Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions
Abstract
1. Introduction
2. Results
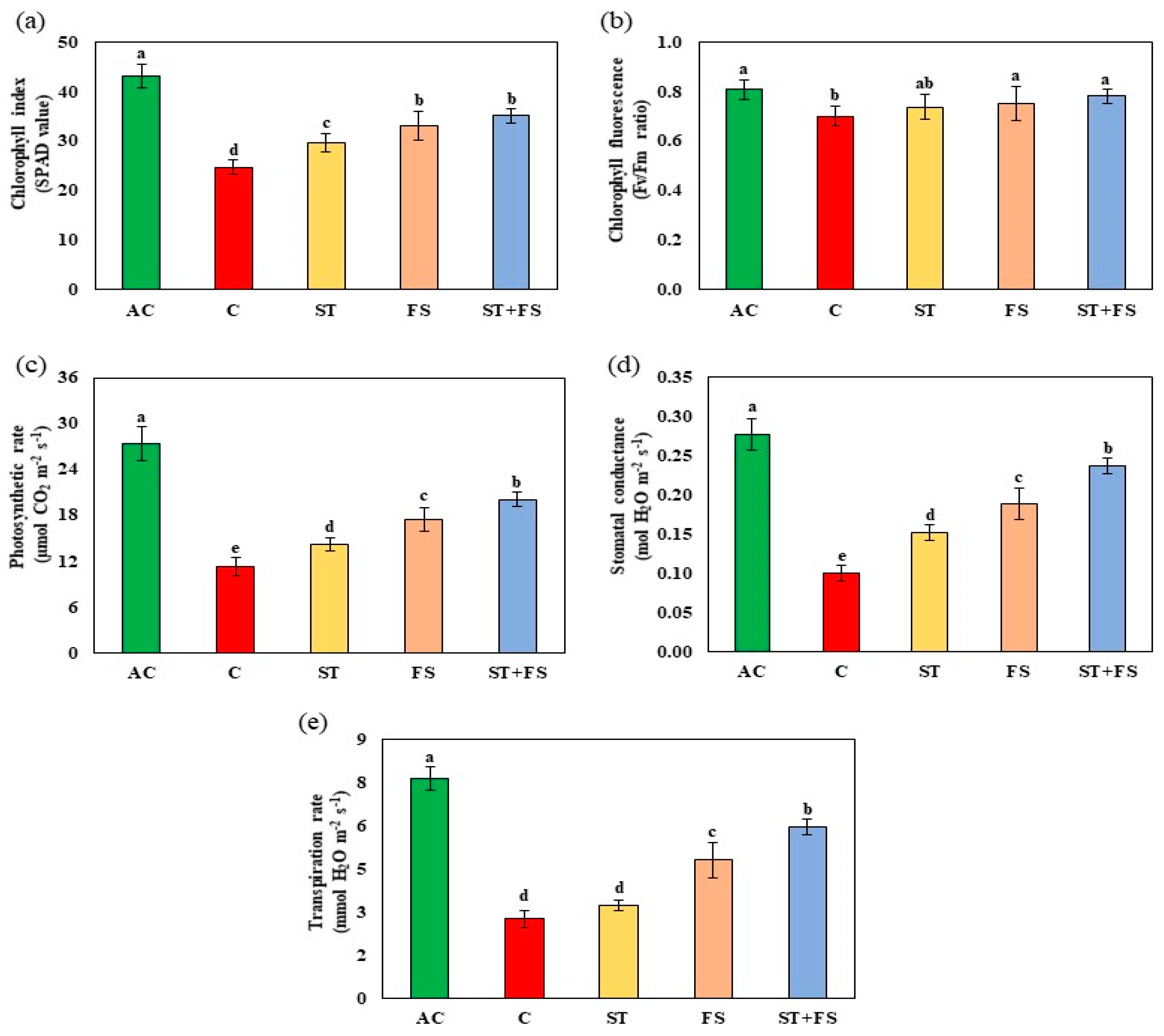
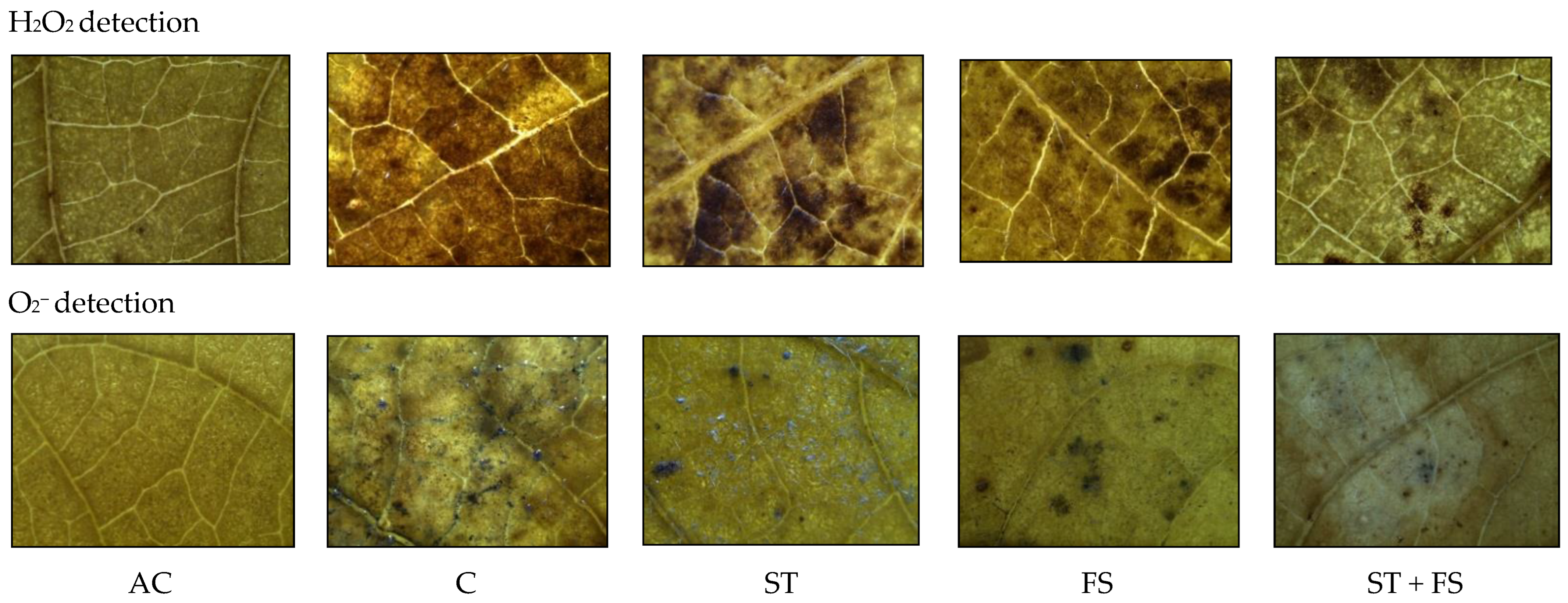
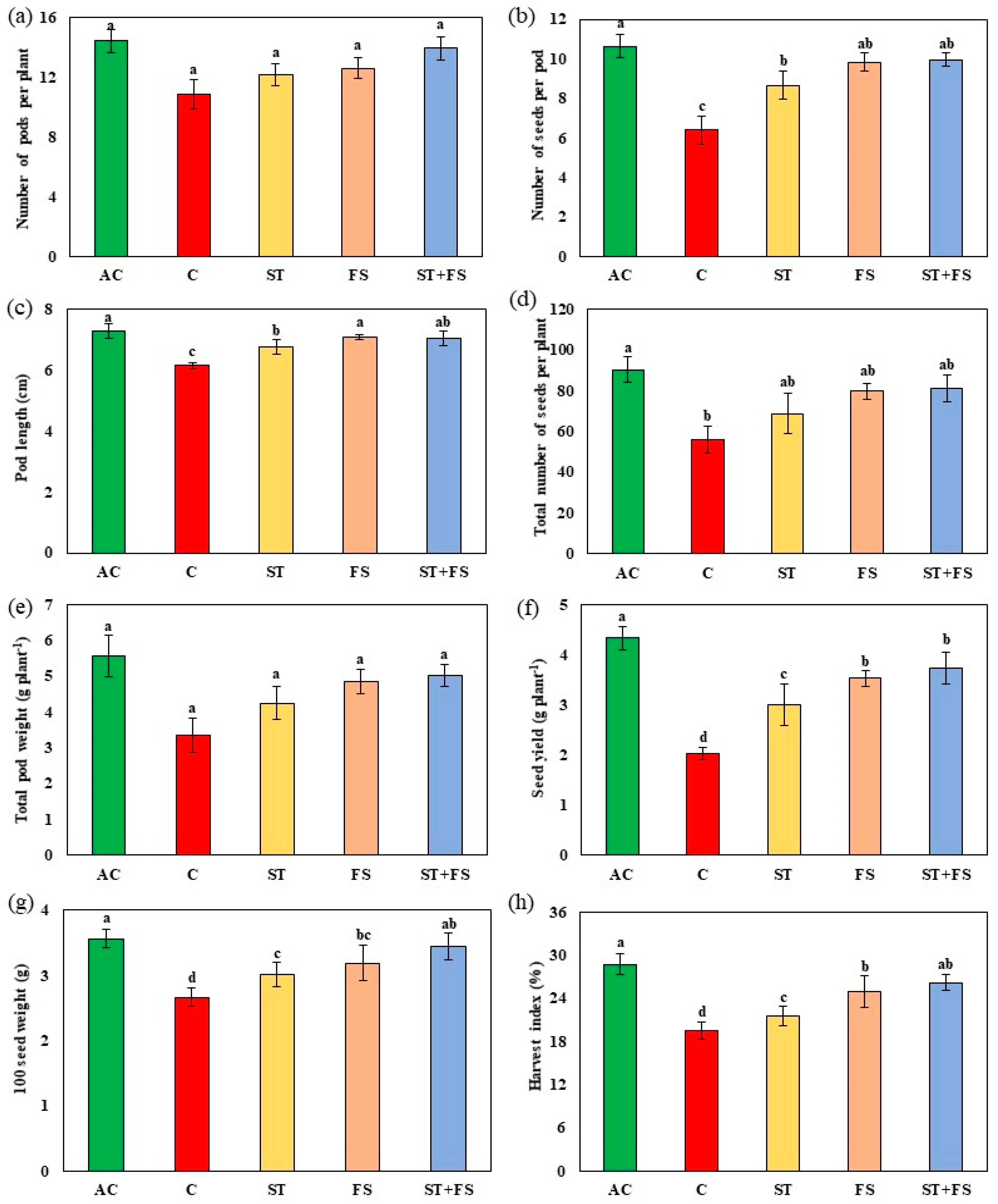
2.1. Effect of Melatonin on Physiological, Biochemical, and Yield Characteristics of Mung Bean under Drought Stress
2.2. Effect of Melatonin on Physiological, Biochemical, and Yield Characteristics of Mung Bean under High-Temperature Stress
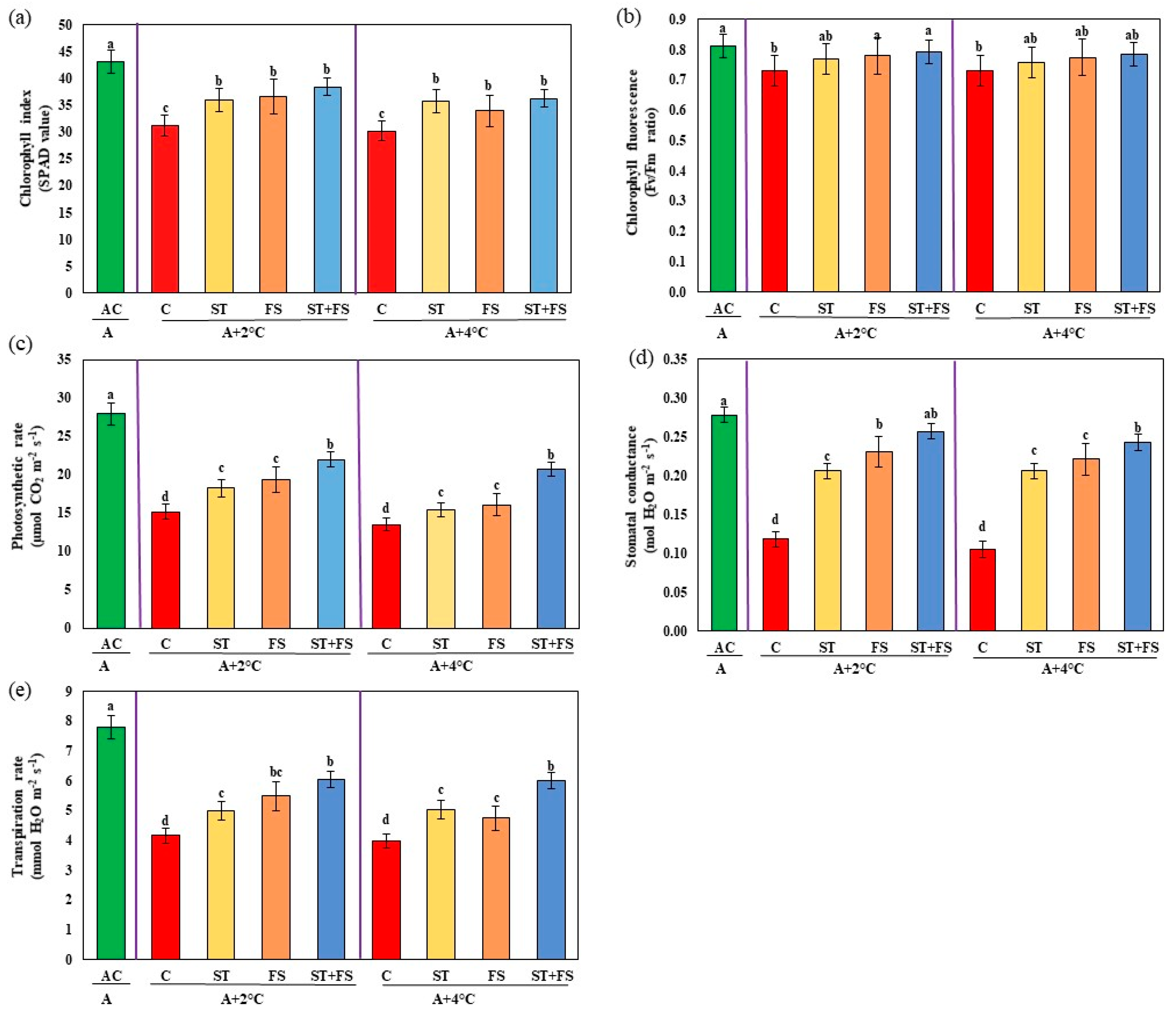
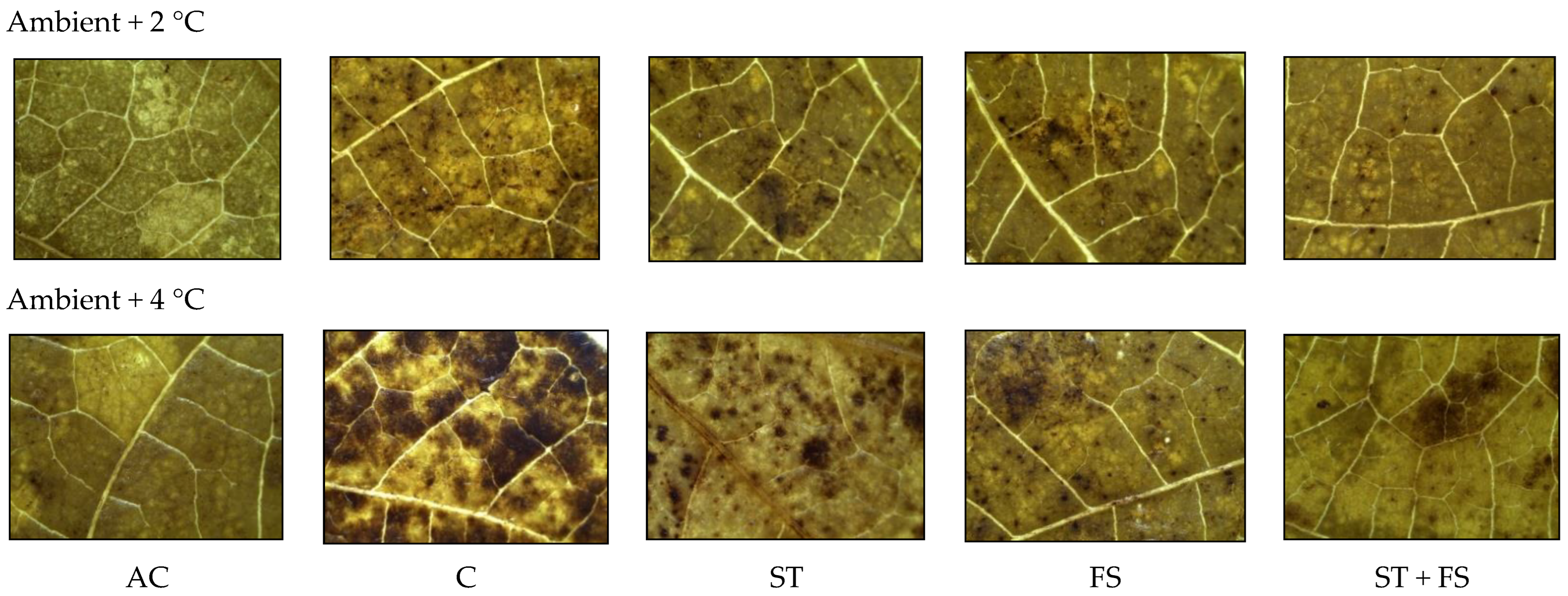
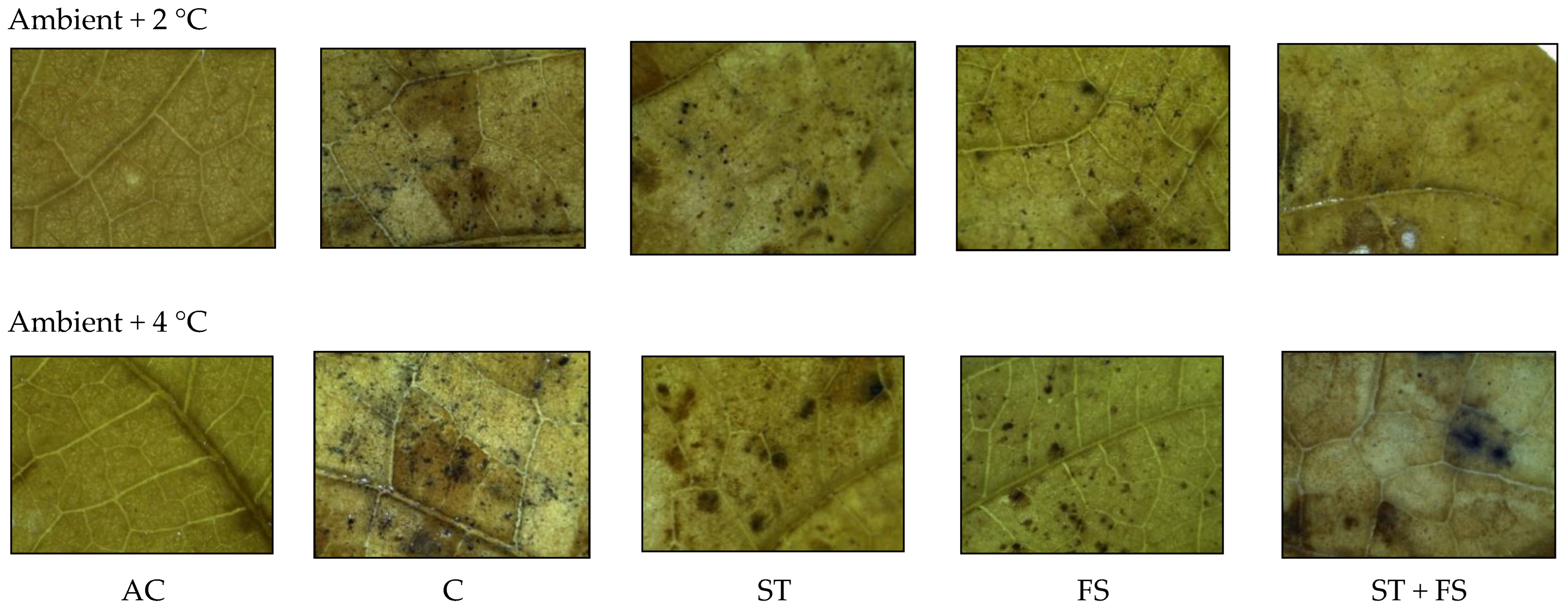
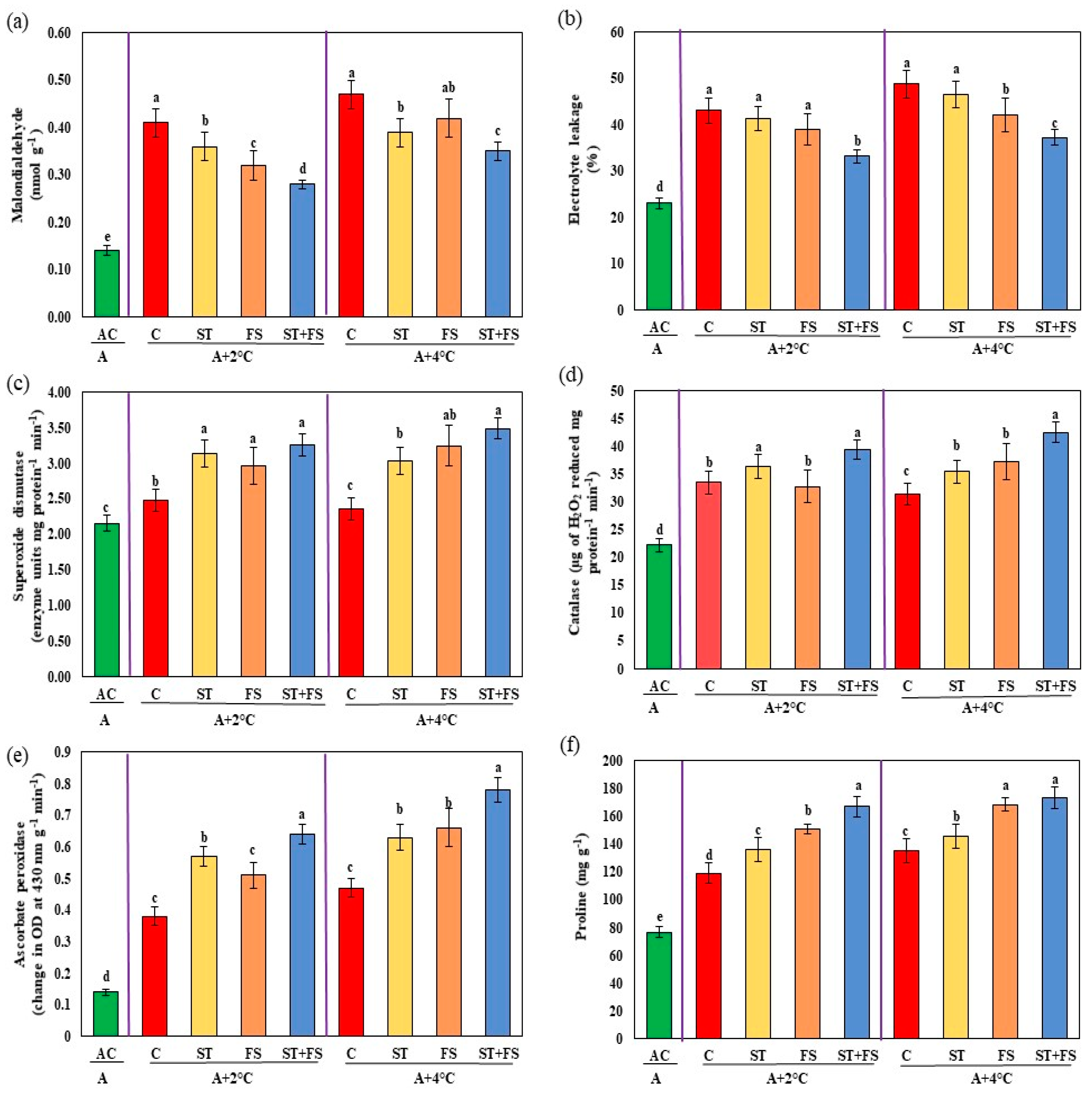
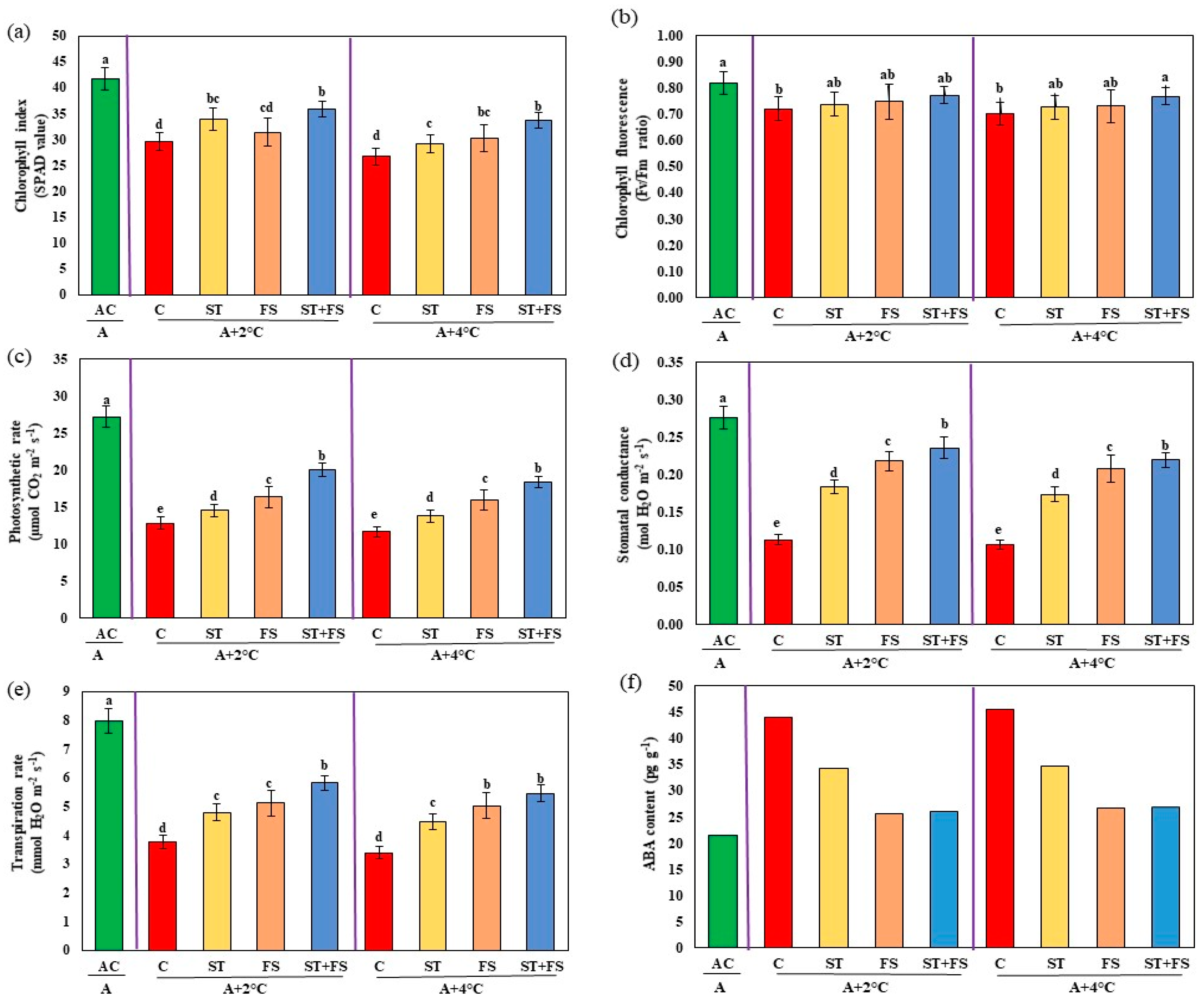
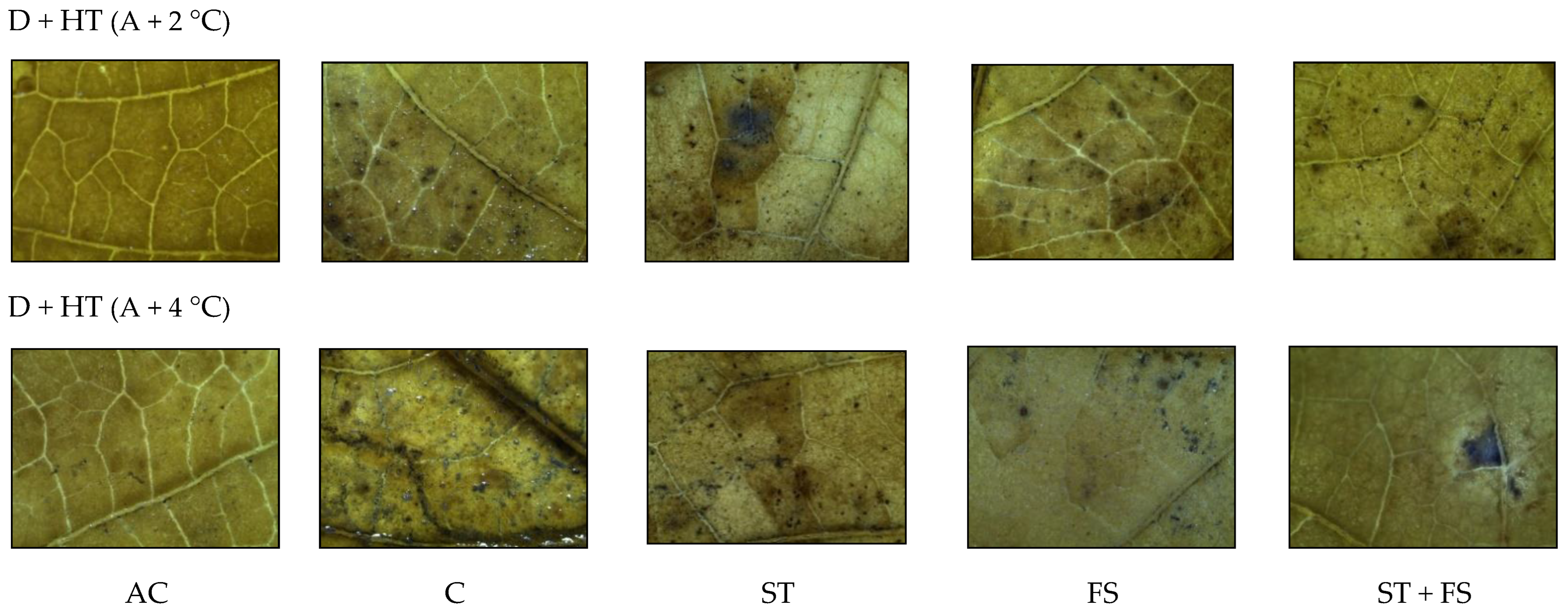
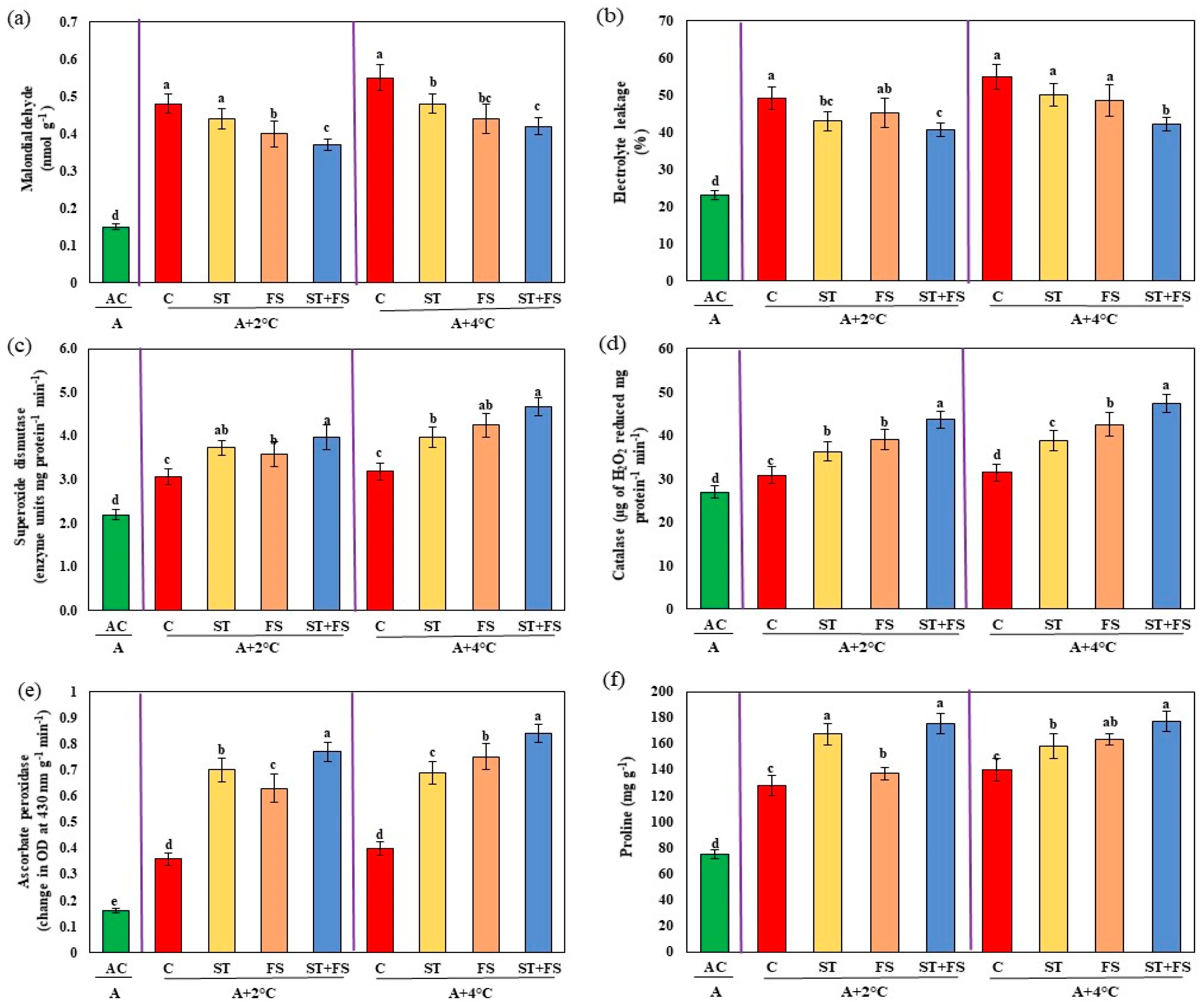
2.3. Effect of Melatonin on Physiological, Biochemical, and Yield Characteristics of Mung Bean under Combined Drought and High-Temperature Stress
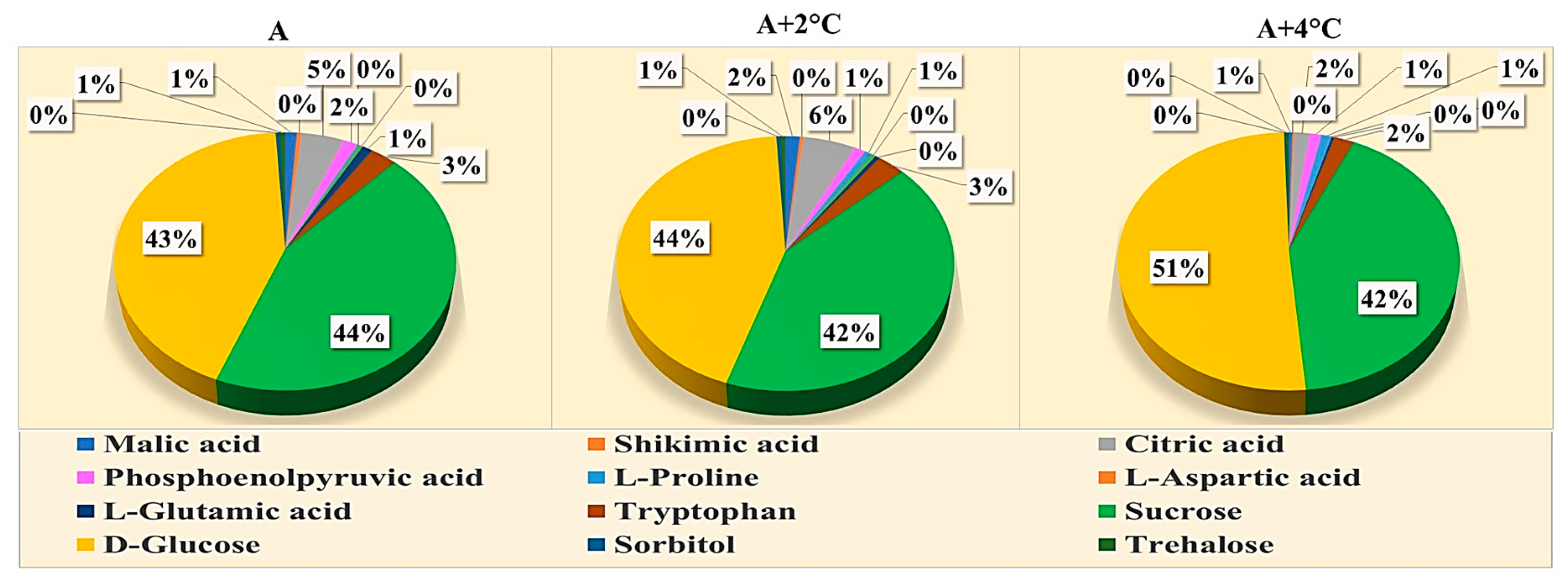
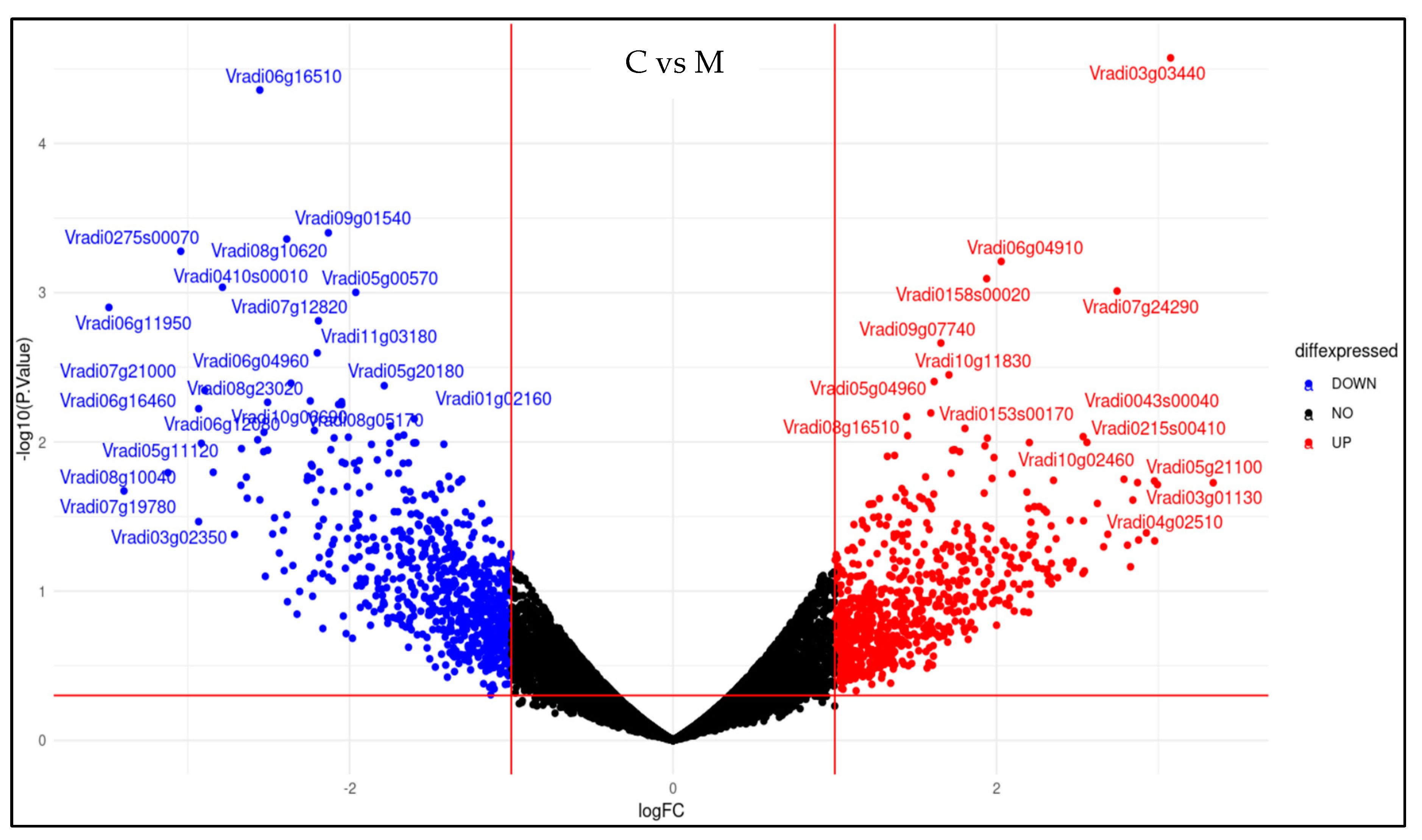
2.4. Effect of Melatonin Treatment on Metabolomics Profiling of Mung Bean under Drought and High-Temperature Stress
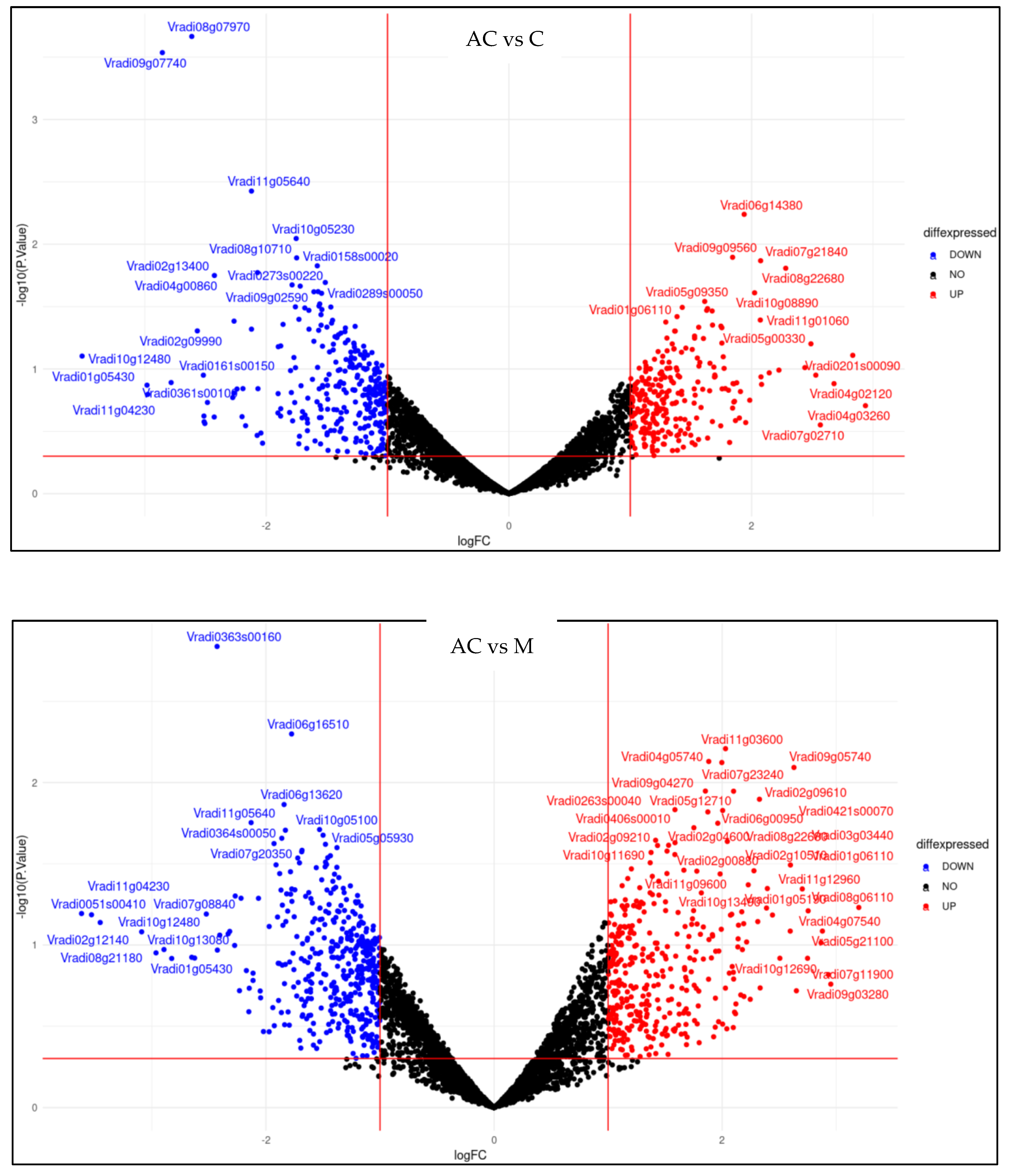
2.5. Effect of Melatonin Treatment on Differentially Expressed Genes of Mung Bean Leaves Exposed to Combined Drought and High-Temperature Stress
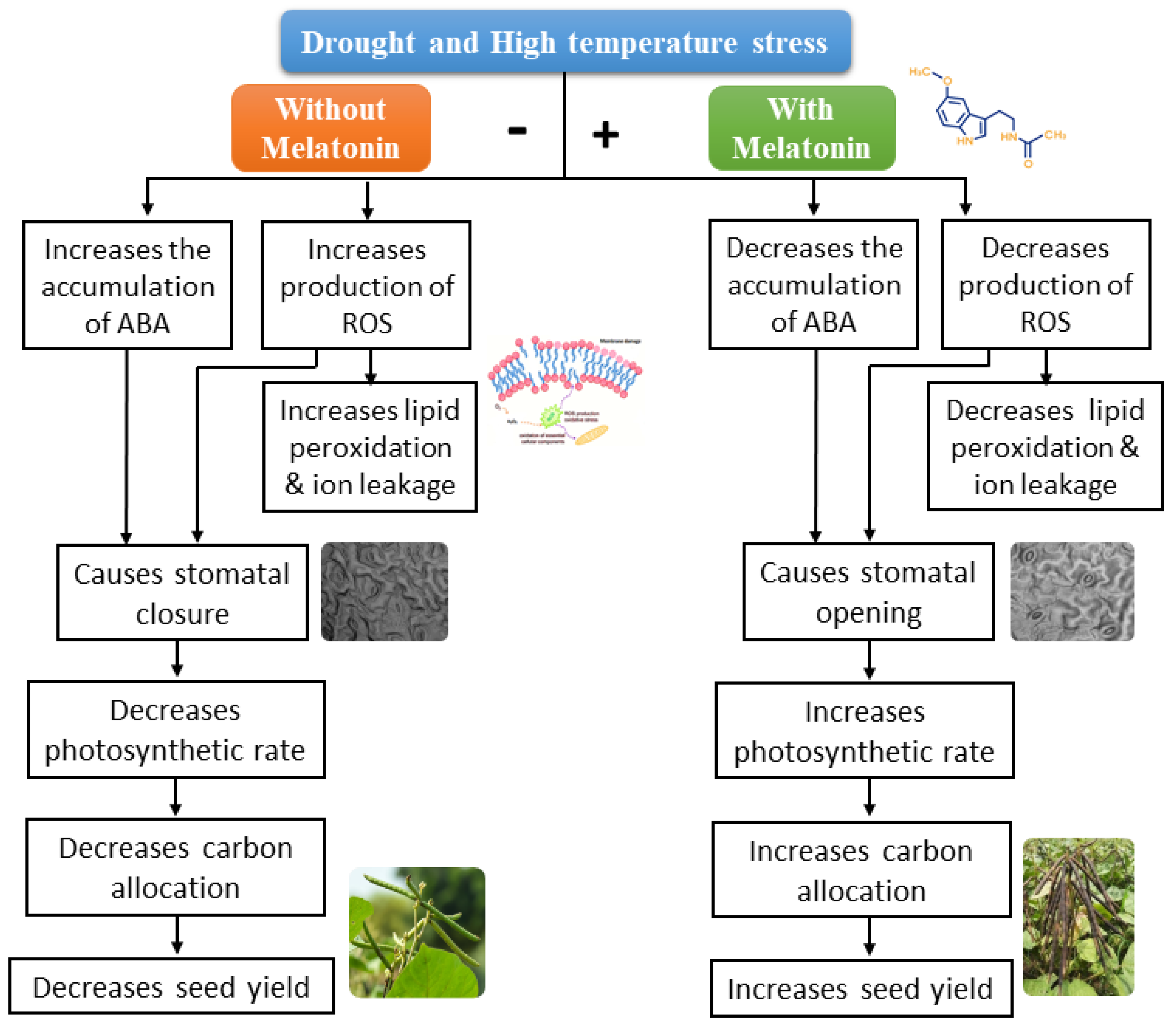
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Treatment Details and Stress Imposition
4.3. Chlorophyll Index (SPAD Value)
4.4. Chlorophyll Fluorescence
4.5. Leaf Gas Exchange Parameters
4.6. Hydrogen Peroxide (H2O2)
4.7. Superoxide Radical (O2−)
4.8. Malondialdehyde
4.9. Electrolyte Leakage
4.10. Antioxidant Enzyme Activity
4.10.1. Superoxide Dismutase
4.10.2. Catalase
4.10.3. Ascorbate Peroxidase
4.11. Proline
4.12. Metabolomics Profiling
4.13. Transcriptomic Analysis
4.14. Yield Parameters
