Ziziphus mauritiana Leaves Normalize Hormonal Profile and Total Cholesterol in Polycystic Ovarian Syndrome Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening
2.2. HPTLC Profile
2.3. Acute Toxicity Studies
2.4. Effect of Different Drug Treatments on Oestrus Cycle
2.5. Effect of Drug Treatments on Body Weight and Ovarian Weight
2.6. Effect of Different Treatments on Plasma Progesterone, LH, Estradiol and Testosterone Levels
2.7. Effect of Different Treatments on Total Cholesterol and Blood Sugar
2.8. Ovarian Histopathology
3. Discussion
4. Materials and Methods
4.1. Collection and Authentication of Plant Material
4.2. Preparation and Extraction of Plant Leaves
4.3. Phytochemical Screening
4.4. High-Performance Thin Layer Chromatography (HPTLC)
4.5. Experimental Animals
4.6. Acute Toxicity
4.7. Animal Grouping and Induction of PCOS
4.8. Oestrus Cycle Determination
4.9. Biochemical Estimation
4.9.1. Serum Hormonal Analysis
4.9.2. Measurement of Total Cholesterol
4.9.3. Determination of Blood Glucose—Oral Glucose Tolerance Test (OGTT)
4.10. Histopathological Changes
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khairwal, A.; Kumar, D. Whom to Blame for Infertility: Semen Analysis of Men from 70 Infertile Couples. Epidemiology 2018, 8, 361. [Google Scholar]
- The Lancet Diabetes & Endocrinology. Empowering women with PCOS. Lancet Diabetes Endocrinol. 2019, 7, 737. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J.; Buschur, E.O.; Chang, A.Y.; Hammer, G.D.; Ramm, C.; Madrigal, D.; Wang, G.; Gonzalez, M.; Xu, X.S.; Smit, J.W.; et al. Abiraterone Acetate to Lower Androgens in Women with Classic 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, M.S.; Shengule, S.; Apte, K.G.; Wani, M.R.; Piprode, V.; Parab, P.B. Anti-obesity activity of Ziziphus mauritiana: A potent pancreatic lipase inhibitor. Anc. Sci. Life 2012, 32, S38. [Google Scholar] [CrossRef]
- Deepak, J.; Anurekha, J. Study of antiobesity activity of polyherbal formulation in correlation with antidiabetic activity. Asian J. Pharm. Clin. Res. 2018, 11, 486–490. [Google Scholar] [CrossRef]
- Talmale, S.; Bhujade, A.; Patil, M. Anti-allergic and anti-inflammatory properties of Zizyphus mauritiana root bark. Food Funct. 2015, 6, 2975–2983. [Google Scholar] [CrossRef]
- Priyanka, C.; Kumar, P.; Bankar, S.P.; Karthik, L. In-Vitro antibacterial activity and gas chromatography–mass spectroscopy analysis of Acacia karoo and Ziziphus mauritiana extracts. J. Taibah Univ. Med. Sci. 2015, 9, 13–19. [Google Scholar] [CrossRef]
- Dahiru, D.; Obidoa, O. Evaluation of the antioxidant effects of Ziziphus mauritiana Lam. Leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr. J. Tradit. Complement. Altern. Med. 2007, 27, 39–45. [Google Scholar] [CrossRef]
