Comparative Cytogenetics and Fluorescent Chromosome Banding in Five Indian Species of Dipcadi Medik
Abstract
1. Introduction
2. Results
2.1. Chromosome Number and Karyotype Analysis
2.2. Fluorochrome Banding Pattern
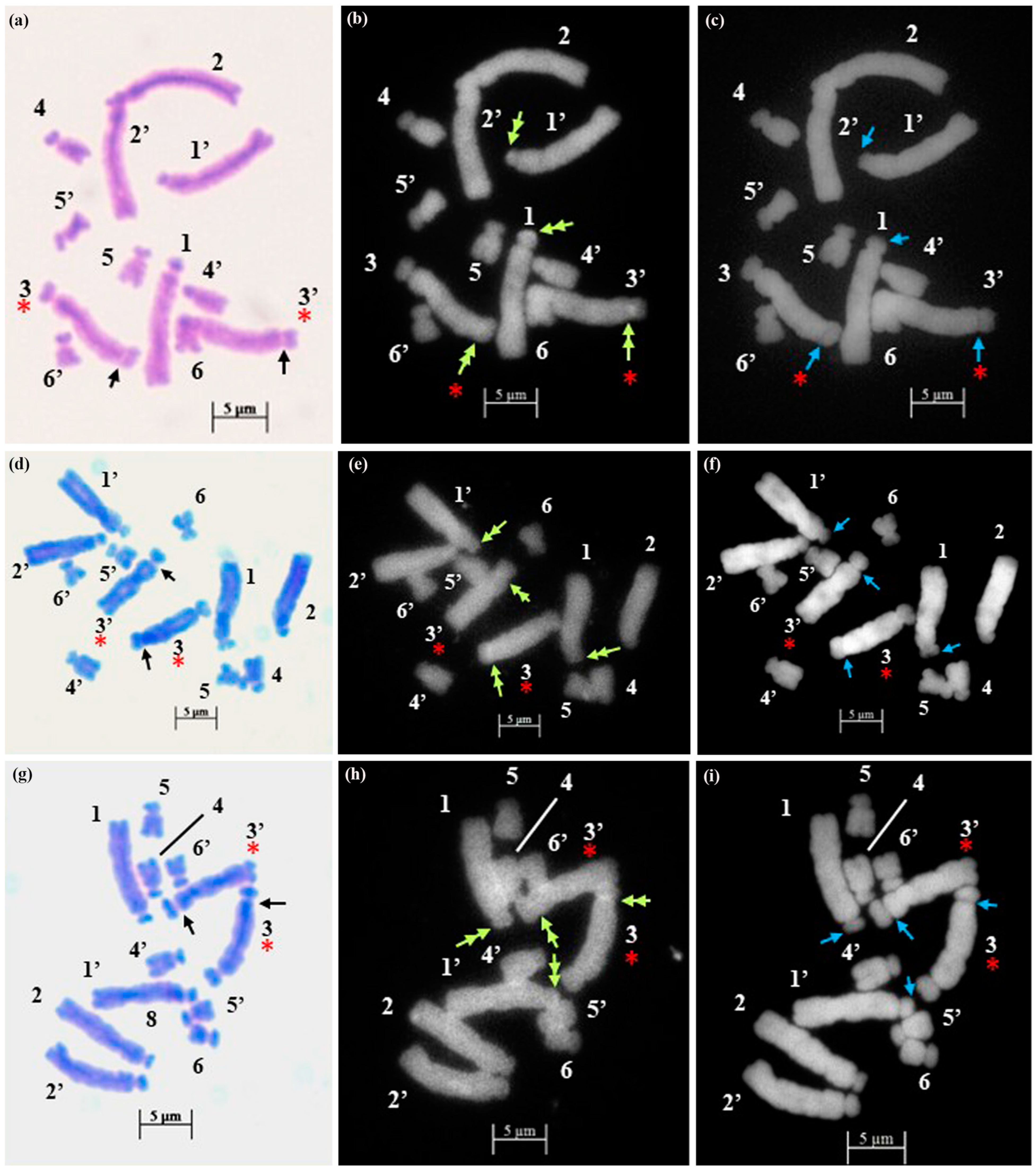
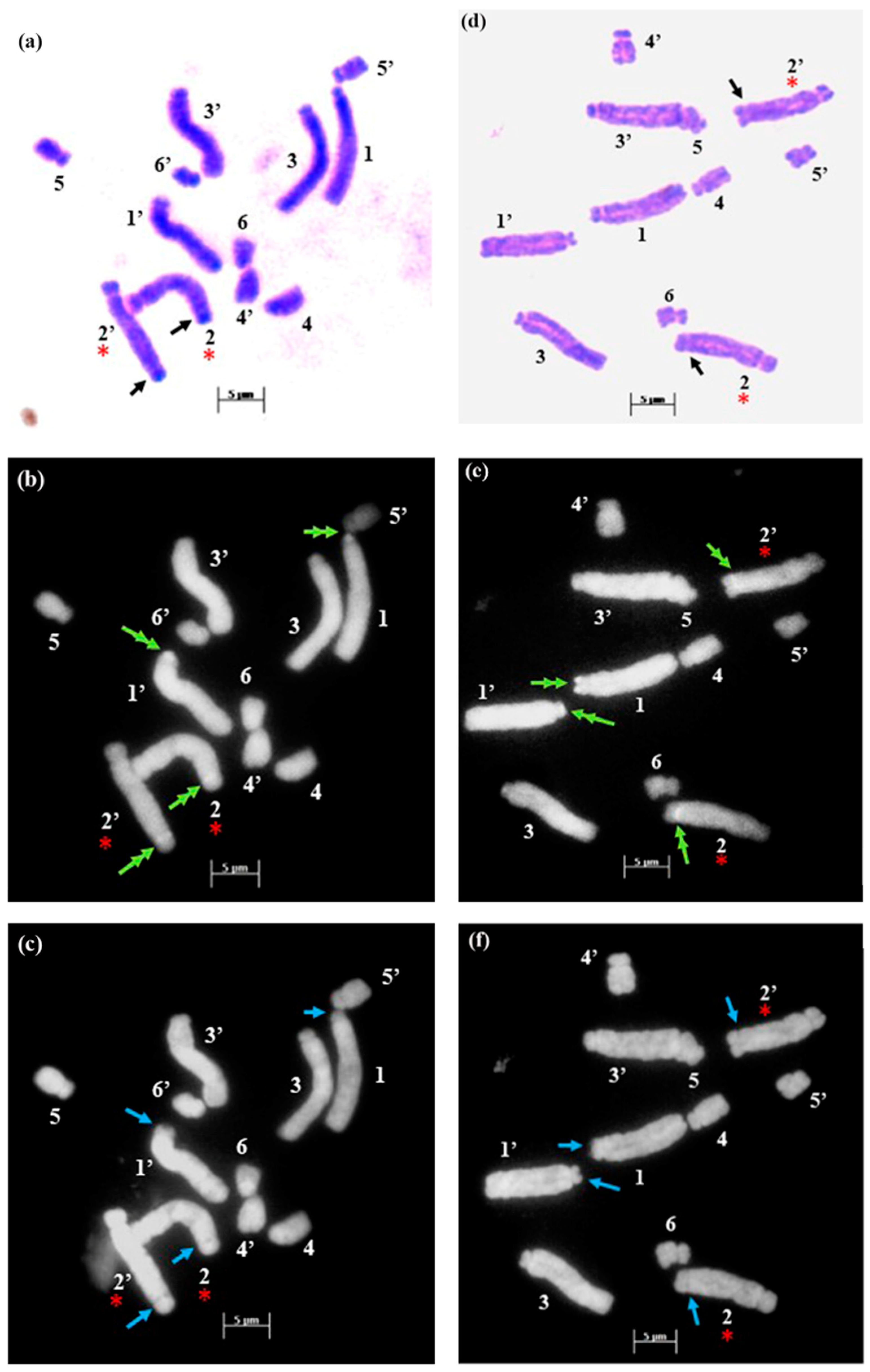
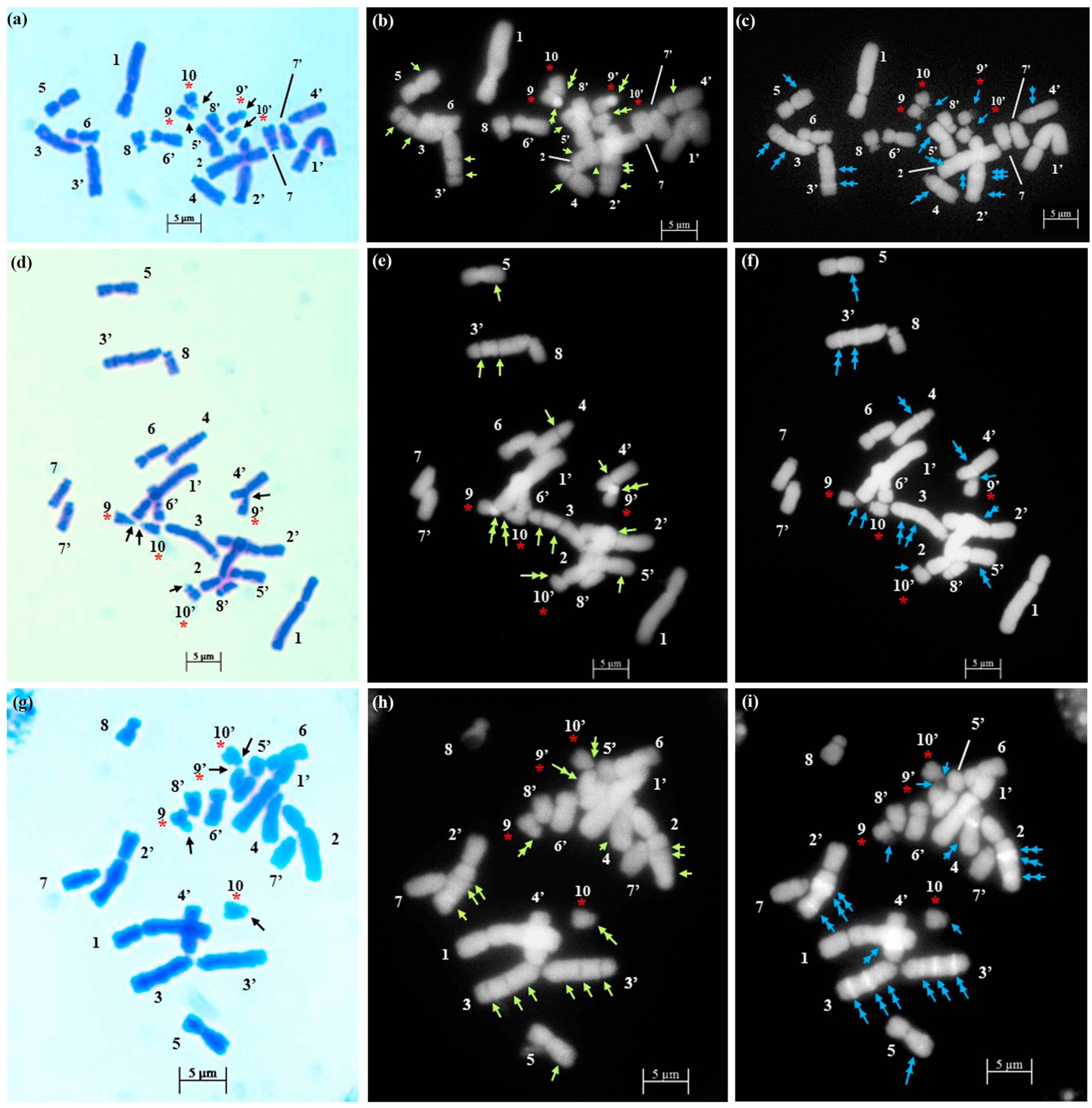


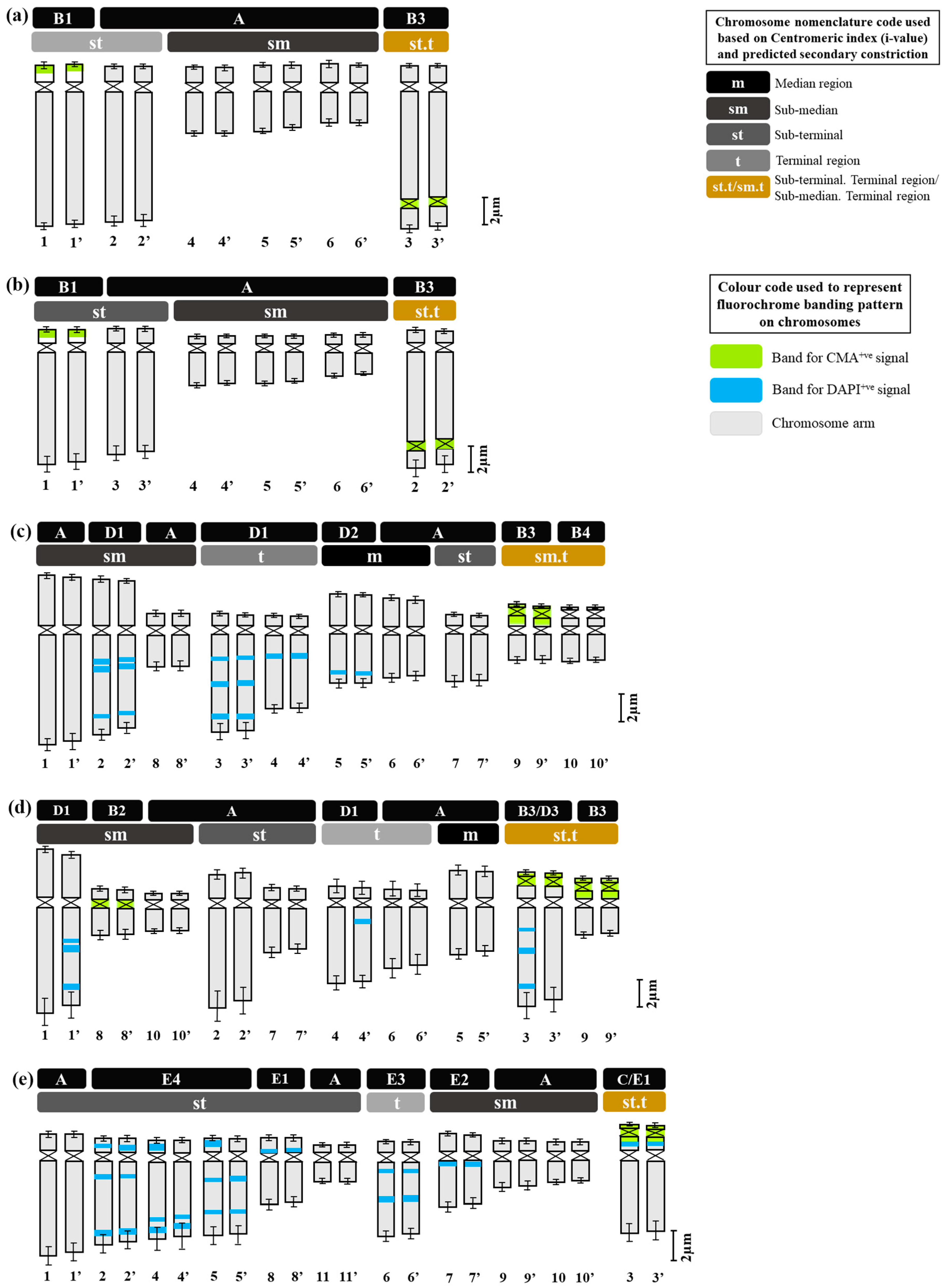
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Their Collection
4.2. Mitotic Chromosome Preparation and Giemsa Staining
4.3. Karyotype Analysis
4.4. Fluorochrome Staining of Somatic Chromosomes
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Speta, F. Systematische Analyse der Gattung Scilla L. s.l. (Hyacinthaceae). Phyton 1998, 38, 1–141. [Google Scholar]
- Pfosser, M.; Speta, F. Phylogenetics of Hyacinthaceae based on plastid DNA sequences. Ann. Mo. Bot. Gard. 1999, 86, 852–875. [Google Scholar] [CrossRef]
- Manning, J.C.; Goldblatt, P.; Fay, M.F. A revised generic synopsis of Hyacinthaceae in sub-Saharan Africa based on molecular evidence, including new combinations and the new tribe Pseudoprospereae. Edinb. J. Bot. 2004, 60, 533–568. [Google Scholar] [CrossRef]
- Martínez-Azorín, M.; Crespo, M.B.; Juan, A.; Fay, M.F. Molecular phylogenetics of subfamily Ornithogaloideae (Hyacinthaceae) based on nuclear and plastid DNA regions, including a new taxonomic arrangement. Ann. Bot. 2011, 107, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Saha, P.S.; Jha, S. Medicinal bulbous plants: Biology, phytochemistry and biotechnology. In Bulbous Plants Biotechnology; Ramawat, K.G., Mérillon, J.M., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 2013; pp. 338–369. [Google Scholar] [CrossRef]
- Jha, S. Bufadienolides. In Phytochemicals in Plant Cell Cultures; Vasil, I.K., Ed.; Academic Press: Cambridge, MA, USA, 1988; pp. 179–191. [Google Scholar]
- Mulholland, D.A.; Schwikkard, S.L.; Crouch, N.R. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165–1210. [Google Scholar] [CrossRef]
- Kamboj, A.; Rathour, A.; Kaur, M. Bufadienolides and their medicinal utility: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 20–27. [Google Scholar]
- Azizi, N.; Amirouche, R.; Amirouche, N. Cytotaxonomic diversity of some medicinal species of Hyacinthaceae from Algeria. Pharmacogn. Commn. 2016, 6, 34–38. [Google Scholar] [CrossRef]
- Pohl, T.S.; Crouch, N.R.; Mulholland, D.A. Southern African Hyacinthaceae chemistry, bioactivity and ethnobotany. Curr. Org. Chem. 2000, 4, 1287–1324. [Google Scholar] [CrossRef]
