Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris
Abstract
1. Introduction
2. Results
3. Discussion
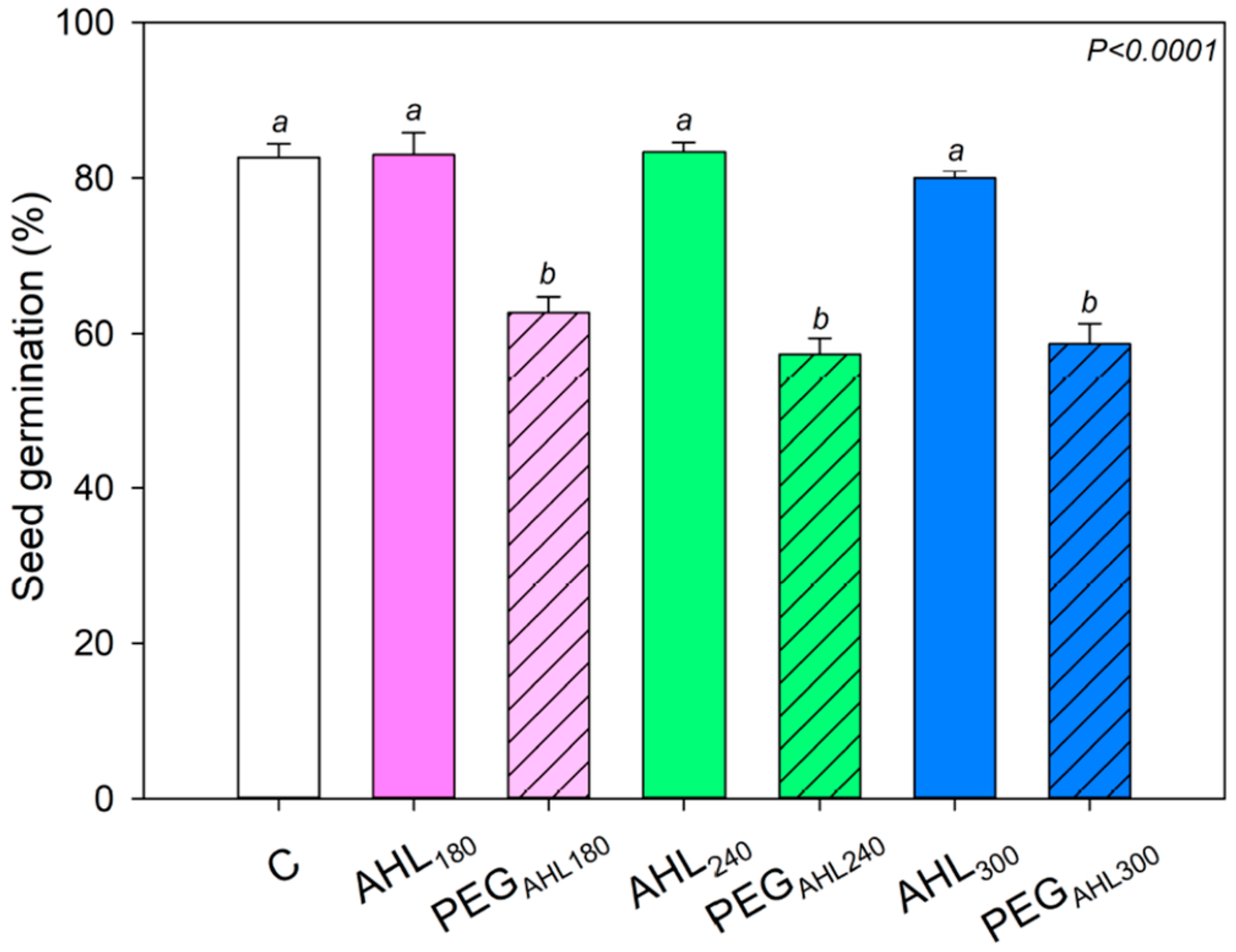
3.1. Effects of the Seed-Priming Treatment with AHL Solutions on Seed Germination
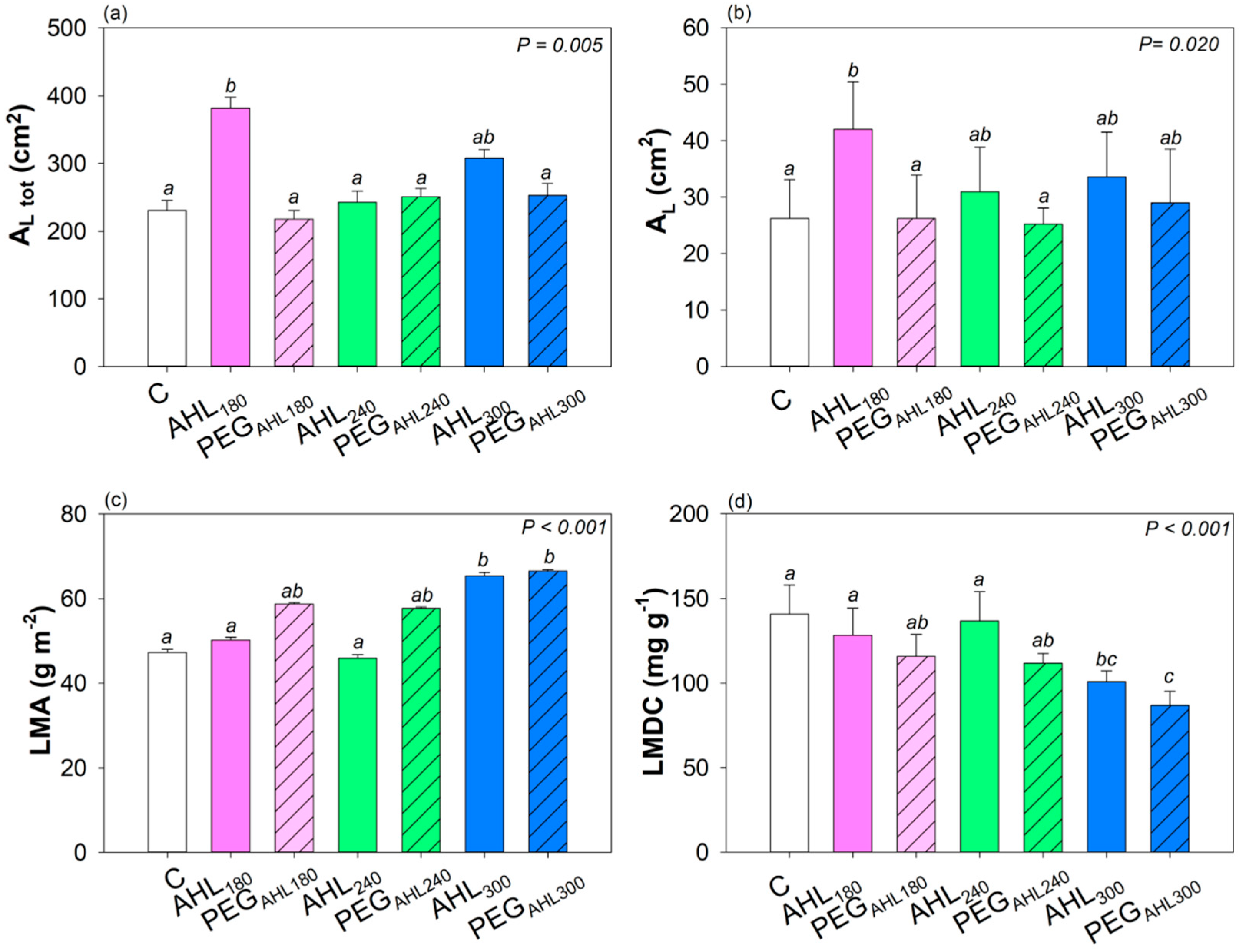
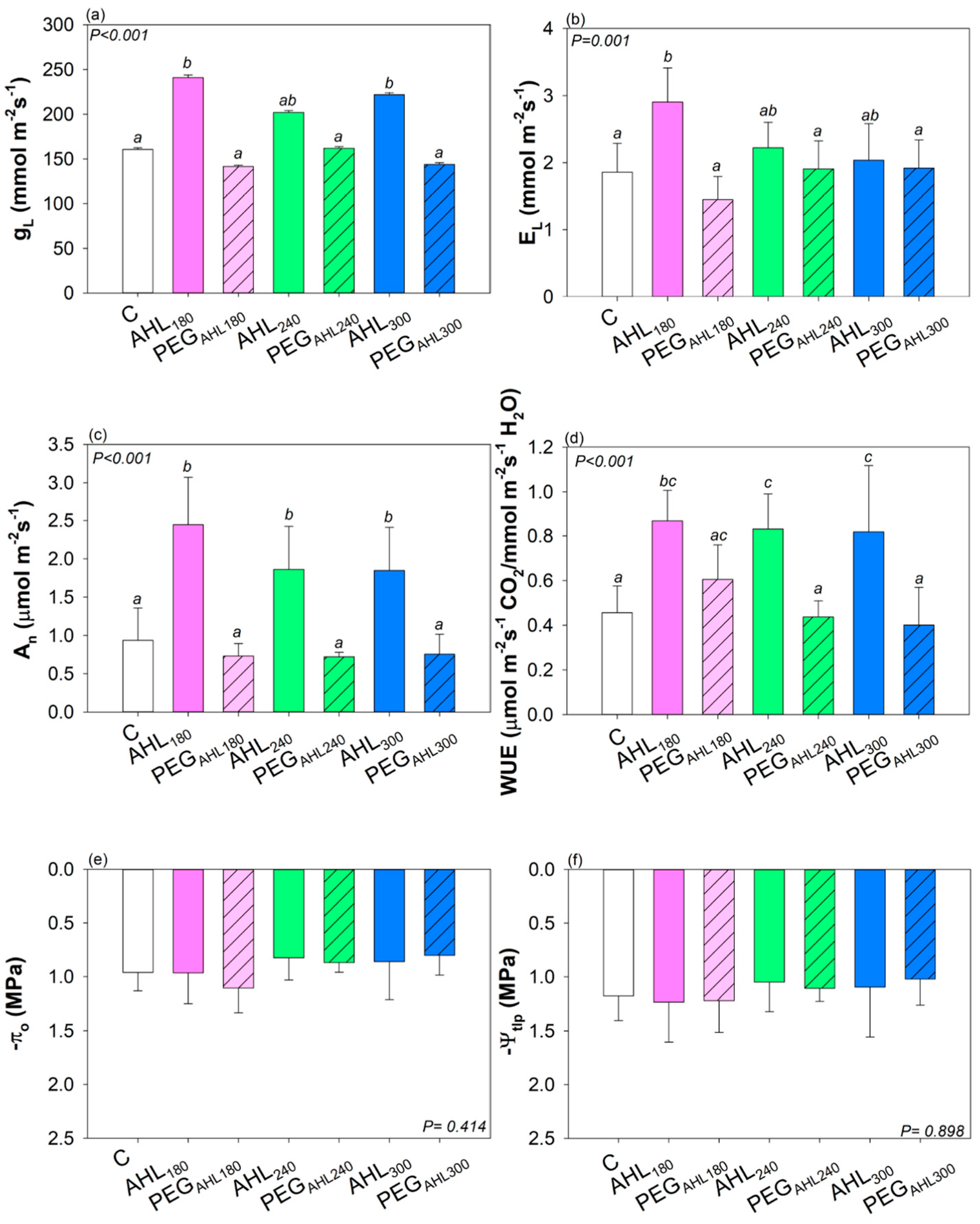
3.2. Effects of the Seed Priming with AHL Solutions on Plant Growth
4. Materials and Methods
4.1. Obtaining AHL Solutions
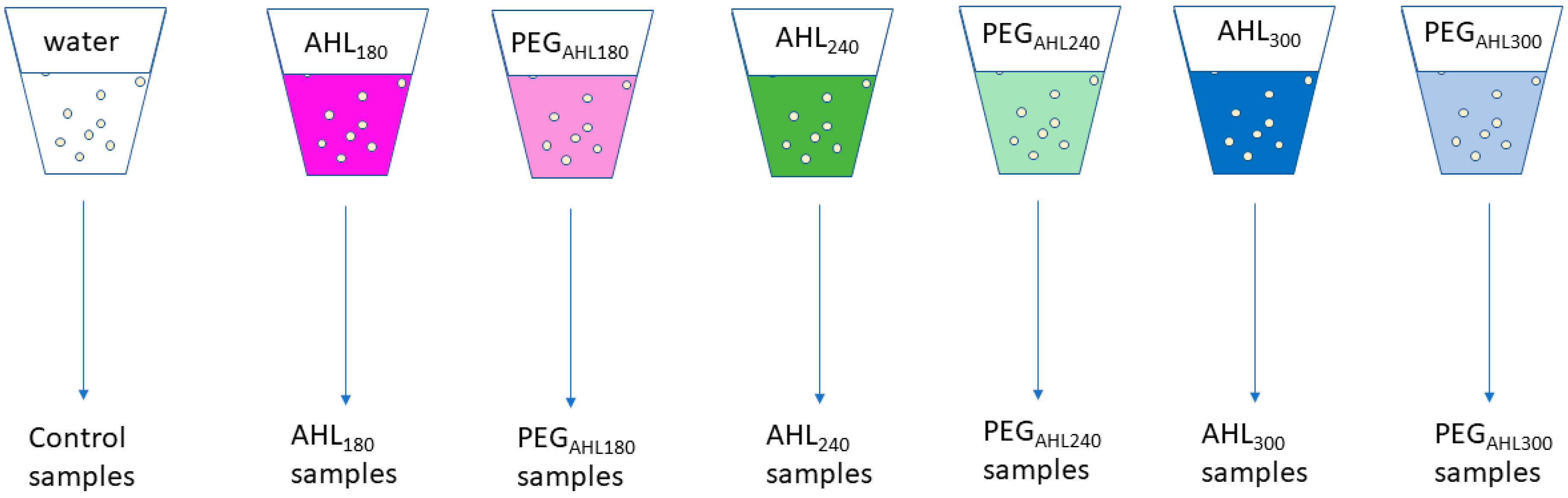
4.2. Seed Priming and Germination
4.3. Plant Growth and Anatomical Traits
4.4. Water Relations Parameters
4.5. Leaf Mineral Contents
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Mitigation of Climate Change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Aznar-Sanchez, J.A.; Belmonte-Urena, L.J.; Velasco-Munoz, J.F.; Valera, D.L. Farmers’ Profiles and Behaviours toward Desalinated Seawater for Irrigation: Insights from South-East Spain. J. Clean. Prod. 2021, 296, 126568. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Sharma, S.; Horn, S.J. Enzymatic Saccharification of Brown Seaweed for Production of Fermentable Sugars. Bioresour. Technol. 2016, 213, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum Extract Biostimulants and Their Role in Enhancing Tolerance to Drought Stress in Tomato Plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; Staden, J. Plant Biostimulants from Seaweed: An Overview. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 31–55. ISBN 978-1-119-35719-3. [Google Scholar]
- Do Rosário Rosa, V.; Farias Dos Santos, A.L.; Alves Da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; De Almeida Silva, M. Increased Soybean Tolerance to Water Deficiency through Biostimulant Based on Fulvic Acids and Ascophyllum nodosum (L.) Seaweed Extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; Van Der Meer, I.M.; Van Der Werf, A.; Karlova, R. The Power of Seaweeds as Plant Biostimulants to Boost Crop Production under Abiotic Stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef]
