel, S.; Airoldi, L. Loss and recovery potential of marine habitats: An experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLoS ONE 2010, 5, e10791. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- Connell, S.D.; Foster, M.S.; Airoldi, L. What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef]
- Strain, E.M.A.; Thomson, R.J.; Micheli, F.; Mancuso, F.P.; Airoldi, L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob. Chang. Biol. 2014, 20, 3300–3312. [Google Scholar] [CrossRef]
- Catra, M.; Alongi, G.; Leonardi, R.; Negri, M.P.; Sanfilippo, R.; Sciuto, F.; Serio, D.; Viola, A.; Rosso, A. Degradation of a photophilic algal community and its associated fauna from eastern Sicily (Mediterranean Sea). Mediterr. Mar. Sci. 2019, 20, 74–89. [Google Scholar] [CrossRef]
- Irving, A.D.; Balata, D.; Colosio, F.; Ferrando, G.A.; Airoldi, L. Light, sediment, temperature, and the early life-history of the habitat-forming alga Cystoseira barbata. Mar. Biol. 2009, 156, 1223–1231. [Google Scholar] [CrossRef]
- Falace, A.; Alongi, G.; Cormaci, M.; Furnari, G.; Curiel, D.; Cecere, E.; Petrocelli, A. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Pitacco, V.; Lipej, L. Loss of canopy-forming algal richness and coverage in the northern Adriatic Sea. Ecol. Indic. 2021, 125, 107501. [Google Scholar] [CrossRef]
- Bonaviri, C.; Vega Fernández, T.; Fanelli, G.; Badalamenti, F.; Gianguzza, P. Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar. Biol. 2011, 158, 2505–2513. [Google Scholar] [CrossRef]
- Hereu, B. Depletion of palatable algae by sea urchins and fishes in a Mediterranean subtidal community. Mar. Ecol. Prog. Ser. 2006, 313, 95–103. [Google Scholar] [CrossRef]
- Knowlton, N. Multiple “stable” states and the conservation of marine ecosystems. Prog. Oceanogr. 2004, 60, 387–396. [Google Scholar] [CrossRef]
- Tamburello, L.; Benedetti-Cecchi, L.; Masini, L.; Bulleri, F. Habitat heterogeneity promotes the coexistence of exotic seaweeds. Oecologia 2013, 172, 505–513. [Google Scholar] [CrossRef]
- Airoldi, L.; Hawkins, S.J. Negative effects of sediment deposition on grazing activity and survival of the limpet Patella vulgata. Mar. Ecol. Prog. Ser. 2007, 332, 235–240. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Alberto, F.; Raimondi, P.T.; Reed, D.C.; Coelho, N.C.; Leblois, R.; Whitmer, A.; Serrão, E.A. Habitat continuity and geographic distance predict population genetic differentiation in giant kelp. Ecology 2010, 91, 49–56. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Pey, A.; Laurent, M.; Martins, G.M.; Airoldi, L.; Mangialajo, L. Threats to large brown algal forests in temperate seas: The overlooked role of native herbivorous fish. Sci. Rep. 2017, 7, 6012. [Google Scholar] [CrossRef]
- Prado, P.; Farina, S.; Tomas, F.; Romero, J.; Alcoverro, T. Marine protection and meadow size alter fish herbivory in seagrass ecosystems. Mar. Ecol. Prog. Ser. 2008, 371, 11–21. [Google Scholar] [CrossRef]
- Savonitto, G.; De La Fuente, G.; Tordoni, E.; Ciriaco, S.; Srijemsi, M.; Bacaro, G.; Chiantore, M.; Falace, A. Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1611–1623. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Savonitto, G.; Asnaghi, V.; Trkov, D.; Pitacco, V.; Šiško, M.; Makovec, T.; Slavinec, P.; Lokovšek, A.; Ciriaco, S.; et al. Where and how—New insight for brown algal forest restoration in the Adriatic. Front. Mar. Sci. 2022, 9, 988584. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Pitacco, V.; Slavinec, P.; Šiško, M.; Makovec, T.; Falace, A. First restoration experiment for Gongolaria barbata in Slovenian coastal waters. What can go wrong? Plants 2021, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, S.; Savonitto, G.; Lipizer, M.; Mancuso, P.; Ciriaco, S.; Srijemsi, M.; Falace, A. Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 2019, 100, e02838. [Google Scholar] [CrossRef]