- Jehan, T.; Vashistha, A.; Yadav, S.R.; Lakhanpaul, S. Genetic diversity and genetic relationships in Hyacinthaceae in India using RAPD and SRAP markers. Physiol. Mol. Biol. Plants. 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Manning, J.C.; Forest, F.; Devey, D.S.; Fay, M.F.; Goldblatt, P. A molecular phylogeny and a revised classification of Ornithogaloideae (Hyacinthaceae) based on an analysis of four plastid DNA regions. Taxon 2009, 58, 77–107. [Google Scholar] [CrossRef]
- Gosavi, K.V.C.; Yadav, U.S.; Janarthanam, M.K.; Yadav, S.R. Karyomorphological analysis of recently described rare species of Dipcadi Medik. (Hyacinthaceae) from Northern Western Ghats. Cytologia 2011, 76, 63–66. [Google Scholar] [CrossRef]
- Deb, D.B.; Dasgupta, S. Liliaceae: Tribe-Scilleae. In Fascicles of Flora of India; Botanical Survey of India, Botanic Garden: Howrah, India, 1981; Volume 7, pp. 2–11. [Google Scholar]
- Karthikeyan, S.; Jain, S.K.; Nayar, M.P.; Sanjappa, M. Florae Indicae Enumeratio: Monocotyledons; BSI: Kolkata, India, 1989. [Google Scholar]
- Patil, S. A note on the occurrence and distribution of Dipcadi concanense (Hyacinthaceae). Nelumbo 2015, 57, 60–63. [Google Scholar] [CrossRef]
- Rawat, D.S.; Chandra, S. Presumed extinct Dipcadi reidii (Asparagaceae) recollected after 127 years from Uttarakhand, India. Rheedea 2014, 24, 1–4. [Google Scholar]
- Prabhugaonkar, A.; Yadav, U.S.; Janarthanam, M.K. Dipcadi goaense (Hyacinthaceae), a new species from the foothills of the Western Ghats, India. Kew Bull. 2009, 64, 743–746. [Google Scholar] [CrossRef]
- Deshpandey, A.S.; Krishnan, S.; Janarthanam, M.K. Systematic position of two endemic Dipcadi spp. (Asparagaceae) from northern Western Ghats of India using molecular markers. Rheedea 2015, 25, 97–102. [Google Scholar]
- Moussaid, M.; Elamrani, A.; Bourhim, N.; Benaissa, M. Contribution to the study of the essential oil of Dipcadi serotinum (L.) Medik. of Morocco. Afr. Sci. Revue Int. Sci. Technol. 2013, 9, 34–42. [Google Scholar]
- El-Shabrawy, M.O.; Marzouk, M.M.; Kawashtya, S.A.; Hosni, H.; El-Garf, I.; Saleh, N.A.M. Flavonoid constituents of Dipcadi erythraeum Webb. & Berthel. Asian Pac. J. Trop. Dis. 2016, 6, 404–405. [Google Scholar] [CrossRef]
- Adly, F.; Moussaid, M.; Berhal, C.; Razik, A.; Elamrani, A.A.; Moussaid, H.; Bourhim, N.; Loutfi, M. Phytochemical screening and biological study of ethanol extractives of Dipcadi serotinum (L.) Medik. EJARBLS 2015, 3, 17–23. [Google Scholar]
- Marzouk, M.M.; Elkhateeb, A.; Latif, R.R.A.; Abdel-Hameed, E.S.; Kawashty, S.A.; Hussein, S.R. C-glycosyl flavonoids-rich extract of Dipcadi erythraeum Webb & Berthel. bulbs: Phytochemical and anticancer evaluations. J. App. Pharm. Sci. 2019, 9, 94–98. [Google Scholar] [CrossRef]