- Prisa, D.; Spagnuolo, D. Evaluation of the Bio-Stimulating Activity of Lake Algae Extracts on Edible Cacti Mammillaria prolifera and Mammillaria glassii. Plants 2022, 11, 3586. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Russo, V.; Manghisi, A.; Di Martino, A.; Morabito, M.; Genovese, G.; Trifilò, P. Screening on the Presence of Plant Growth Regulators in High Biomass Forming Seaweeds from the Ionian Sea (Mediterranean Sea). Sustainability 2022, 14, 3914. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, Q.; Quek, A.; Ai, N.; Zeng, G.; Wang, J. Hydrothermal Carbonization of Macroalgae and the Effects of Experimental Parameters on the Properties of Hydrochars. ACS Sustain. Chem. Eng. 2013, 1, 1092–1101. [Google Scholar] [CrossRef]
- Patel, N.; Acharya, B.; Basu, P. Hydrothermal Carbonization (HTC) of Seaweed (Macroalgae) for Producing Hydrochar. Energies 2021, 14, 1805. [Google Scholar] [CrossRef]
- Shrestha, A.; Acharya, B.; Farooque, A.A. Study of Hydrochar and Process Water from Hydrothermal Carbonization of Sea Lettuce. Renew. Energy 2021, 163, 589–598. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Ning, X.; Wall, D.; Murphy, J.D. Co-Production of Hydrochar, Levulinic Acid and Value-Added Chemicals by Microwave-Assisted Hydrothermal Carbonization of Seaweed. Chem. Eng. J. 2022, 441, 135915. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Flórez-Fernández, N.; Dolores Torres, M.; Domínguez, H.; Rodríguez-Jasso, R.M.; Ruiz, H.A. Hydrothermal Systems to Obtain High Value-Added Compounds from Macroalgae for Bioeconomy and Biorefineries. Bioresour. Technol. 