- Verdura, J.; Santamaría, J.; Ballesteros, E.; Smale, D.A.; Cefalì, M.E.; Golo, R.; de Caralt, S.; Vergés, A.; Cebrian, E. Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 2021, 109, 1758–1773. [Google Scholar] [CrossRef]
- Gouvêa, L.P.; Schubert, N.; Martins, C.D.L.; Sissini, M.; Ramlov, F.; Rodrigues, E.R.d.O.; Bastos, E.O.; Freire, V.C.; Maraschin, M.; Carlos Simonassi, J.; et al. Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnol. Oceanogr. 2017, 62, 2056–2075. [Google Scholar] [CrossRef]
- De Bettignies, T.; Wernberg, T.; Gurgel, C.F.D. Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front. Mar. Sci. 2018, 5, 218. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Straub, S.C.; Wernberg, T.; Thomsen, M.S.; Moore, P.J.; Burrows, M.T.; Harvey, B.P.; Smale, D.A. Resistance, Extinction, and Everything in Between—The Diverse Responses of Seaweeds to Marine Heatwaves. Front. Mar. Sci. 2019, 6, 763. [Google Scholar] [CrossRef]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast A tlantic perspective. Ecol. Evol. 2013, 3, 4016–4038. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Convention on the Conservation of European Wildlife and Natural Habitats (ETS No. 104); European Treaty Series—No. 104; Council of Europe: Bern, Switzerland, 1979. [Google Scholar]
- Gubbay, S.N.S.; Haynes, T.; Janssen, J.A.M.; Rodwell, J.R.; Nieto, A.; García Criado, M.; Beal, S.; Borg, J.; Kennedy, M.; Micu, D.; et al. Red List of European Habitats. European Commission; DG Environment: Brussels, Belgium, 2016. [Google Scholar]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Medrano, A.; Linares, C.; Aspillaga, E.; Capdevila, P.; Montero-Serra, I.; Pages-Escola, M.; Zabala, M.; Hereu, B. Long-term monitoring of temperate macroalgal assemblages inside and outside a No take marine reserve. Mar. Environ. Res. 2020, 153, 104826. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union. EU Biodiversity Strategy for 2030. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. In Document 52020DC0380; European Commision: Brussels, Belgium, 2020. [Google Scholar]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J. International principles and standards for the practice of ecological restoration. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Falace, A.; Zanelli, E.; Bressan, G. Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull. Mar. Sci. 2006, 78, 161–166. [Google Scholar]
- Susini, M.; Mangialajo, L.; Thibaut, T.; Meinesz, A. Development of a transplantation technique of Cystoseira amentacea var. stricta and Cystoseira compressa. Hydrobiologia 2007, 580, 241–244. [Google Scholar] [CrossRef]
- Robvieux, P. Conservation des Populations de Cystoseira en Régions Provence-Alpes-Côte-d’Azur et Corse; Université Nice Sophia Antipolis: Nice, France, 2013. [Google Scholar]
- Perkol-Finkel, S.; Ferrario, F.; Nicotera, V.; Airoldi, L. Conservation challenges in urban seascapes: Promoting the growth of threatened species on coastal infrastructures. J. Appl. Ecol. 2012, 49, 1457–1466. [Google Scholar] [CrossRef]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a canopy-forming alga based on recruitment enhancement: Methods and long-term success assessment. Front. Plant Sci. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Falace, A.; Kaleb, S.; De La Fuente, G.; Asnaghi, V.; Chiantore, M. Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS ONE 2018, 13, e0193011. [Google Scholar] [CrossRef] [PubMed]