- Nath, S.; Sarkar, S.; Patil, S.D.; Saha, S.S.; Lekhak, M.M.; Ray, S.; Rao, S.R.; Yadav, S.R.; Verma, R.C.; Dhar, M.K.; et al. Cytogenetic diversity in Scilloideae (Asparagaceae): A comprehensive recollection and exploration of karyo-evolutionary trends. Bot. Rev. 2023, 89, 158–200. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J.C. A review of chromosome cytology in Hyacinthaceae subfamily Ornithogaloideae (Albuca, Dipcadi, Ornithogalum and Pseudogaltonia) in sub-Saharan Africa. S. Afr. J. Bot. 2011, 77, 581–591. [Google Scholar] [CrossRef]
- Mahabale, T.S.; Chennaveeraiah, M.S. Karyotype in Dipcadi Medik. Curr. Sci. 1954, 23, 367–368. [Google Scholar]
- Kanmani, B.N. Karyotype study in Dipcadi concanense Dalz. Curr. Sci. 1975, 44, 278–279. [Google Scholar]
- Dixit, G.B.; Yadav, S.R.; Patil, K.S. Cytological studies in Dipcadi concanense (Dalz.) Baker. J. Cytol. Genet. 1992, 27, 91–94. [Google Scholar]
- Rawat, D.; Sharma, S.K.; Mahmoudi, A.; Rao, S.R. Cytogenetic rationale for probable amphidiploid origin of Dipcadi erythraeum Webb. & Berth.—A rare and endemic plant of Indian Thar desert. Caryologia 2011, 64, 75–83. [Google Scholar] [CrossRef]
- Jakhi, P.S.; Desai, N.S.; Dixit, G.B. Karyogical studies in Dipcadi erythraeum. J. Cytol. Genet. 1994, 29, 89–93. [Google Scholar]
- Naik, V.N. Cytological studies in two species of Dipcadi Medic. from India. Cytologia 1974, 39, 591–596. [Google Scholar] [CrossRef]
- Rejón, M.R.; Rejón, C.R.; Pascual, L. The chromosome system of Dipcadi serotinum (Liliaceae): A natural species with unusual cytogenetic characteristics. Caryologia 1981, 34, 419–426. [Google Scholar] [CrossRef]
- Fukui, K. Plant chromosomes at mitosis. In Plant Chromosomes: Laboratory Methods; Fukui, K., Nakayama, S., Eds.; CRC Press: Boca Raton, CA, USA, 1996; pp. 1–17. [Google Scholar]
- Jha, T.B. Karyotype diversity in cultivated and wild Indian rice through EMA-based chromosome analysis. J. Genet. 2021, 100, 81. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 1976, 58, 307–324. [Google Scholar] [CrossRef]
- Nath, S.; Jha, T.B.; Mallick, S.K.; Jha, S. Karyological relationships in Indian species of Drimia based on fluorescent chromosome banding and nuclear DNA amount. Protoplasma 2015, 252, 283–299. [Google Scholar] [CrossRef]
- Saha, P.S.; Jha, S. A molecular phylogeny of the genus Drimia (Asparagaceae: Scilloideae: Urgineeae) in India inferred from non-coding chloroplast and nuclear ribosomal DNA sequences. Sci. Rep. 2019, 9, 7563. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Mitrenina, E.; Erst, A.; Peruzzi, L.; Skaptsov, M.; Ikeda, H.; Nikulin, V.; Wang, W. Karyotype and genome size variation in white-flowered Eranthis sect. Shibateranthis (Ranunculaceae). PhytoKeys 2021, 187, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Barros e Silva, A.E.; Guerra, M. The meaning of DAPI bands observed after C-banding and FISH procedures. Biotech. Histochem. 