2022, 343, 126017. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal Carbonization: Process Water Characterization and Effects of Water Recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Espro, C.; Satira, A.; Mauriello, F.; Anajafi, Z.; Moulaee, K.; Iannazzo, D.; Neri, G. Orange Peels-Derived Hydrochar for Chemical Sensing Applications. Sens. Actuators B Chem. 2021, 341, 130016. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Leng, S.; Chen, J.; Yang, L.; Li, H.; Jiang, S.; Huang, H. Bioenergy Recovery from Wastewater Produced by Hydrothermal Processing Biomass: Progress, Challenges, and Opportunities. Sci. Total Environ. 2020, 748, 142383. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Taufer, N.L.; Benedetti, V.; Pecchi, M.; Matsumura, Y.; Baratieri, M. Coupling Hydrothermal Carbonization of Digestate and Supercritical Water Gasification of Liquid Products. Renew. Energy 2021, 173, 934–941. [Google Scholar] [CrossRef]
- Satira, A.; Paone, E.; Bressi, V.; Iannazzo, D.; Marra, F.; Calabrò, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Appl. Sci. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Celletti, S.; Lanz, M.; Bergamo, A.; Benedetti, V.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Evaluating the Aqueous Phase From Hydrothermal Carbonization of Cow Manure Digestate as Possible Fertilizer Solution for Plant Growth. Front. Plant Sci. 2021, 12, 687434. [Google Scholar] [CrossRef]