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indiastat. Available online: https://www.indiastat.com/data/agriculture/area-under-food-crops/data-year/2022 (accessed on 2 March 2023).
- Kaur, L.; Kaur, A.; Brar, A. Water use efficiency of green gram (Vigna radiata L.) impacted by paddy straw mulch and irrigation regimes in north-western India. Agric. Water Manag. 2021, 258, 107184. [Google Scholar] [CrossRef]
- Banerjee, P.; Mukherjee, B.; Venugopalan, V.K.; Nath, R.; Chandran, M.A.S.; Dessoky, E.S.; Ismail, I.A.; El-Hallous, E.; Hossain, A. Thermal response of spring–summer-grown black gram (Vigna mungo L. Hepper) in Indian subtropics. Atmosphere 2021, 12, 1489. [Google Scholar] [CrossRef]
- Chaudhary, S.; Priya, M.; Jha, U.C.; Pratap, A.; HanumanthaRao, B.; Singh, I.; Prasad, P.; Siddique, K.H.; Nayyar, H. Approaches toward developing heat and drought tolerance in mungbean. In Developing Climate Resilient Grain Forage Legumes; Springer: Singapore, 2022; pp. 205–234. [Google Scholar] [CrossRef]
- Bal, S.K.; Sandeep, V.; Kumar, P.V.; Rao, A.S.; Pramod, V.; Manikandan, N.; Rao, C.S.; Singh, N.P.; Bhaskar, S. Assessing impact of dry spells on the principal rainfed crops in major dryland regions of India. Agric. For. Meteorol. 2022, 313, 108768. [Google Scholar] [CrossRef]
- IPPC. Climate Change Synthesis Report Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022; Volume 151. [Google Scholar]
- Jha, U.C.; Nayyar, H.; Jha, R.; Singh, P.K.; Dixit, G.P.; Kumar, Y.; Mondal, B.; Srivastava, A.K.; Von Wettberg, E.J.; Paul, P.J. Improving chickpea genetic gain under rising drought and heat stress using breeding approaches and modern technologies. In Developing Climate Resilient Grain and Forage Legumes; Springer: Singapore, 2022; pp. 1–25. [Google Scholar]