2010, 85, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, H.W.; Schneeweiss, G.M. Karyotype diversity and evolutionary trends in Angiosperms. In Plant Genome Diversity; Greilhuber, J., Doležel, J., Wendel, J.F., Eds.; Springer: Vienna, Austria, 2013; Volume 2, pp. 209–230. [Google Scholar]
- Winterfeld, G.; Becher, H.; Voshell, S.; Hilu, K.; Röser, M. Karyotype evolution in Phalaris (Poaceae): The role of reductional dysploidy, polyploidy and chromosome alteration in a wide-spread and diverse genus. PLoS ONE 2018, 13, e0192869. [Google Scholar] [CrossRef]
- Wen-Guang, S.; Hang, S.; Zhi-Min, L. Chromosome data mining and its application in plant diversity research. Plant Sci. J. 2019, 37, 260–269. [Google Scholar] [CrossRef]
- Knytl, M.; Fornaini, N.R. Measurement of chromosomal arms and FISH reveal complex genome architecture and standardized karyotype of model fish, genus Carassius. Cells 2021, 10, 2343. [Google Scholar] [CrossRef]
- Deanna, R.; Acosta, M.C.; Scaldaferro, M.; Chiarini, F. Chromosome Evolution in the Family Solanaceae. Front. Plant Sci. 2022, 12, 787590. [Google Scholar] [CrossRef]
- Carta, A.; Escudero, M. Karyotypic diversity: A neglected trait to explain angiosperm diversification? Evolution 2023, 77, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Knytl, M.; Fornaini, N.R.; Bergelov, B.; Gvoždík, V.; Cernohorskà, H.; Kubíckova, S.; Fokam, E.B.; Evans, B.J.; Krylov, V. Divergent subgenome evolution in the allotetraploid frog Xenopus calcaratus. Gene 2023, 851, 146974. [Google Scholar] [CrossRef]
- Guerra, M. Cytotaxonomy: The end of childhood. Plant Biosyst. 2012, 146, 703–710. [Google Scholar] [CrossRef]
- Guerra, M. Reviewing the chromosome nomenclature of Levan et al. Genet. and Mol. Biol. 1986, 9, 741–743. [Google Scholar]
- Rawat, D.S. Dipcadi Medik. (Liliaceae): A disappearing genus in India. Natl. Acad. Sci. Lett. USA 2009, 32, 211–212. [Google Scholar]
- Guerra, M. Chromosome number in plant cytotaxonomy: Concepts and implications. Cytogenet. Genome Res. 2008, 120, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lima-De-Faria, A. The chromosome fields: I. Prediction of the location of ribosomal cistrons. Hereditas 1976, 83, 1–22. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.S.; Schwarzacher, T. The plant genome: An evolutionary view on structure and function; Organisation of the plant genome in chromosomes. Plant J. 2011, 66, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M. Patterns of heterochromatin distribution in plant chromosomes. Genet. Mol. Biol. 2000, 23, 1029–1041. [Google Scholar] [CrossRef]