- Langone, M.; Basso, D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Gu, C.; Han, Y.; Zan, S.; Wu, S. Effects of Process Water Recirculation on Solid and Liquid Products from Hydrothermal Carbonization of Laminaria. Bioresour. Technol. 2019, 292, 121996. [Google Scholar] [CrossRef] [PubMed]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; Van Genuchten, M.T. Seed Priming Alleviated Salinity Stress during Germination and Emergence of Wheat (Triticum aestivum L.). Agric. Water Manag. 2020, 231, 106022. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming Memory Invokes Seed Stress-Tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘Primeomics’: Plants Memorize Their Germination under Stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Michelet, S.; Bruno, F.; Castelli, G.; Vitale, F.; Sfriso, A.; Morabito, M.; Genovese, G. Bioactivity of Phycocolloids against the Mediterranean Protozoan Leishmania infantum: An Inceptive Study. Sustainability 2016, 8, 1131. [Google Scholar] [CrossRef]
- Jandl, G.; Eckhardt, K.-U.; Bargmann, I.; Kücke, M.; Greef, J.-M.; Knicker, H.; Leinweber, P. Hydrothermal Carbonization of Biomass Residues: Mass Spectrometric Characterization for Ecological Effects in the Soil-Plant System. J. Environ. Qual. 2013, 42, 199–207. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Vozhdayev, G.V.; Spokas, K.A.; Molde, J.S.; Heilmann, S.M.; Wood, B.M.; Valentas, K.J. Response of Maize Germination and Growth to Hydrothermal Carbonization Filtrate Type and Amount. Plant Soil 2015, 396, 127–136. [Google Scholar] [CrossRef]
- Mau, V.; Arye, G.; Gross, A. Wetting Properties of Poultry Litter and Derived Hydrochar. PLoS ONE 2018, 13, e0206299. [Google Scholar] [CrossRef]
- Levine, R.B.; Bollas, A.; Savage, P.E. Process Improvements for the Supercritical in Situ Transesterification of Carbonized Algal Biomass. Bioresour. Technol. 2013, 136, 556–564. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Miguel, T.B.A.R.; De Castro Miguel, E.; De Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity Evaluation of Process Water from Hydrothermal Carbonization of Sugarcane Industry By-Products. Environ. Sci. Pollut. Res. 2019, 26, 27579–27589. [Google Scholar] [CrossRef]