- Akhtar, N.; Ijaz, S.; Khan, H.M.S.; Uzair, B.; Reich, A.; Khan, B.A. Ziziphus mauritiana leaf extract emulsion for skin rejuvenation. Trop. J. Pharm. Res. 2016, 15, 929–936. [Google Scholar] [CrossRef]
- Shiv, K.; Ganachari, M.S.; Banappa Nagoor, V.S. Antiinflammatory activity of Ziziphus jujuba Lamk. Leaves extract in rats. J. Nat. Remedies 2004, 4, 183–185. [Google Scholar]
- Ganachari, M.S.; Shiv, K. Anti-ulcer properties of Ziziphus jujube Lam leaves extract in rats. J. Nat. Remedies 2004, 4, 103–108. [Google Scholar]
- Gupta, M.K.; Bhandari, A.K.; Singh, R.K. Pharmacognostical evaluations of the leaves of Ziziphus mauritiana. Int. J. Pharm. Sci. Res. 2012, 3, 818–821. [Google Scholar]
- Chin, W.J.; Wa, X.E. A new neo-lignan, a prostaglandin I2 inducer from the leaves of Ziziphus jujuba. Planta Med. 1986, 6, 501–502. [Google Scholar]
- De Medeiros, S.F. Risks, benefits size and clinical implications of combined oral contraceptive use in women with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Gambineri, A. Insulin-sensitizing agents in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2006, 154, 763–765. [Google Scholar] [CrossRef]
- Mitchell, S.Y.; Fletcher, H.M.; Williams, E. Ovarian hyperstimulation syndrome associated with clomiphene citrate. West Indian Med J. 2001, 50, 227–229. [Google Scholar]
- Kafali, H.; Iriadam, M.; Ozardali, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med Res. 2014, 35, 103–108. [Google Scholar] [CrossRef]
- Brown, J.; Farquhar, C. Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome. Cochrane Database Syst. Rev. 2016, 12, CD002249. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 23, 2278. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef]
- Banaszewska, B.; Duleba, A.J.; Spaczynski, R.Z.; Pawelczyk, L. Lipids in polycystic ovary syndrome: Role of hyperinsulinemia and effects of metformin. Am. J. Obstet. Gynecol. 2006, 194, 1266–1272. [Google Scholar] [CrossRef]
- Prakash, O.; Usmani, S.; Singh, R.; Singh, N.; Gupta, A.; Ved, A. A panoramic view on phytochemical, nutritional, and therapeutic attributes of Ziziphus mauritiana Lam.: A comprehensive review. Phytother. Res. 2020, 35, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, F.P.F.; Hajizadeh-Sharafabad, F.; Vaezi, M.; Jafari-Vayghan, H.; Alizadeh, M.; Maleki, V. Quercetin and polycystic ovary syndrome, current evidence and future directions: A systematic review. J. Ovarian Res. 2020, 13, 11. [Google Scholar] [CrossRef]
- Neisy, A.; Zal, F.; Seghatoleslam, A.; Alaee, S. Amelioration by quercetin of insulin resistance and uterine GLUT4 and ERα gene expression in rats with polycystic ovary syndrome (PCOS). Reprod. Fertil. Dev. 2019, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef]