- Tamburello, L.; Papa, L.; Guarnieri, G.; Basconi, L.; Zampardi, S.; Scipione, M.B.; Terlizzi, A.; Zupo, V.; Fraschetti, S. Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PLoS ONE 2019, 14, e0224477. [Google Scholar] [CrossRef] [PubMed]
- Lardi, P.I.; Varkitzi, I.; Tsiamis, K.; Orfanidis, S.; Koutsoubas, D.; Falace, A.; Salomidi, M. Early development of Gongolaria montagnei (Fucales, Phaeophyta) germlings under laboratory conditions, with a view to enhancing restoration potential in the Eastern Mediterranean. Bot. Mar. 2022, 65, 279–287. [Google Scholar] [CrossRef]
- Clausing, R.J.; De La Fuente, G.; Falace, A.; Chiantore, M. Accounting for environmental stress in restoration of intertidal foundation species. J. Appl. Ecol. 2022, 60, 305–318. [Google Scholar] [CrossRef]
- Keough, M.J.; Raimondi, P.T. Responses of settling invertebrate larvae to bioorganic films: Effects of large-scale variation in films. J. Exp. Mar. Biol. Ecol. 1996, 207, 59–78. [Google Scholar] [CrossRef]
- Kim, A.; Park, S.-Y.; Lee, C.-H.; Lee, C.-H.; Lee, J.-K. Quorum quenching bacteria isolated from the sludge of a wastewater treatment plant and their application for controlling biofilm formation. J. Microbiol. Biotechnol. 2014, 24, 1574–1582. [Google Scholar] [CrossRef]
- Yallop, M.L.; de Winder, B.; Paterson, D.M.; Stal, L.J. Comparative structure, primary production and biogenic stabilization of cohesive and non-cohesive marine sediments inhabited by microphytobenthos. Estuar. Coast. Shelf Sci. 1994, 39, 565–582. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between microbial biofilms and marine fouling algae: A mini review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef]
- Leflaive, J.; Ten-Hage, L. Allelopathic interactions in benthic biofilms: Effects of abiotic conditions on production of and sensitivity to allelochemicals. J. N. Am. Benthol. Soc. 2009, 28, 273–282. [Google Scholar] [CrossRef]
- Monserrat, M.; Verdura, J.; Comeau, S.; Cottalorda, J.-M.; Priouzeau, F.; Romero, G.; Mangialajo, L. The role of grazers in early-life stages of Cystoseira sensu lato can be crucial in the restoration of marine forests. Front. Mar. Sci. 2023, 10, 1176780. [Google Scholar] [CrossRef]
- Ogrin, D. Podnebje in izredni vremenski dogodki ob Tržaškem zalivu pred letom 1841. Geogr. Obz. 2012, 3, 23–30. [Google Scholar]
- Boicourt, W.; Licer, M.; Li, M.; Vodopivec, M.; Malacic, V. Sea State: Recent Progress in the Context of Climate Change. In Coastal Ecosystems in Transition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 21–48. [Google Scholar] [CrossRef]
- Turk, R. An assessment of the vulnerability of the Slovene coastal belt and its categorisation in view of (in)admissible human pressure, various activities and land-use. Ann.-Ser. Hist. Nat. 1999, 9, 37–50. [Google Scholar]
- Orlando-Bonaca, M.; Rotter, A. Any signs of replacement of canopy-forming algae by turf-forming algae in the northern Adriatic Sea? Ecol. Indic. 2018, 87, 272–284. [Google Scholar] [CrossRef]
- Mair, P.; Wilcox, R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Millar, R.B.; Anderson, M.J. Remedies for pseudoreplication. Fish. Res. 2004, 70, 397–407. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef]