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C.W. Hydrothermal Carbonization and Liquefaction for Sustainable Production of Hydrochar and Aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Liu, Q. Fate and Distribution of Nutrients and Heavy Metals during Hydrothermal Carbonization of Sewage Sludge with Implication to Land Application. J. Clean. Prod. 2019, 225, 972–983. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. Production of Bio-Coal, Bio-Methane and Fertilizer from Seaweed via Hydrothermal Carbonisation. Algal Res. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative Study of the Effects of Mannitol and PEG Osmotic Stress on Growth and Solute Accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.A.A.; Abdelly, C. Influence of PEG Induced Drought Stress on Molecular and Biochemical Constituents and Seedling Growth of Egyptian Barley Cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Plaut, Z.; Federman, E. A Simple Procedure to Overcome Polyethelene Glycol Toxicity on Whole Plants. Plant Physiol. 1985, 79, 559–561. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, C.; Li, S.; Li, X.; Tai, F. Effects of Different Plant Hormones or PEG Seed Soaking on Maize Resistance to Drought Stress. Can. J. Plant Sci. 2014, 94, 1491–1499. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A.; Veres, S. Physiology of Soybean as Affected by PEG-Induced Drought Stress. Curr. Plant Biol. 2020, 22, 100135. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Caruso, A.; Chefdor, F.; Carpin, S.; Depierreux, C.; Delmotte, F.M.; Kahlem, G.; Morabito, D. Physiological Characterization and Identification of Genes Differentially Expressed in Response to Drought Induced by PEG 6000 in Populus canadensis Leaves. J. Plant Physiol. 2008, 165, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.-H.; Yin, H.; Wang, W.-X.; Zhao, X.-M.; Du, Y.-G. Alginate Oligosaccharides Enhanced Triticum aestivum L. Tolerance to Drought Stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic Adjustment Is a Prime Drought Stress Adaptive Engine in Support of Plant Production: Osmotic Adjustment and Plant Production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline Metabolic Dynamics and Implications in Drought Tolerance of Peanut Plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. From One Side to Two Sides: The Effects of Stomatal Distribution on Photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Banerjee, S.; Roy, P.; Nandi, S.; Roy, S. Advanced Biotechnological Strategies towards the Development of Crops with Enhanced Micronutrient Content. Plant Growth Regul. 2023, 100, 355–371. [Google Scholar] [CrossRef]
- North, G.B.; Nobel, P.S. Changes in Hydraulic Conductivity and Anatomy Caused by Drying and Rewetting Roots of Agave deserti (Agavaceae). Am. J. Bot. 1991, 78, 906–915. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root Trait Responses to Drought Are More Heterogeneous than Leaf Trait Responses. Funct. Ecol. 2020, 34, 2224–2235. [Google Scholar] [CrossRef]
- Trifilo, P.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A.; Salleo, S. Drought Resistance of Ailanthus altissima: Root Hydraulics and Water Relations. Tree Physiol. 2004, 24, 107–114. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining Root Water Transport Drives Stomatal Closure in Olive under Moderate Water Stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef]