- Jahan, S.; Abid, A.; Khalid, S.; Afsar, T.; Ain, Q.U.; Shaheen, G.; Almajwal, A.; Razak, S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J. Ovarian Res. 2018, 11, 26. [Google Scholar] [CrossRef]
- Khorshidi, M.; Moini, A.; Alipoor, E.; Rezvan, N.; Gorgani-Firuzjaee, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother Res. 2018, 32, 2282–2289. [Google Scholar] [CrossRef]
- Khorchani, M.J.; Zal, F.; Neisy, A. The phytoestrogen, quercetin, in serum, uterus and ovary as a potential treatment for dehydroepiandrosterone-induced polycystic ovary syndrome in the rat. Reprod. Fertil. Dev. 2020, 32, 313–321. [Google Scholar] [CrossRef]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kokate, C.K. A Text Book of Practical Pharmacognosy, 5th ed.; Vallabh Prakashan: New Delhi, India, 2005; pp. 107–111. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 17th ed.; Bahiv Tinal: London, UK, 1985; p. 149. [Google Scholar]
- Khandewal, K.R. Practical Pharmacognosy, 19th ed.; Nirali Prakashan: Pune, India, 2008. [Google Scholar]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Technique of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Wagner, H.; Baldt, S. Plant Drug Analysis; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ecobichon, D.J. The Basis of Toxicology Testing, 2nd ed.; CRC Press: New York, NY, USA, 1997; pp. 43–60. [Google Scholar]
- Russell Westwood, F. The Female Rat Reproductive Cycle: A Practical Histological Guide to Staging. Toxicol. Pathol. 2008, 36, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague–Dawley rats. Biomed. Res. Int. 2014, 2014, 160980–160991. [Google Scholar] [CrossRef] [PubMed]

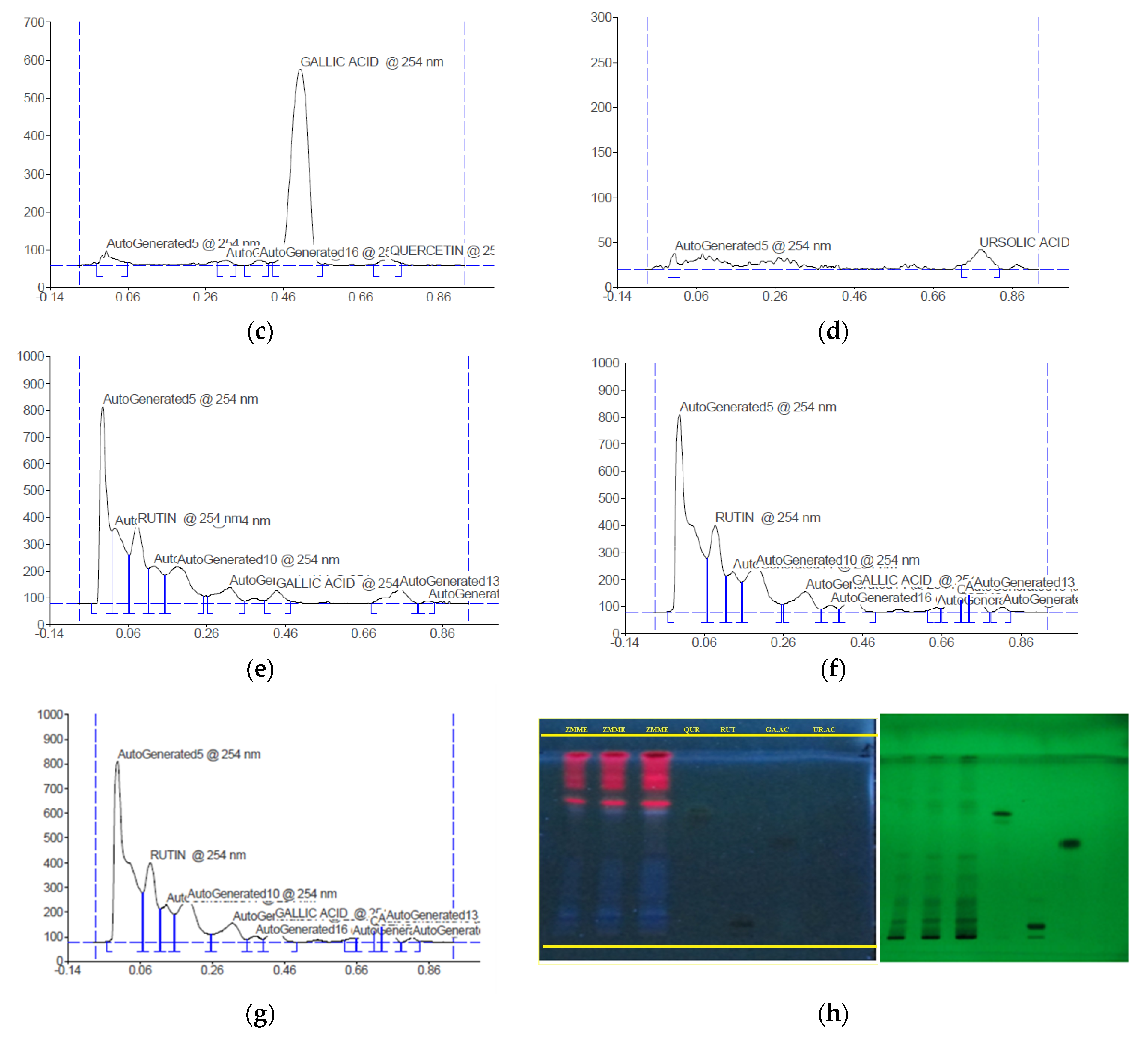

| Rf | Height | Area (1/100) | Maximum % Concentration | Assigned Substances |
|---|---|---|---|---|
| 0.05 | 634.1 | 141.60 | 59.54 | Rutin |
| 0.68 | 564.3 | 113.79 | 58.27 | Quercetin |
| 0.50 | 518.4 | 181.44 | 85.77 | Gallic acid |
| 0.78 | 22.3 | 85.50 | 54.96 | Ursolic acid |
| 0.00 | 18.3 | 23.48 | 45.04 | Unknown |
| 0.02 | 281.6 | 74.09 | 16.02 | Unknown |
| 0.19 | 249.6 | 125.68 | 10.22 | Unknown |
| 0.13 | 229.2 | 55.27 | 9.38 | Unknown |
| 0.63 | 202.8 | 56.68 | 20.94 | Unknown |
| 0.74 | 129.5 | 27.84 | 5.30 | Unknown |
| 0.31 | 119.5 | 55.95 | 4.89 | Unknown |
| 0.72 | 109.5 | 13.71 | 4.48 | Unknown |
| 0.38 | 68.9 | 18.86 | 2.82 | Unknown |
| 0.10 | 56.2 | 11.24 | 5.28 | Unknown |
| 0.81 | 35.8 | 8.68 | 1.47 | Unknown |
| 0.54 | 17.2 | 5.25 | 0.71 | Unknown |
| 0.34 | 17.0 | 3.71 | 1.75 | Unknown |
| 0.85 | 12.3 | 1.86 | 0.50 | Unknown |
| Treatment and Dose | Body Weight (g) | Ovary Weight (g) |
|---|---|---|
| Normal control | 102.6 ± 1.04 | 0.08 ± 0.0 |
| PCOS control | 132.5 ± 2.05 ## | 0.10 ± 0.0 ## |