- Ahmad, H.M.; Wang, X.; Fiaz, S.; Azeem, F.; Shaheen, T. Morphological and physiological response of Helianthus annuus L. to drought stress and correlation of wax contents for drought tolerance traits. Arab. J. Sci. Eng. 2022, 47, 6747–6761. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Kumar, M.; Siddique, K.H. Metabolic engineering for understanding abiotic stress tolerance in plants. In Molecular Response and Genetic Engineering for Stress in Plants, Volume 1: Abiotic Stress; IOP Publishing: Bristol, UK, 2022; pp. 2–38. [Google Scholar]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Ji, Y.; Wu, X.; Xu, X.; Wu, P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J. Plant Growth Regul. 2022, 42, 2232–2245. [Google Scholar] [CrossRef]
- Dadasoglu, E.; Turan, M.; Ekinci, M.; Argin, S.; Yildirim, E. Alleviation mechanism of melatonin in chickpea (Cicer arietinum L.) under the salt stress conditions. Horticulturae 2022, 8, 1066. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Liu, K.; Wang, Y.; Ai, X.; Bi, H. Melatonin positively regulates both dark-and age-induced leaf senescence by reducing ROS accumulation and modulating abscisic acid and auxin biosynthesis in cucumber plants. Int. J. Mol. Sci. 2022, 23, 3576. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Jan, S.; Singh, B.; Bhardwaj, R.; Singh, R.; Mansoor, S.; Ahmad, P. Recent advances on the pragmatic roles of phytomelatonin and its exogenous application for abiotic stress management in plants. J. Plant Growth Regul. 2022, 1, 1–16. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.; Ibrahim, M.F. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.W.; Khan, A.L.; Lee, I.J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.; Wang, J. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2022, 41, 2108–2121. [Google Scholar] [CrossRef]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C.; Cao, L.; Zhang, Y. Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean. PLoS ONE 2020, 15, e0243537. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R.; Talebi, M.; Hesami, M. Exogenous melatonin protects lime plants from drought stress-induced damage by maintaining cell membrane structure, detoxifying ROS and regulating antioxidant systems. Horticulturae 2022, 8, 257. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef] [PubMed]
- Sezer, I.; Kiremit, M.S.; Ozturk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, W.; Lv, H.; Liang, B.; Jin, S.; Li, J.; Zhou, W. Animal-derived plant biostimulant alleviates drought stress by regulating photosynthesis, osmotic adjustment, and antioxidant systems in tomato plants. Sci. Hortic. 2022, 305, 111365. [Google Scholar] [CrossRef]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial effect of melatonin on growth and chlorophyll content in wheat (Triticum aestivum L.) grown under salt stress conditions. Gesunde Pflanz. 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Lin, S.; Song, X.F.; Mao, H.T.; Li, S.-Q.; Gan, J.Y.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y.; Su, Y.Q. Exogenous melatonin improved photosynthetic efficiency of photosystem II by reversible phosphorylation of thylakoid proteins in wheat under osmotic stress. Front. Plant Sci. 2022, 13, 966181. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Ancin, M.; Fakhet, D.; Gonzalez-Torralba, J.; Gamez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2022, 74, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Shi, S.; Huang, L.; Zhu, M.; Li, F. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 327–336. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef]