- Marques, A.; Fuchs, J.; Ma, L.; Heckmann, S.; Guerra, M.; Houben, A. Characterization of eu-and heterochromatin of Citrus with a focus on the condensation behavior of 45S rDNA chromatin. Cytogenet. Genome Res. 2011, 134, 72–82. [Google Scholar] [CrossRef]
- Eriksson, S.; Kim, S.K.; Kubista, M.; Norden, B. Binding of 4’,6-diamidino-2-phenylindole (DAPI) to AT regions of DNA: Evidence for an allosteric conformational change. Biochemistry 1993, 32, 2987–2998. [Google Scholar] [CrossRef]
- Roa, F.; Guerra, M. Distribution of 45S rDNA sites in chromosomes of plants: Structural and evolutionary implications. BMC Evol. Biol. 2012, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Vanzela, A.L.L.; Cuadrado, A.; Guerra, M. Localization of 45S rDNA and telomeric sites on holocentric chromosomes of Rhyncospora tenuis Link (Cyperaceae). Genet. Mol. Biol. 2003, 26, 199–201. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tominaga, S. Chromosome identification in haploid clementine (Citrus clementina hort. ex. Tanaka) by fluorescent staining. Sci Hortic. 2004, 101, 201–206. [Google Scholar] [CrossRef]
- Yamamoto, M.; Abkenar, A.A.; Matsumoto, R.; Kubo, T.; Tominaga, S. CMA staining analysis of chromosomes in several species of Aurantioideae. Genet. Resour. Crop Evol. 2008, 55, 1167–1173. [Google Scholar] [CrossRef]
- Alam, S.S.; Jahan, N.; Habib, M.A.; Islam, M.N. Cytogenetical and molecular characterization of five commercial varieties in Trichosanthes anguina L. Cytologia 2012, 77, 155–162. [Google Scholar] [CrossRef]
- Bareka, P.; Siljak-Yakovlev, S.; Kamari, G. Molecular cytogenetics of Bellevalia (Hyacinthaceae) species occurring in Greece. Plant Syst. Evol. 2012, 298, 421–430. [Google Scholar] [CrossRef]
- Kuroki, Y.; Shibata, F.; Hizume, M. Chromosome bandings and signal pattern of FISH using rDNAs in Bellevalia romana. Cytologia 2013, 78, 399–401. [Google Scholar] [CrossRef]
- Cunado, N.; Herrán, R.D.L.; Santos, J.L.; Rejón, C.R.; Garrido-Ramos, M.A.; Rejón, M.R. The evolution of the ribosomal loci in the subgenus Leopoldia of the genus Muscari (Hyacinthaceae). Plant Syst. Evol. 2000, 221, 245–252. [Google Scholar] [CrossRef]
- Rejon, C.R.; Lozano, R.; Rejon, M.R. A cytogenetic analysis of the chromosomes in two related species of the genus Muscari (Liliaceae). Genome 2011, 33, 729–732. [Google Scholar] [CrossRef]
- Herrán, R.D.L.; Cunado, F.R.N.; Santos, J.L.; Rejón, M.R.; Garrido-Ramos, M.A.; Rejón, C.R. A heterochromatic satellite DNA is highly amplified in a single chromosome of Muscari (Hyacinthaceae). Chromosoma 2001, 110, 197–202. [Google Scholar] [CrossRef]