| ZMME 100 mg/kg | 124.5 ± 2.2 ** | 0.09 ± 0.0 * |
| ZMME 200 mg/kg | 118.6 ± 2.2 ** | 0.08 ± 0.0 ** |
| Clomiphene citrate 2 mg/kg | 113 ± 1.7 ** | 0.08 ± 0.0 ** |
| Group | Progesterone (ng/mL) | LH (ng/mL) | Testosterone (ng/mL) | Estradiol (ng/mL) |
|---|---|---|---|---|
| Normal control | 48.83 ± 2.32 | 07.17 ± 1.47 | 02.33 ± 1.03 | 768.67 ± 1.37 |
| PCOS control | 17.67 ± 1.03 ## | 24.33 ± 2.07 ## | 07.83 ± 1.17 ## | 127.33 ± 1.63 ## |
| ZMME 100 mg/kg | 38.83 ± 2.31 ** | 17.83 ± 1.17 * | 04.83 ± 0.75 ** | 548.83 ± 1.16 ** |
| ZMME 200 mg/kg | 47.83 ± 2.48 ** | 14.67 ± 1.86 ** | 04.56 ± 1.47 ** | 638.66 ± 2.58 ** |
| Clomiphene citrate 2 mg/kg | 46.33 ± 1.03 ** | 10.33 ± 1.03 ** | 04.16 ± 0.75 ** | 650.16 ± 1.47 ** |
| Group | Total Cholesterol (mg/dL) |
|---|---|
| Normal control | 71.33 ± 1.03 |
| PCOS control | 116.33 ± 1.36 ## |
| ZMME 100 mg/kg | 81.33 ± 1.03 ** |
| ZMME 200 mg/kg | 72.50 ± 0.83 ** |
| Clomiphene citrate 2 mg/kg | 74.00 ± 1.41 ** |
| Treatment and Dose | Concentration of Blood Glucose (mg/dL) | ||||
|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | |
| Normal control | 79 ± 4.5 | 118.3 ± 7.6 | 130.2 ± 6.04 | 157.5 ± 7.5 | 145.8 ± 5.5 |
| PCOS control | 75.8 ± 3.7 | 120.2 ± 7.3 | 132.7 ± 7.3 | 159.3 ± 4.2 | 149.8 ± 6.9 |
| ZMME 100 mg/kg | 79.5 ± 3.7 | 115.5 ± 13.9 | 126.7 ± 6.8 | 162.5 ± 4.2 | 141.8 ± 6.7 |
| ZMME 200 mg/kg | 79.5 ± 7.4 | 115.2 ± 11.6 | 126.3 ± 5.04 | 163.3 ± 6.4 | 152 ± 7.5 |
| Clomiphene citrate 2 mg/kg | 78.5 ± 7.2 | 115.2 ± 7.05 | 130.8 ± 5.5 | 161 ± 7.8 | 155 ± 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivanandappa, T.B.; Chinnadhurai, M.; Kandasamy, G.; Vasudevan, R.; Sam, G.; Karunakarannair, A. Ziziphus mauritiana Leaves Normalize Hormonal Profile and Total Cholesterol in Polycystic Ovarian Syndrome Rats. Plants 2023, 12, 2599. https://doi.org/10.3390/plants12142599
Shivanandappa TB, Chinnadhurai M, Kandasamy G, Vasudevan R, Sam G, Karunakarannair A. Ziziphus mauritiana Leaves Normalize Hormonal Profile and Total Cholesterol in Polycystic Ovarian Syndrome Rats. Plants. 2023; 12(14):2599. https://doi.org/10.3390/plants12142599
Chicago/Turabian StyleShivanandappa, Thippeswamy Boreddy, Maheswari Chinnadhurai, Geetha Kandasamy, Rajalakshimi Vasudevan, Gigi Sam, and Anjana Karunakarannair. 2023. "Ziziphus mauritiana Leaves Normalize Hormonal Profile and Total Cholesterol in Polycystic Ovarian Syndrome Rats" Plants 12, no. 14: 2599. https://doi.org/10.3390/plants12142599
APA StyleShivanandappa, T. B., Chinnadhurai, M., Kandasamy, G., Vasudevan, R., Sam, G., & Karunakarannair, A. (2023). Ziziphus mauritiana Leaves Normalize Hormonal Profile and Total Cholesterol in Polycystic Ovarian Syndrome Rats. Plants, 12(14), 2599. https://doi.org/10.3390/plants12142599






