Cyclic Hexapeptide from Bouvardia ternifolia (Cav.) Schltdl. and Neuroprotective Effects of Root Extracts
Abstract
1. Introduction
2. Results
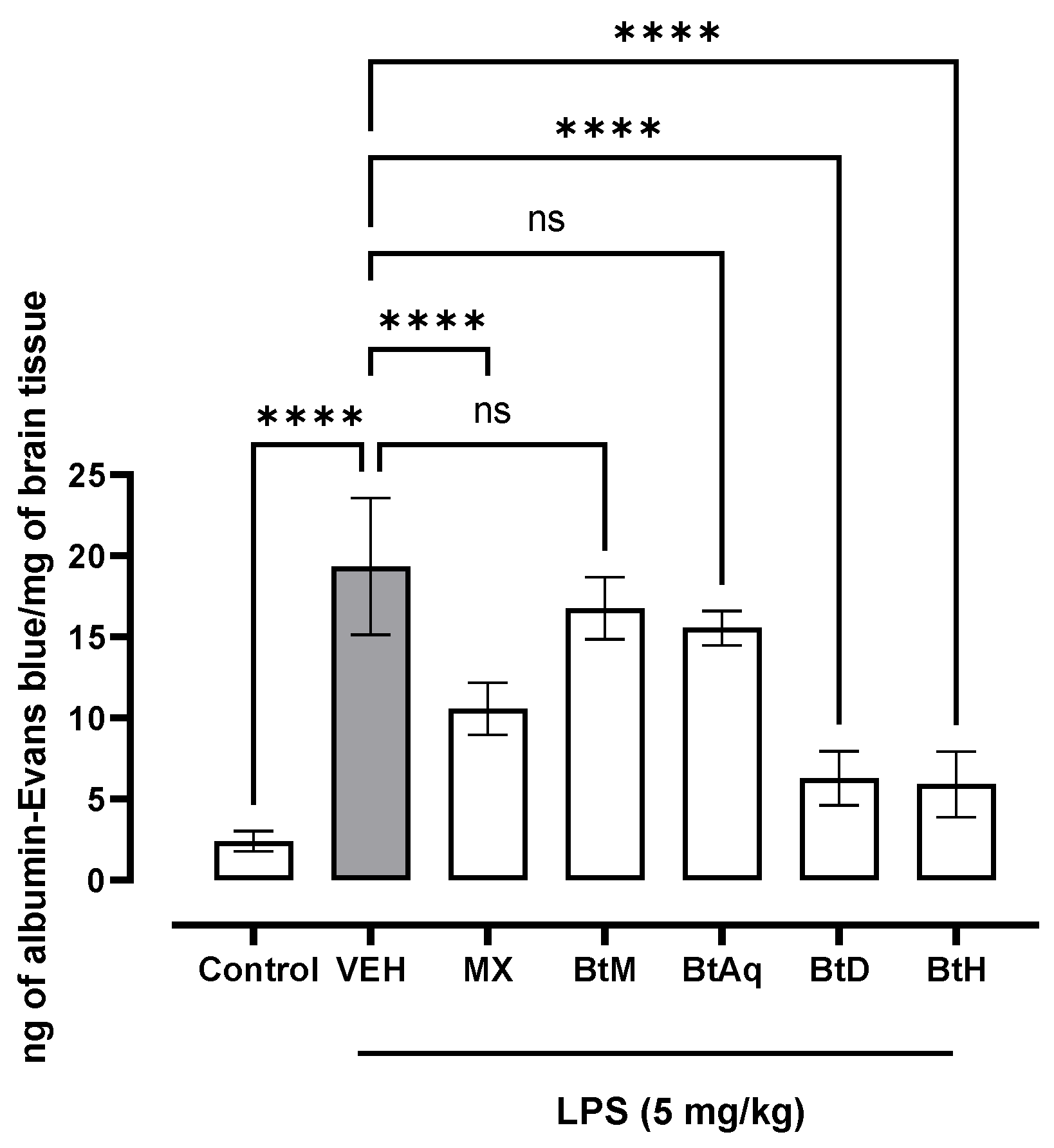
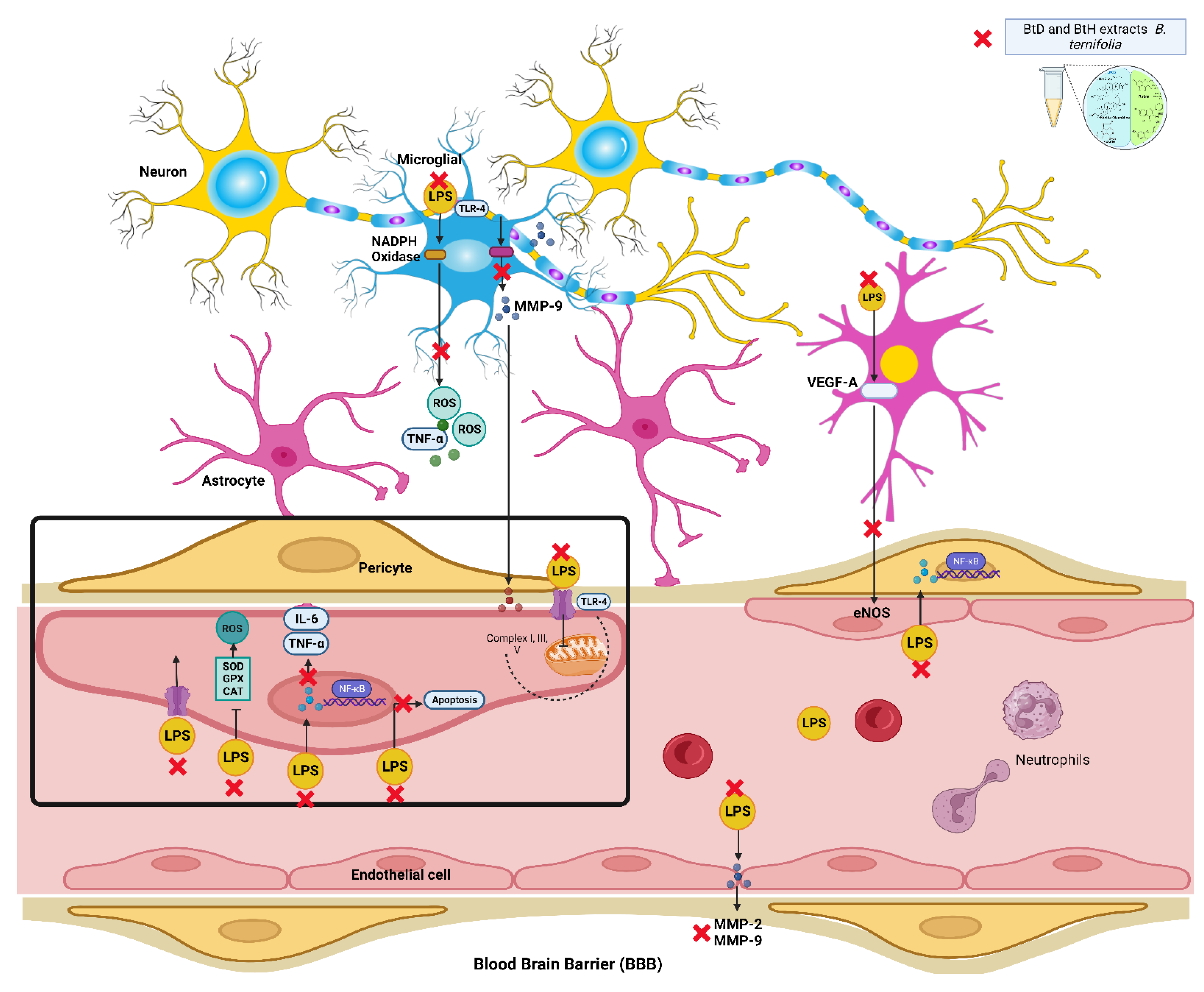
2.1. Protective Effect of B. termifolia Root Extracts on Blood-Brain Barrier
2.2. Effect of B. ternifolia Root Extracts on Cytokine Levels in the Brain of LPS-Administered Mice
2.3. Chemical Analysis
2.3.1. GC-MS Analysis of Dichloromethane Extract
2.3.2. GC–MS Analysis of Hexane Extract
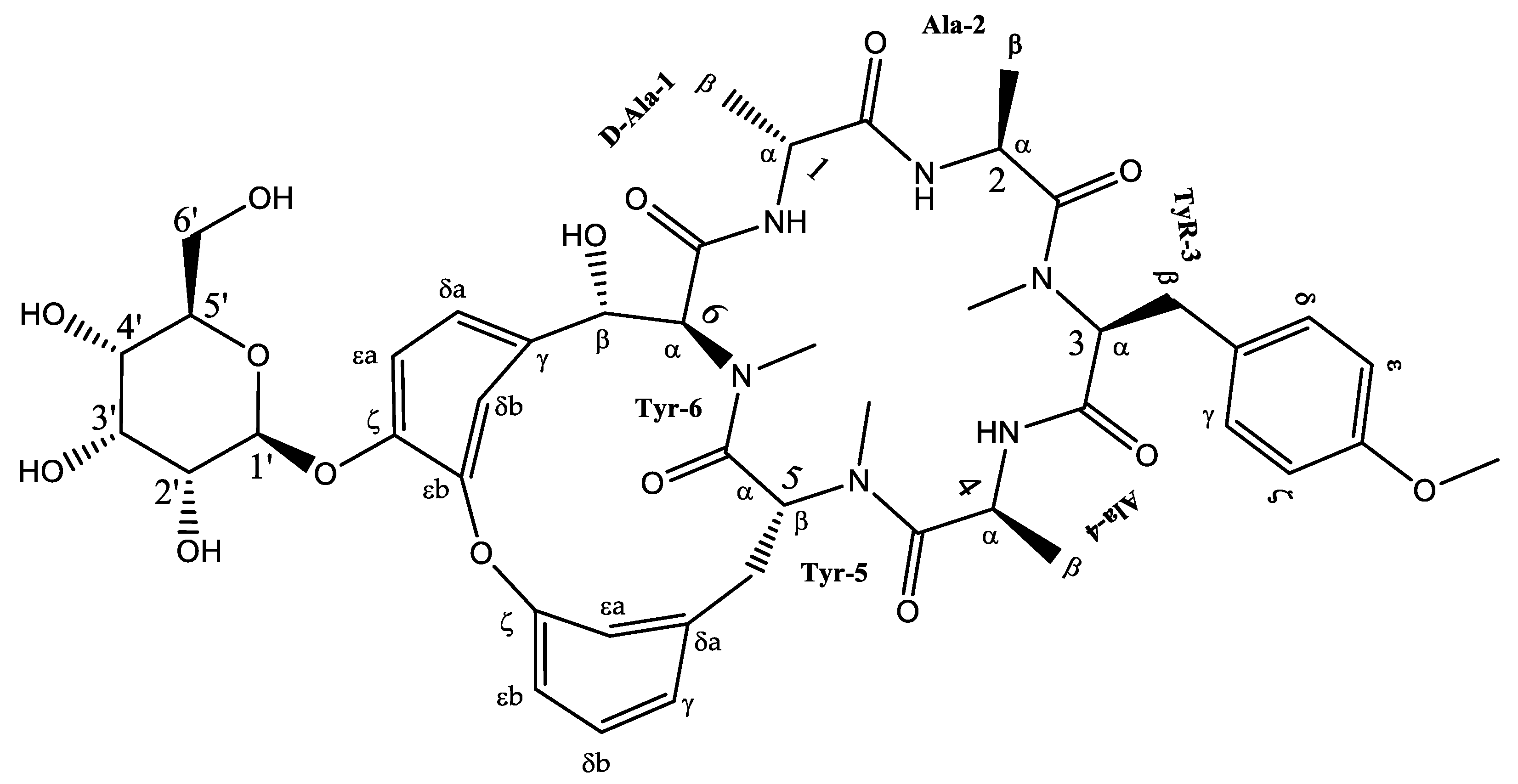
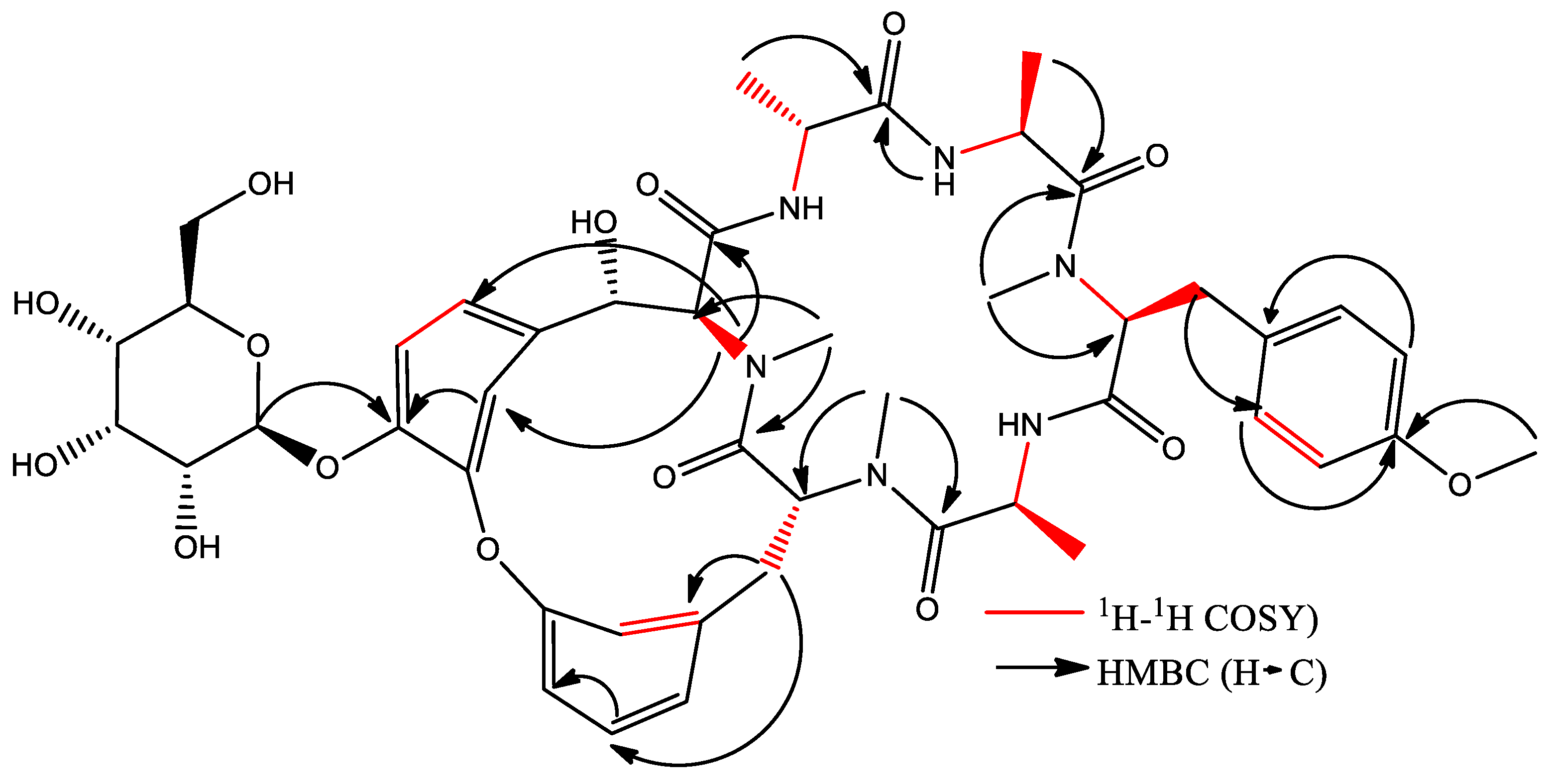
2.3.3. Isolation of Cyclic Hexapeptide
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. LPS-Induced Acute Brain Inflammation Model in Mice
4.2.1. Quantification of Evans Blue Extravasation
4.2.2. Quantification of Cytokines
4.3. GC-Mass Spectrometry (CG-MS)
4.4. Fractionation of the BtD Extract
Isolation and Identification of Cyclic Hexapeptide
4.5. Nuclear Magnetic Resonance
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcos-Burgos, M.; Lopera, F.; Sepulveda-Falla, D.; Mastronardi, C. Neural Plasticity during Aging. Neural Plats 2019, 2019, 6042132. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bidram, E.; Łos, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting autophagy, oxidative stress, and ER stress for neurodegenerative disease treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 2470547022107639. [Google Scholar] [CrossRef] [PubMed]
- Regen, F.; Hellmann-Regen, J.; Costantini, E.; Reale, M. Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr. Alzheimer Res. 2017, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr. Top. Med. Chem. 2017, 17, 3146–3169. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar]
- Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar]
- Zhao, H.; Cheng, L.; Liu, Y.; Zhang, W.; Maharjan, S.; Cui, Z.; Wang, X.; Tang, D.; Nie, L. Mechanisms of anti-inflammatory property of conserved dopamine neurotrophic factor: Inhibition of JNK signaling in lipopolysaccharide-induced microglia. J. Mol. Neurosci. 2014, 52, 186–192. [Google Scholar] [CrossRef]
- Standley, P.C. Trees and Shrubs of Mexico; Govt. Print. Off.: Washington, DC, USA, 1920; p. 1651. [Google Scholar]
- Jolad, S.D.; Hoffmann, J.J.; Torrance, S.J.; Wiedhopf, R.M.; Cole, J.R.; Arora, S.K.; Bates, R.B.; Gargiulo, R.L.; Kriek, G.R. Bouvardin and deoxybouvardin, antitumor cyclic hexapeptides from Bouvardia ternifolia (Rubiaceae). J. Am. Chem. Soc. 1977, 99, 8040–8044. [Google Scholar] [CrossRef]
- Jiménez-Ferrer, J.E.; Pérez-Terán, Y.Y.; Román-Ramos, R.; Tortoriello, J. Antitoxin activity of plants used in Mexican traditional medicine against scorpion poisoning. Phytomedicine 2005, 12, 116–122. [Google Scholar] [CrossRef]
- Zapata Lopera, Y.M.; Jiménez-Ferrer, E.; Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Rosas-Salgado, G.; Santillán-Urquiza, M.A.; Trejo-Tapia, G.; Jiménez-Aparicio, A.R. New Chromones from Bouvardia ternifolia (Cav.) Schltdl. with Anti-Inflammatory and Immunomodulatory Activity. Plants 2022, 12, 1. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Garcia-Morales, G.; Zamilpa, A.; Gonzalez-Cortazar, M.; Tortoriello, J.; Ventura-Zapata, E.; Jimenez-Ferrer, E. Inhibition of acetylcholinesterase activity by hidroalcoholic extract and their fractions of Bouvardia ternifolia (Cav.) Shcltdl (Rubiaceae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 526–541. [Google Scholar]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Román-Ramos, R. Anti-inflammatory, antioxidant and anti-acetylcholinesterase activities of Bouvardia ternifolia: Potential implications in Alzheimer’s disease. Arch. Pharm. Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.B.; Cole, J.R.; Hoffmann, J.J.; Kriek, G.R.; Linz, G.S.; Torrance, S.J. Solution forms of bouvardin and relatives from NMR studies. 6-O-Methylbouvardin. J. Am. Chem. Soc. 1983, 105, 1343–1347. [Google Scholar] [CrossRef]
- Jiménez-Ferrer, E.; Reynosa-Zapata, I.; Pérez-Torres, Y.; Tortoriello, J. The secretagogue effect of the poison from Centruroides limpidus limpidus on the pancreas of mice and the antagonistic action of the Bouvardia ternifolia extract. Phytomedicine 2005, 12, 65–71. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. BioMed Res. 2020, 2020, 7532306. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Sarker, S.D. Alzheimer’s disease: Natural products as inhibitors of neuroinflammation. Inflammopharmacology 2020, 28, 1439–1455. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. An in vivo assay to test blood vessel permeability. J. Vis. Exp. 2013, 73, e50062. [Google Scholar]
- Chen, W.-Q.; Zhao, X.-L.; Hou, Y.; Li, S.-T.; Hong, Y.; Wang, D.-L.; Cheng, Y.Y. Protective effects of green tea polyphenols on cognitive impairments induced by psychological stress in rats. Behav. Brain Res. 2009, 202, 71–76. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef]