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-induced resilience strategies against the damaging impacts of drought stress in rice. Agronomy 2022, 12, 813. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.; Liu, H.; Cai, Z.; Lu, C.; Chen, Y. A novel ABA-insensitive mutant in Arabidopsis reveals molecular network of ABA-induced anthocyanin accumulation and abiotic stress tolerance. J. Plant Physiol. 2022, 278, 153810. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Gul, F.; Zahoor, S.; Nosheen, A.; Yasmin, H.; Keyani, R.; Shahid, M.; Hassan, M.; Siddiqui, M.; Batool, S. Interactive effects of hydrogen sulphide and silicon enhance drought and heat tolerance by modulating hormones, antioxidant defence enzymes and redox status in barley (Hordeum vulgare L.). Plant Biol. 2022, 24, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive oxygen, nitrogen, carbonyl and sulfur species and their roles in plant abiotic stress responses and tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Kranzlein, M.; Geilfus, C.M.; Franzisky, B.L.; Zhang, X.; Wimmer, M.A.; Zorb, C. Physiological responses of contrasting maize (Zea mays L.) hybrids to repeated drought. J. Plant Growth Regul. 2022, 41, 2708–2718. [Google Scholar] [CrossRef]
- Hannachi, S.; Signore, A.; Adnan, M.; Mechi, L. Single and associated effects of drought and heat stresses on physiological, biochemical and antioxidant machinery of four eggplant cultivars. Plants 2022, 11, 2404. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; He, H.; Raza, A.; Liu, Z.; Xiaoyu, D.; Guijuan, W. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression. Plant Signal. 2022, 17, e2129289. [Google Scholar] [CrossRef]
- Roy, R.; Sultana, S.; Begum, N.; Fornara, D.; Barmon, M.; Zhang, R.; Sarker, T.; Rabbany, M.G. Exogenous melatonin reduces water deficit-induced oxidative stress and improves growth performance of Althaea rosea grown on coal mine spoils. Environ. Sci. Pollut. Res. 2022, 29, 61550–61560. [Google Scholar] [CrossRef]