- Haridas, D.; Atreya, C.D. The microbicidal potential of visible blue light in clinical medicine and public health. Front. Med. 2022, 9, 905606. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhão, F.J.M. A Selection of Platforms to Evaluate Surface Adhesion and Biofilm Formation in Controlled Hydrodynamic Conditions. Microorganisms 2021, 9, 1993. [Google Scholar] [CrossRef]
- Thomsen, M.; McGlathery, K. Facilitation of macroalgae by the sedimentary tube forming polychaete Diopatra cuprea. Estuar. Coast. Shelf Sci. 2005, 62, 63–73. [Google Scholar] [CrossRef]
- Merz, R.A. Textures and traction: How tube-dwelling polychaetes get a leg up. Invertebr. Biol. A Q. J. Am. Microsc. Soc. Div. Invertebr. Zool./ASZ 2015, 134, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.M. Behavioural and secretory activity during tube construction by Platynereis dumerilii Aud & M. Edw. [Polychaeta: Nereidae]. J. Mar. Biol. Assoc. UK 1973, 53, 521–529. [Google Scholar]
- Carpizo-Ituarte, E.; Hadfield, M.G. Stimulation of metamorphosis in the polychaete Hydroides elegans Haswell (Serpulidae). Biol. Bull. 1998, 194, 14–24. [Google Scholar] [CrossRef]
- Pinedo, S.; Sardá, R.; Rey, C.; Bhaud, M. Effect of sediment particle size on recruitment of Owenia fusiformis in the Bay of Blanes (NW Mediterranean Sea): An experimental approach to explain field distribution. Mar. Ecol. Prog. Ser. 2000, 203, 205–213. [Google Scholar] [CrossRef]
- D’Antonio, C. Epiphytes on the rocky intertidal red alga RhodomelaLarix (Turner) C. Agardh: Negative effects on the host and food for herbivores? J. Exp. Mar. Biol. Ecol. 1985, 86, 197–218. [Google Scholar] [CrossRef]
- Brawley, S.; Adey, W. The effect of micrograzers on algal community structure in a coral reef microcosm. Mar. Biol. 1981, 61, 167–177. [Google Scholar] [CrossRef]
- Macic, V.; Svirčev, Z. Macroepiphytes on Cystoseira Species (Phaeophyceae) on the Coast of Montenegro. Fresenius Environ. Bull. 2014, 23, 29–34. [Google Scholar] [CrossRef]
- Baghdadli, D.; Tremblin, G.; Pellegrini, M.; Coudret, A. Effects of environmental parameters on net photosynthesis of a free-living brown seaweed, Cystoseira barbata formarepens: Determination of optimal photosynthetic culture conditions. J. Appl. Phycol. 1990, 2, 281–287. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of ambient temperature on the photosynthetic activity and phenolic content of the intertidal Cystoseira compressa along the Italian coastline. J. Appl. Phycol. 2019, 31, 3069–3076. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2005, 43, 1–91. [Google Scholar]
- Abdala-Díaz, R.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Álvarez, R.C.; Figueroa, F. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Sjøtun, K.; Øvreås, L. Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquat. Microb. Ecol. 2010, 60, 71–83. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; González, N.; Echevarría, F.; García, C.M. Distribution and Fluxes of Dissolved Nutrients in the Strait of Gibraltar and its Relationships to Microphytoplankton Biomass. Estuar. Coast. Shelf Sci. 2000, 51, 439–449. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Williams, S.L. Mass transfer limitation of photosynthesis of coral reef algal turfs. Mar. Biol. 2007, 151, 435–450. [Google Scholar] [CrossRef]
- Falter, J.L.; Atkinson, M.J.; Merrifield, M.A. Mass-transfer limitation of nutrient uptake by a wave-dominated reef flat community. Limnol. Oceanogr. 2004, 49, 1820–1831. [Google Scholar] [CrossRef]