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 2021, 88, 108552. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Hyun, K.-Y. 2-Nonadecanone Alleviates Depression through Inflammation Relief in SD Rat. Biomed. Sci. Lett. 2018, 24, 206–212. [Google Scholar] [CrossRef]
- Kim, S.K.; Karadeniz, F. Biological importance, and applications of squalene and squalene. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chon-droprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Munda, S.; Pandey, S.K.; Dutta, S.; Baruah, J.; Lal, M. Antioxidant Activity, Antibacterial Activity and Chemical Compo-sition of Essential Oil of Artemisia vulgaris L. Leaves from Northeast India. J. Essent. Oil Bear. Plants 2019, 22, 368–379. [Google Scholar] [CrossRef]
- Badshah, H.; Ali, T.; Rehman, S.; Amin, F.U.; Ullah, F.; Kim, T.H.; Kim, M.O. Protective Effect of Lupeol Against Lipopolysaccharide-Induced Neuroinflammation via the p38/c-Jun N-Terminal Kinase Pathway in the Adult Mouse Brain. J. Neuroimmune Pharmacol. 2016, 11, 48–60. [Google Scholar] [CrossRef]
- Oliveira-Junior, M.S.; Pereira, E.P.; de Amorim, V.C.M.; Reis, L.T.C.; do Nascimento, R.P.; da Silva, V.D.A.; da Silva, V.D.A.; Costa, S.L. Lupeol inhibits LPS-induced neuroinflammation in cerebellar cultures and induces neuroprotection associated to the modulation of astrocyte response and expression of neurotrophic and inflammatory factors. Int. Immunopharmacol. 2019, 70, 302–312. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Fan, J.T.; Su, J.; Peng, Y.M.; Li, Y.; Li, J.; Zhou, Y.B.; Zeng, G.Z.; Yan, H.; Tan, N.H. Rubiyunnanins C-H, cytotoxic cyclic hexapeptides from Rubia yunnanensis inhibiting nitric oxide production and NF-κB activation. Bioorg. Med. Chem. 2010, 18, 8226–8234. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Ghisi, D.; Aprile, P.L.; Lapi, F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: Latest evidence and clinical implications. Ther. Adv. Drug Saf. 2017, 8, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hakan, T.; Toklu, H.Z.; Biber, N.; Ozevren, H.; Solakoglu, S.; Demirturk, P.; Aker, F.V. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol. Res. 2010, 32, 629–635. [Google Scholar] [CrossRef]
- Alves da Silva, J.A.; Oliveira, K.C.; Camillo, M.A.P. Gyroxin increases blood-brain barrier permeability to Evans blue dye in mice. Toxicon 2011, 57, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jiménez-Nevárez, Y.B.; Angulo-Escalante, M.A.; Montes-Avila, J.; Guerrero-Alonso, A.; Christen, J.G.; Hurtado-Díaz, I.; Heredia, J.B.; Quintana-Obregón, E.A.; Alvarez, L. Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts. Plants 2023, 12, 560. [Google Scholar] [CrossRef]

| No | RT (min) | Chemical Structure | Compound Name | Formula | Molecular Weight (g/mol) | Area (%) | Spectral Information ID |
|---|---|---|---|---|---|---|---|
| 1 | 6.8 |  | (M) 3-Carene | C10H16 | 136 | 1.2 | 103751 |
| 2 | 19.8 | 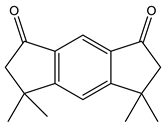 | (M) s-indacene-1,7-dione,2,3,5,6-tetrahydro-3,3,5,5-tetramethyl | C16H18O2 | 242 | 14.3 | 31005 |
| 3 | 20.2 |  | (M) 2-Nonadecanone | C19H38O | 282 | 2.9 | 114582 |