- Wang, Y.; Gun, S.; Li, Y.; Qu, L. Meta-analysis of effects of melatonin treatment on plant drought stress alleviation. Agriculture 2022, 12, 1335. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Gul, N.; Haq, Z.U.; Ali, H.; Munsif, F.; Hassan, S.S.U.; Bungau, S. Melatonin pretreatment alleviated inhibitory effects of drought stress by enhancing antioxidant activities and accumulation of higher proline and plant pigments and improving maize productivity. Agronomy 2022, 12, 2398. [Google Scholar] [CrossRef]
- Ghosh, U.; Islam, M.; Siddiqui, M.; Cao, X.; Khan, M. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Peixoto, B.; Baena-Gonzalez, E. Management of plant central metabolism by SnRK1 protein kinases. J. Exp. Bot. 2022, 73, 7068–7082. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, Y.; Zeng, X.; Wang, Y.; Zhang, Y. Quantitative proteomics analysis reveals proteins associated with high melatonin content in barley seeds under NaCl-induced salt stress. J. Agric. Food Chem. 2022, 70, 8492–8510. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, T.; Zhao, X.; Wang, Z.; Chen, Y. Comparative physiological and full-length transcriptome analyses reveal the molecular mechanism of melatonin-mediated salt tolerance in okra (Abelmoschus esculentus L.). BMC Plant Biol. 2021, 21, 180. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Ji, J.; He, X.; Zhang, J.; Shang, Y.; Liu, H.; Xu, J.; Liang, B. Ionomic combined with transcriptomic and metabolomic analyses to explore the mechanism underlying the effect of melatonin in relieving nutrient stress in apple. Int. J. Mol. Sci. 2022, 23, 9855. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Pramanick, K. Melatonin mediated activation of MAP kinase pathway may reduce DNA damage stress in plants: A review. BioFactors 2022, 48, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jie, Z.; Xiangjun, K.; Ullah, N.; Short, A.W.; Diao, Y.; Zhou, R.; Xiong, Y.C. Pretreatment of melatonin rescues cotton seedlings from cadmium toxicity by regulating key physio-biochemical and molecular pathways. J. Hazard. Mater. 2022, 445, 130530. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Liu, T.; Shi, J.; Qi, M.; Liu, Y.; Li, T. Integrated physiological, transcriptomic, and proteomic analyses reveal the regulatory role of melatonin in tomato plants response to low night temperature. Antioxidants 2022, 11, 2060. [Google Scholar] [CrossRef]
- Behera, B.; Kancheti, M.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R. Mechanistic insight on boron-mediated toxicity in plant vis-a-vis its mitigation strategies: A review. Int. J. Phytoremediation 2022, 1, 9–26. [Google Scholar] [CrossRef]
- Hu, C.H.; Zheng, Y.; Tong, C.L.; Zhang, D.J. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; Garcia-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillen, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead–cadmium stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef]
- Oliveira-Spolaor, B.; Chiari-Bertoli, S.; Silva-Sukert, D.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, I.R.; Lima-Moro, A. Exogenous melatonin induces tolerance to drought stress damage in seedlings and soybean plants. Chil. J. Agric. Res. 2022, 82, 515–526. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Shah, D.; Pal, M.; Jagadish, S. High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink-strength in rice. Sci. Rep. 2017, 7, 8227. [Google Scholar] [CrossRef] [PubMed]
- Minolta, C. Manual for Chlorophyll Meter SPAD-502; Minolta Radiometric Instruments Divisions: Osaka, Japan, 1989. [Google Scholar] [CrossRef]