- Hamatani, S.; Tagashira, N.; Kondo, K. Molecular cytogenetic analysis in seven species of Lachenalia (Liliaceae) with chromosome numbers of 2n = 18, 22, 23,26, and 28 by DAPI staining and FISH using 5S rDNA and 18S rDNA probes. Chromosome Bot. 2010, 5, 55–59. [Google Scholar] [CrossRef]
- Hamatani, S.; Masuda, Y.; Uchida, M.; Tagashira, N.; Kusaba, M.; Kondo, K. Molecular cytogenetical and phylogenetical studies of Lachenalia congesta (Asparagaceae). Chromosome Bot. 2012, 7, 47–52. [Google Scholar] [CrossRef]
- Pedrosa, A.; Jantsch, M.F.; Moscone, E.A.; Ambros, P.F.; Schweizer, D. Characterisation of pericentromeric and sticky intercalary heterochromatin in Ornithogalum longibracteatum (Hyacinthaceae). Chromosoma 2001, 110, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Astuti, G.; Roma-Marzio, F.; Peruzzi, L. Traditional karyomorphological studies: Can they still provide a solid basis in plant systematics? Flora Mediterr. 2017, 27, 91–98. [Google Scholar]
- Mraz, P.; Filipas, L.; Bărbos, M.I.; Kadlecova, J.; Paštova, L.; Belyayev, A.; Fehrer, J. An unexpected new diploid Hieracium from Europe: Integrative taxonomic approach with a phylogeny of diploid Hieracium taxa. Taxon 2019, 68, 1258–1277. [Google Scholar] [CrossRef]
- Erst, A.S.; Sukhorukov, A.P.; Mitrenina, E.Y.; Skaptsov, M.V.; Kostikova, V.A.; Chernisheva, O.A.; Troshkina, V.I.; Kushunina, M.A.; Krivenko, D.A.; Ikeda, H.; et al. An integrative taxonomic approach reveals a new species of Eranthis (Ranunculaceae) in North Asia. PhytoKeys 2020, 140, 75–100. [Google Scholar] [CrossRef]
- Jha, T.B.; Bhowmick, B.K. Unraveling the genetic diversity and phylogenetic relationships of Indian Capsicum through fluorescent banding. Genet. Resour. Crop Evol. 2021, 68, 205–225. [Google Scholar] [CrossRef]
- Baltisberger, M.; Widmer, A. Chromosome numbers and karyotypes within the Ranunculus alpestris-group (Ranunculaceae). Org. Divers. Evol. 2009, 9, 232–243. [Google Scholar] [CrossRef]
- Jha, T.B.; Bhowmick, B.; Roy, P. Analysis of CMA-DAPI bands and preparation of fluorescent karyotypes in thirty Indian cultivars of Lens culinaris. Caryologia 2021, 74, 65–77. [Google Scholar] [CrossRef]
| Species | Population | Site of Collection | Geographic Details | Chromosome Number (2n) | TCL (Mean ± µm) * |
|---|---|---|---|---|---|
| D. concanense | Dcon1 | Rajapur, Maharashtra | 16.6571° N, 73.5211° E | 12 | 85.68 ± 0.53 b |
| Dcon2 | Ratnagiri, Maharashtra | 16.9902° N, 73.3120° E | 12 | 86.20 ± 0.71 b | |
| D. goaense | Dgoa1 | Quepem District, South Goa | 15.2282° N, 74.0647° E | 12 | 81.98 ± 0.72 a |
| D. ursulae | Durs1 | Thosegar, Maharashtra | 17.6031° N, 73.8478° E | 20 | 110.58 ± 1.38 c |
| Durs2 | Panhala, Maharashtra | 16.8107° N, 74.1181° E | 20 | 117.00 ± 0.87 f | |