| 4 | 21.1 | 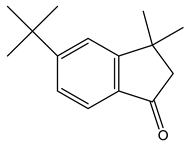 | (M) 1H-Inden-1 one,5-(1,1-dimethylethyl)-2,3-dihydro-3,3-dimethyl | C15H20O | 216 | 11.3 | JP005240 |
| 5 | 28.8 | 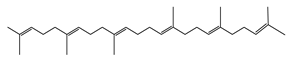 | (R) Squalene | C30H50 | 410 | 9.13 | 2140 |
| 6 | 32.7 | 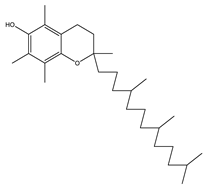 | (M) D, α-Tocopherol | C29H50O2 | 430 | 35.6 | 30334 |
| 7 | 34.7 | 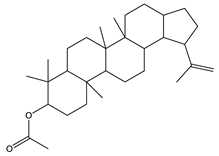 | (M) Lup-20(29)-en-3-ol-acetate, (3β) | C32H52O2 | 468 | 5.4 | 194307 |
| No | RT (min) | Chemical Structure | Compound Name | Formula | Molecular Weight (g/mol) | Area (%) | Spectral Information ID |
|---|---|---|---|---|---|---|---|
| 8 | 18.4 |  | (R) Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 3.2 | 1656 |
| 9 | 19.1 | 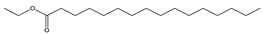 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 0.8 | 27658 |
| 10 | 20.1 |  | (M) 9,12-octadecadienoic acid (z-z)-methyl ester | C19H34O2 | 294 | 3.4 | 112-63-0 |
| 11 | 20.1 | 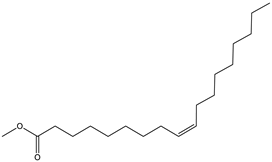 | (M) 9- octadecadienoic acid (z)-methyl ester | C19H36O | 296 | 2.4 | JP005355 |
| 12 | 20.3 | 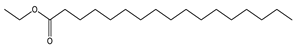 | (R) Octadecanoic acid, methyl ester | C19H38O2 | 298 | 0.6 | 1815 |
| 13 | 20.7 | 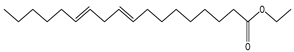 | (M) 9,12 Octadecanoic acid, ethyl ester | C20H36O2 | 308 | 1.2 | 249157 |
| 14 | 20.7 | 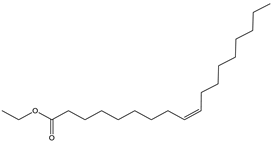 | (R) Ethyl oleate | C20H38O2 | 310 | 1.4 | JP002528 |
| 15 | 22.4 | 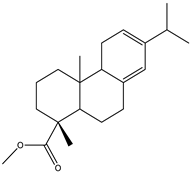 | (M) Phenanthrenecarboxilic acid 1,2,3,4,4a,9,10,10a-dimethyl-7-(1-methylethyl)-methyl ester, [1R-(1α,4aβ,10aα)] | C20H26O3 | 316 | 3.8 | 3513-69-7 |