- Monje, O.A.; Bugbee, B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Pfundel, E.; Klughammer, C.; Schreiber, U. Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl. Notes 2008, 1, 21–24. [Google Scholar]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Karabal, E.; Yucel, M.; Oktem, H.A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003, 164, 925–933. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Arginase induction by heat treatment contributes to amelioration of chilling injury and activation of antioxidant enzymes in tomato fruit. Postharvest Biol. Technol. 2013, 79, 1–8. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Hugo, A.; Lester, P. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hill, C.B.; Roessner, U. Metabolic profiling of plants by GC-MS. In The Handbook of Plant Metabolomics; Weckwerth, W., Kahl, G., Eds.; Metabolite Profiling Networking; Wiley-VCH: Weinheim, Germany, 2013; pp. 1–23. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535. https://doi.org/10.3390/plants12132535
Kuppusamy A, Alagarswamy S, Karuppusami KM, Maduraimuthu D, Natesan S, Ramalingam K, Muniyappan U, Subramanian M, Kanagarajan S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants. 2023; 12(13):2535. https://doi.org/10.3390/plants12132535
Chicago/Turabian StyleKuppusamy, Anitha, Senthil Alagarswamy, Kalarani M. Karuppusami, Djanaguiraman Maduraimuthu, Senthil Natesan, Kuttimani Ramalingam, Umapathi Muniyappan, Marimuthu Subramanian, and Selvaraju Kanagarajan. 2023. "Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions" Plants 12, no. 13: 2535. https://doi.org/10.3390/plants12132535
APA StyleKuppusamy, A., Alagarswamy, S., Karuppusami, K. M., Maduraimuthu, D., Natesan, S., Ramalingam, K., Muniyappan, U., Subramanian, M., & Kanagarajan, S. (2023). Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants, 12(13), 2535. https://doi.org/10.3390/plants12132535