- Abate, E.; Nardini, A.; Petruzzellis, F.; Trifilò, P. Too Dry to Survive: Leaf Hydraulic Failure in Two Salvia Species Can Be Predicted on the Basis of Water Content. Plant Physiol. Biochem. 2021, 166, 215–224. [Google Scholar] [CrossRef]
- Bacher, H.; Sharaby, Y.; Walia, H.; Peleg, Z. Modifying Root-to-Shoot Ratio Improves Root Water Influxes in Wheat under Drought Stress. J. Exp. Bot. 2022, 73, 1643–1654. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-Induced Changes in Root Biomass Largely Result from Altered Root Morphological Traits: Evidence from a Synthesis of Global Field Trials: Effects of Drought on Root Traits. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of Leaf-trait Variation along Environmental Gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Nardini, A. Hard and Tough: The Coordination between Leaf Mechanical Resistance and Drought Tolerance. Flora 2022, 288, 152023. [Google Scholar] [CrossRef]
- Grassi, F.; Labra, M.; Minuto, L. Molecular diversity in Ligurian local races of common bean (Phaseolus vulgaris L.). Plant Biosyst. 2006, 140, 17–20. [Google Scholar] [CrossRef]
- Monalisa, S.P.; Beura, J.K.; Tarai, R.K.; Naik, M. Seed Quality Enhancement through Biopriming in Common Bean (Phaseolus vulgaris. L). J. Appl. Nat. Sci. 2017, 9, 1740–1743. [Google Scholar] [CrossRef][Green Version]
- Lo Gullo, M.A.; Trifilo, P.; Raimondo, F. Hydraulic Characteristics and Water Relations in Pigment-Less Mutant Shoots of an Orange Tree. Tree Physiol. 2007, 27, 209–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petruzzellis, F.; Savi, T.; Bacaro, G.; Nardini, A. A Simplified Framework for Fast and Reliable Measurement of Leaf Turgor Loss Point. Plant Physiol. Biochem. 2019, 139, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef] [PubMed]