- Gerard, V.A. In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera. Mar. Biol. 1982, 69, 51–54. [Google Scholar] [CrossRef]
- Monserrat, M.; Comeau, S.; Verdura, J.; Alliouane, S.; Spennato, G.; Priouzeau, F.; Romero, G.; Mangialajo, L. Climate change and species facilitation affect the recruitment of macroalgal marine forests. Sci. Rep. 2022, 12, 18103. [Google Scholar] [CrossRef]


| Day | Sample_ID | Sample Treatment | Counting Method | Volume of Seawater [μL] | CFU | CFU/L |
|---|---|---|---|---|---|---|
| 2 | 1 | SW | On agar plate | 100 | 22 | 220,000 |
| 2 | 2 | SW | On filter | 10,000 | >250 | TNTC |
| 2 | 3 | 1× UVC | On agar plate | 100 | 0 | 0 |
| 2 | 4 | 1× UVC | On filter | 50,000 | 11 | 220 |
| 2 | 5 | 5× UVC | On agar plate | 100 | 0 | 0 |
| 2 | 6 | 5× UVC | On filter | 50,000 | 1 | 20 |
| 3 | 1 | SW | On agar plate | 100 | 26 | 260,000 |
| 3 | 2 | SW | On filter | 10,000 | >250 | TNTC |
| 3 | 3 | 1× UVC | On agar plate | 100 | 0 | 0 |
| 3 | 4 | 1× UVC | On filter | 50,000 | 12 | 240 |
| 3 | 5 | 5× UV | On agar plate | 100 | 1 | 10,000 |
| 3 | 6 | 5× UV | On filter | 50,000 | 2 | 40 |
| Date and Time | Depth [m] | T [°C] | Salinity | pH | O2 Saturation [%] | PO4 [mg/L] | NO2 [mg/L] | NO3 [mg/L] | NH4 [mg/L] | SiO3 [mg/L] |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 March 2022 09:40 | 3 | 10.31 | 38.86 | 8.18 | 102.6 | 0.01 | 0.098 | 0.12 | 0.03 | 2.06 |
| 4 April 2022 09:00 | 3 | 11.09 | 38.46 | 8.21 | 100.89 | 0.01 | 0.006 | 0.36 | 0.03 | 2.68 |
| 12 April 2022 10:20 | 3 | 12.05 | 38.31 | 8.19 | 103.08 | 0.01 | 0.028 | 0.72 | 0.03 | 2.85 |
| 26 April 2022 08:50 | 3 | 13.02 | 38.46 | 8.19 | 104.78 | 0.02 | 0.006 | 0.03 | 0.03 | 1.8 |
| 17 May 2022 09:10 | 3 | 20.61 | 38.36 | 8.16 | 108.97 | 0.02 | 0.006 | 0.03 | 0.03 | 2.07 |
| 31 May 2022 09:50 | 3 | 19.3 | 38.47 | 8.16 | 104.37 | 0.03 | 0.006 | 0.08 | 0.03 | 1.37 |
| 14 June 2022 13:40 | 3 | 21.43 | 38.24 | 8.15 | 109.91 | 0.01 | 0.014 | 0.12 | 0.03 | 0.56 |
| 28 June 2022 08:45 | 3 | 25.96 | 38.09 | 8.14 | 104.41 | 0.01 | 0.016 | 0.17 | 0.03 | 0.78 |
| 12 July 2022 09:50 | 3 | 24.64 | 38.16 | 8.02 | 104.42 | 0.01 | 0.01 | 0.18 | 0.04 | 1.11 |
| 27 July 2022 11:40 | 3 | 26.22 | 38.1 | 8.15 | 104.01 | 0.01 | 0.006 | 0.07 | 0.03 | 0.5 |
| 17 August 2022 09:30 | 3 | 24.97 | 38.42 | 8.15 | 102.35 | 0.01 | 0.006 | 0.11 | 0.03 | 0.94 |
| 30 August 2022 10:00 | 3 | 25.22 | 38.42 | 8.15 | 102.16 | 0.01 | 0.006 | 0.18 | 0.21 | 0.84 |
| 13 September 2022 09:13 | 3 | 24.79 | 38.36 | 8.15 | 99.9 | 0.01 | 0.022 | 0.26 | 0.67 | 1.08 |
| 27 September 2022 10:43 | 3 | 21.78 | 38.27 | 8.12 | 95.59 | 0.01 | 0.07 | 0.55 | 0.84 | 3.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokovšek, A.; Pitacco, V.; Trkov, D.; Zamuda, L.L.; Falace, A.; Orlando-Bonaca, M. Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests. Plants 2023, 12, 2615. https://doi.org/10.3390/plants12142615
Lokovšek A, Pitacco V, Trkov D, Zamuda LL, Falace A, Orlando-Bonaca M. Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests. Plants. 2023; 12(14):2615. https://doi.org/10.3390/plants12142615
Chicago/Turabian StyleLokovšek, Ana, Valentina Pitacco, Domen Trkov, Leon Lojze Zamuda, Annalisa Falace, and Martina Orlando-Bonaca. 2023. "Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests" Plants 12, no. 14: 2615. https://doi.org/10.3390/plants12142615
APA StyleLokovšek, A., Pitacco, V., Trkov, D., Zamuda, L. L., Falace, A., & Orlando-Bonaca, M. (2023). Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests. Plants, 12(14), 2615. https://doi.org/10.3390/plants12142615