| 16 | 22.9 |  | (R) methyl abietate | C21H32O2 | 316 | 0.6 | JP003373 |
| 17 | 28.8 |  | (R) Scualene | C30H50 | 410 | 20 | 2140 |
| 18 | 32.6 | 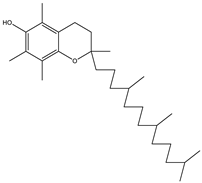 | (M) D, α-Tocopherol | C29H50O2 | 430 | 44.8 | 30334 |
| 19 | 35.6 |  | (R) β-Sitosterol | C29H50O | 414 | 4.8 | 2300 |
| 20 | 44.7 |  | (T) Urs-12-en-28-al,3-(acetyloxy)-(3β) | C32H50O3 | 482 | 6.2 | 86996-88-5 |
| Position | 1H | 13C | |
|---|---|---|---|
| D-Ala1 | α | 4.48 (overlap) | 48.3 d |
| β | 1.23 (overlap) | 19.9 q | |
| C=O | 172.4 s | ||
| Ala2 | α | 4.71 (overlap) | 46.6 d |
| β | 1.28 (overlap) | 17.5 q | |
| C=O | 174.8 s | ||
| Tyr3 | α | 3.76 (overlap) | 67.4 d |
| βa | 3.27 (m) | 32.4 t | |
| γ | 130.7 s | ||
| δ*2 | 7.10 (8.5) | 130.2 d | |
| ε*2 | 6.88 (8.7) | 114.7 d | |
| ζ | 159.3 s | ||
| C=O | 172 s | ||
| NMe | 2.96 (s) | 38.8 q | |
| OMe | 3.77 (s) | 54.2 q | |
| Ala4 | α | 4.71 (overlap) | 47.02 d |
| β | 1.12 (d,6.6) | 17.5 q | |
| C=O | 172.2 s | ||
| Tyr5 | α | 5.46 (dd,11.6,3.1) | 57.5 t |
| βa | 2.63 (d,11.2) | 38.9 | |
| βb | 3.57 (overlap) | ||
| γ | 139.3 s | ||
| δa | 7.13 (overlap) | 130.9 d | |
| δb | 7.52 (dd,8.6,2.0) | 130.2 d | |
| εa | 7.31 (dd,8.3,2.3) | 123.9 d | |
| εb | 7.42 (dd,8.5,2.2) | 125.5 d | |
| ζ | 159.3 s | ||
| C=O | 172.1 s | ||
| NMe | 3.2 (s) | 29.2 q | |
| Tyr6 | α | 4.55 (overlap) | 67.4 d |
| βa | 4.48 (d,6.9) | 73.5 d | |
| βb | |||
| γ | 135.8 s | ||
| δa | 6.95 (dd,8.6,2.4) | 125.5 d | |
| δb | 5.0 (d,7.8) | 117.4 d | |
| εa | 7.10 (d,8.5) | 121.3 d | |
| eb | 153.1 s | ||
| ζ | 144.2 s | ||
| C=O | 170.5 s | ||
| NMe | 2.3 (s) | 32.5 s | |
| Glucose | 1′ | 5.0 (d,7.8) | 101.5 d |