| Durs3 | Satara, Maharashtra | 17.6805° N, 74.0183° E | 20 | 118.08 ± 1.00 g | |
| D. montanum | Dmon1 | Ajara, Maharashtra | 16.1159° N, 74.2106° E | 20 | 118.84 ± 0.43 g |
| Dmon2 | Badami, Karnataka | 15.9186° N, 75.6761° E | 20 | 112.00 ± 0.64 d | |
| D. erythraeum | Dery1 | Jaisalmer, Rajasthan | 26.9157° N, 70.9083° E | 22 | 112.24 ± 0.43 d |
| Dery2 | Jodhpur, Rajasthan | 26.2389° N, 73.0243° E | 22 | 113.30 ± 0.98 e |
| Species & Population | Absolute Length of Longest Chromosome (Mean ± SD in µm) * | Absolute Length of Shortest Chromosome (Mean ± SD in µm) * | ACL (Mean ± SD in µm) * | No. of SAT Chromosome & Ordering No. of SAT Bearing Pair | Diploid Karyotype Formula | Diagrammatic Representation of Karyotype (Haploid Set) | |
|---|---|---|---|---|---|---|---|
| D. concanense (Dalzell) Baker 2n = 12 | Dcon1 | 11.25 ± 0.30 b | 3.10 ± 0.20 u | 7.15 ± 0.40 z | 2 (3rd pair) | 4st + 6sm + 2st.t | 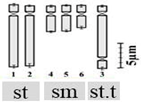 |
| Dcon2 | 11.14 ± 0.45 b | 3.19 ± 0.14 u | 7.18 ± 0.31 z | ||||
| D. goaense Prabhug. 2n = 12 | Dgoa1 | 10.93 ± 1.36 b | 3.05 ± 0.47 u | 6.83 ± 0.81 yz | 2 (2nd pair) | 4st + 6sm + 2st.t | 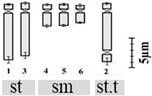 |
| D. montanum (Dalzell) Baker 2n = 20 | Dmon1 | 11.79 ± 0.87 b | 2.35 ± 0.18 st | 5.94 ± 0.55 xy | 4 (9th & 10th pair) | 6sm + 4t + 4m + 2st + 4sm.t | 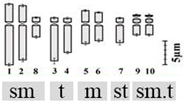 |
| Dmon2 | 11.66 ± 0.68 b | 2.40 ± 0.15 t | 5.60 ± 0.54 x | ||||
| D. ursulae Blatt. 2n = 20 | Durs1 | 11.29 ± 1.66 b | 2.01 ± 0.29 qr | 5.52 ± 0.86 x | 4 (3rd & 9th pair) | 6sm + 4st + 4t + 2m + 4st.t | 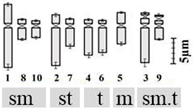 |
| Durs2 | 11.54 ± 0.32 b | 2.05 ± 0.22 qr | 5.85 ± 0.92 xy | ||||
| Durs3 | 11.79 ± 0.87 b | 2.01 ± 0.28 qr | 5.90 ± 0.74 xy | ||||
| D. erythraeum Webb & Berthel. 2n = 22 | Dery1 | 7.88 ± 1.02 a | 1.66 ± 0.20 p | 5.10 ± 1.05 x | 2 (3rd pair) | 12st + 2t + 6sm + 2st.t | 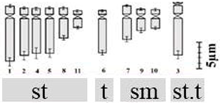 |
| Dery2 | 7.93 ± 1.58 a | 1.72 ± 0.16 pq | 5.15 ± 0.75 x | ||||
| Major Band/Signal Types Based on CMA/DAPI Staining | Sub-Types Based on Position of Bands/Signals on Chromosomes | Position of Bands/Signal(s) on Chromosome Arms | Diagrammatic Representation | Species Name |
|---|---|---|---|---|
| Type A CMA0/DAPI0 | A | No distinct signal |  | In all species |
| Type B CMA+ve/DAPI−ve | B1 | Short arm, distal to constriction |  | D. concanense, D. goaense |
| B2 | Centromeric region |  | D. ursulae | |
| B3 | Nucleolar |  | D. concanense, D. goaense | |
| Nucleolar, extended to terminal satellite |  | D. ursulae | ||
| Nucleolar, extended to short arm and terminal satellite |  | D. montanum, D. ursulae | ||
| B4 | Short arm and terminal satellite |  | D. montanum | |