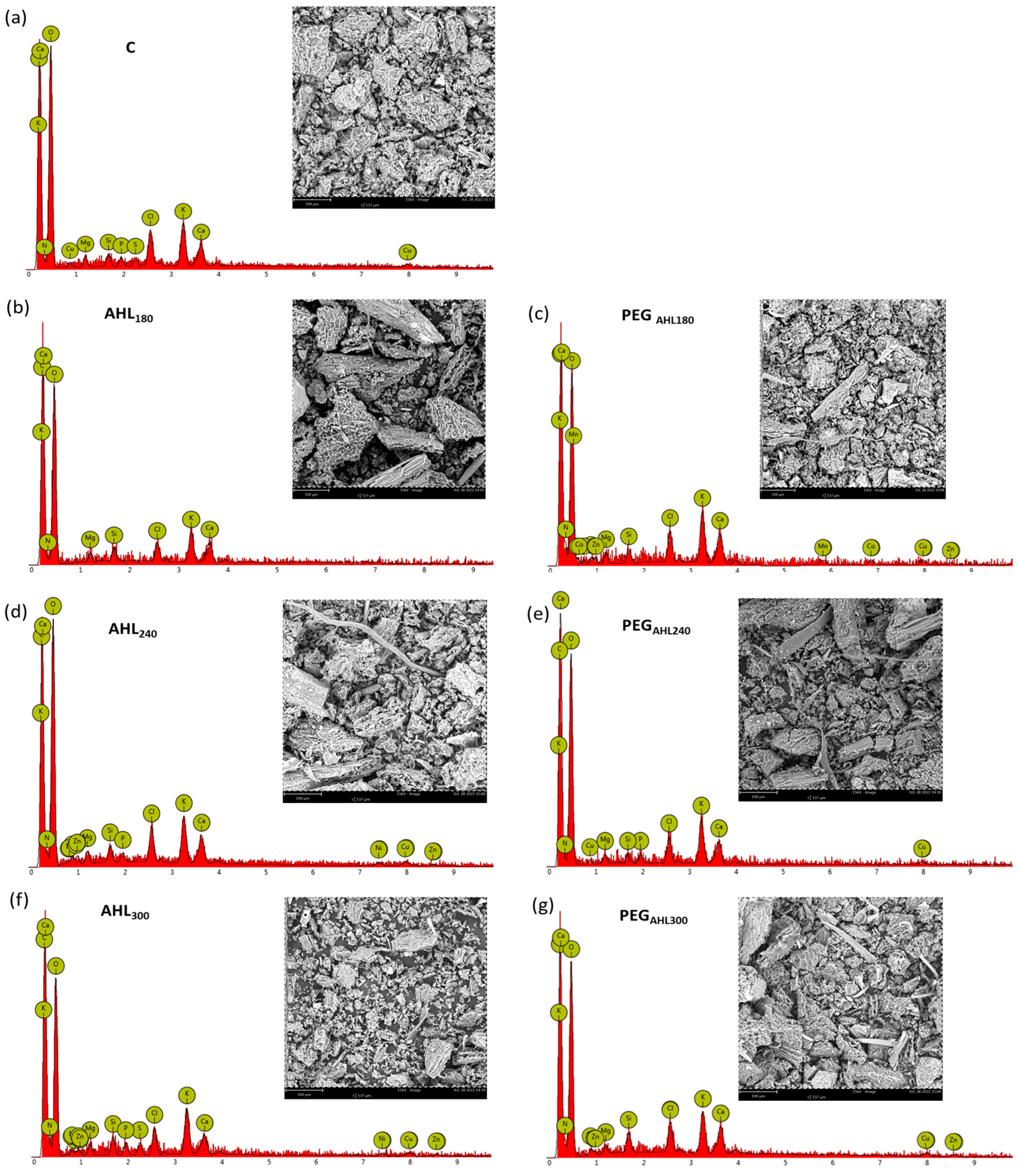
| Stomatal Density (m−2) | C (mg g−1) | C (%) | N (mg g−1) | N (%) | C:N | |
|---|---|---|---|---|---|---|
| C | 148.4 ± 9.9 a | 76.83 | 41.83 | 5.141 | 2.726 | 15.3 |
| AHL180 | 176.7 ± 10.2 ab | 73.80 | 41.93 | 5.644 | 3.124 | 13.4 |
| PEGAHL180 | 131.0 ± 28.5 ac | 78.94 | 41.96 | 5.262 | 2.725 | 15.4 |
| AHL240 | 182.6 ± 8.4 ab | 72.49 | 41.74 | 5.272 | 2.956 | 14.1 |
| PEGAHL240 | 104.7 ± 8.3 c | 67.64 | 41.62 | 5.8 | 3.476 | 12 |
| AHL300 | 188.2 ± 9.5 b | 69.41 | 41.73 | 6.544 | 3.833 | 10.9 |
| PEGAHL300 | 148.7 ± 2.5 a | 77.85 | 41.7 | 7.167 | 3.74 | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnuolo, D.; Bressi, V.; Chiofalo, M.T.; Morabito, M.; Espro, C.; Genovese, G.; Iannazzo, D.; Trifilò, P. Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris. Plants 2023, 12, 2745. https://doi.org/10.3390/plants12142745
Spagnuolo D, Bressi V, Chiofalo MT, Morabito M, Espro C, Genovese G, Iannazzo D, Trifilò P. Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris. Plants. 2023; 12(14):2745. https://doi.org/10.3390/plants12142745
Chicago/Turabian StyleSpagnuolo, Damiano, Viviana Bressi, Maria Teresa Chiofalo, Marina Morabito, Claudia Espro, Giuseppa Genovese, Daniela Iannazzo, and Patrizia Trifilò. 2023. "Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris" Plants 12, no. 14: 2745. https://doi.org/10.3390/plants12142745
APA StyleSpagnuolo, D., Bressi, V., Chiofalo, M. T., Morabito, M., Espro, C., Genovese, G., Iannazzo, D., & Trifilò, P. (2023). Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris. Plants, 12(14), 2745. https://doi.org/10.3390/plants12142745