| 2′ | 3.56 (overlap) | 76.5 d | |
| 3′ | 3.48 (m) | 76.9 d | |
| 4′ | 3.44 (overlap) | 70.0 d | |
| 5′ | 3.44 (overlap) | 77.6 d | |
| 6′ | 3.77 (dd,12.0, 4.8) | 61.1 t | |
| 3.87 (overlap) |
| Group | Oral Treatment | Doses |
|---|---|---|
| Control | Water | 100 µL/10 g |
| VEH | Water + LPS * | 5 mg/kg * |
| Experimental | MX BtM BtAq BtD BtH | 25 mg/kg for 5 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopera, Y.M.Z.; Trejo-Tapia, G.; González-Cortazar, M.; Herrera-Ruiz, M.; Zamilpa, A.; Jiménez-Ferrer, E. Cyclic Hexapeptide from Bouvardia ternifolia (Cav.) Schltdl. and Neuroprotective Effects of Root Extracts. Plants 2023, 12, 2600. https://doi.org/10.3390/plants12142600
Lopera YMZ, Trejo-Tapia G, González-Cortazar M, Herrera-Ruiz M, Zamilpa A, Jiménez-Ferrer E. Cyclic Hexapeptide from Bouvardia ternifolia (Cav.) Schltdl. and Neuroprotective Effects of Root Extracts. Plants. 2023; 12(14):2600. https://doi.org/10.3390/plants12142600
Chicago/Turabian StyleLopera, Yury Maritza Zapata, Gabriela Trejo-Tapia, Manasés González-Cortazar, Maribel Herrera-Ruiz, Alejandro Zamilpa, and Enrique Jiménez-Ferrer. 2023. "Cyclic Hexapeptide from Bouvardia ternifolia (Cav.) Schltdl. and Neuroprotective Effects of Root Extracts" Plants 12, no. 14: 2600. https://doi.org/10.3390/plants12142600
APA StyleLopera, Y. M. Z., Trejo-Tapia, G., González-Cortazar, M., Herrera-Ruiz, M., Zamilpa, A., & Jiménez-Ferrer, E. (2023). Cyclic Hexapeptide from Bouvardia ternifolia (Cav.) Schltdl. and Neuroprotective Effects of Root Extracts. Plants, 12(14), 2600. https://doi.org/10.3390/plants12142600