| Type C CMA+ve/DAPI0 | C | Nucleolar, extended to short arm and terminal satellite |  | D. erythraeum * |
| Type D DAPI+ve/CMA−ve | D1 | Long arm, interstitial, 1–3 in number |  | D. montanum, D. ursulae |
| D2 | Long arm, Distal |  | D. montanum | |
| Type E DAPI+ve/CMA0 | E1 | Short arm, interstitial |  | D. erythraeum * |
| E2 | Long arm, proximal to constriction |  | D. erythraeum | |
| E3 | Long arm, interstitial, 2 in number (2 bands) |  | D. erythraeum | |
| E4 | Both arms, interstitial, 3 in number |  | D. erythraeum |
| Sl. No. | Species & Chromosome Number | Order of Nucleolar Pair | CMA+ve Bands | DAPI+ve Bands | Total No. CMA+ve & DAPI+ve Bands/2n | Fluorescent Karyotype (2n) * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Chromosome Pair (p)/Single (s) | Type | No. | Chromosome Pair (p)/Single (s) | Type | |||||
| 1. | D. concanense (2n = 12) | 3rd | 2 | 1st (p) | B1 | Nil | -- | -- | 4 | 8A + 4B |
| 2 | 3rd (p) | B3 | ||||||||
| 2. | D. goaense (2n = 12) | 2nd | 2 | 1st (p) | B1 | Nil | -- | -- | 4 | 8A + 4B |
| 2 | 2nd (p) | B3 | ||||||||
| 3. | D. montanum (2n = 20) | 9th 10th | 2 | 9th (p) | B3 | 6 | 2nd (p) | D1 | 20 | 8A + 4B + 8D |
| 2 | 10th (p) | B4 | 6 | 3rd (p) | D1 | |||||
| 2 | 4th (p) | D1 | ||||||||
| 2 | 5th (p) | D2 | ||||||||
| 4. | D. ursulae (2n = 20) | 3rd 9th | 2 | 3rd (p) | B3 | 3 | 1st (s) | D1 | 13 | 10A + 4B + 2B/D + 4D |
| 2 | 8th (p) | B2 | 3 | 3rd (s) | D3 | |||||
| 2 | 9th (p) | B3 | 1 | 4th (s) | D1 | |||||
| 5. | D. erythraeum (2n = 22) | 3rd | 2 | 3rd (p) | C | 6 | 2nd (p) | E4 | 28 | 8A + 2C/E+12E |
| 2 | 3rd (p) | E1 | ||||||||
| 4 + 1 | 4th (2p + 1s) | E4 | ||||||||
| 4 + 1 | 5th (2p + 1s) | E4 | ||||||||
| 4 | 6th (p) | E3 | ||||||||
| 2 | 7th (p) | E2 | ||||||||
| 2 | 8th (p) | E1 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, T.; Jha, T.B.; Ray, S.; Jha, S. Comparative Cytogenetics and Fluorescent Chromosome Banding in Five Indian Species of Dipcadi Medik. Plants 2023, 12, 2534. https://doi.org/10.3390/plants12132534
Samanta T, Jha TB, Ray S, Jha S. Comparative Cytogenetics and Fluorescent Chromosome Banding in Five Indian Species of Dipcadi Medik. Plants. 2023; 12(13):2534. https://doi.org/10.3390/plants12132534
Chicago/Turabian StyleSamanta, Tundra, Timir B. Jha, Sudipta Ray, and Sumita Jha. 2023. "Comparative Cytogenetics and Fluorescent Chromosome Banding in Five Indian Species of Dipcadi Medik" Plants 12, no. 13: 2534. https://doi.org/10.3390/plants12132534
APA StyleSamanta, T., Jha, T. B., Ray, S., & Jha, S. (2023). Comparative Cytogenetics and Fluorescent Chromosome Banding in Five Indian Species of Dipcadi Medik. Plants, 12(13), 2534. https://doi.org/10.3390/plants12132534






