Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oil Yield Seasonal Variation
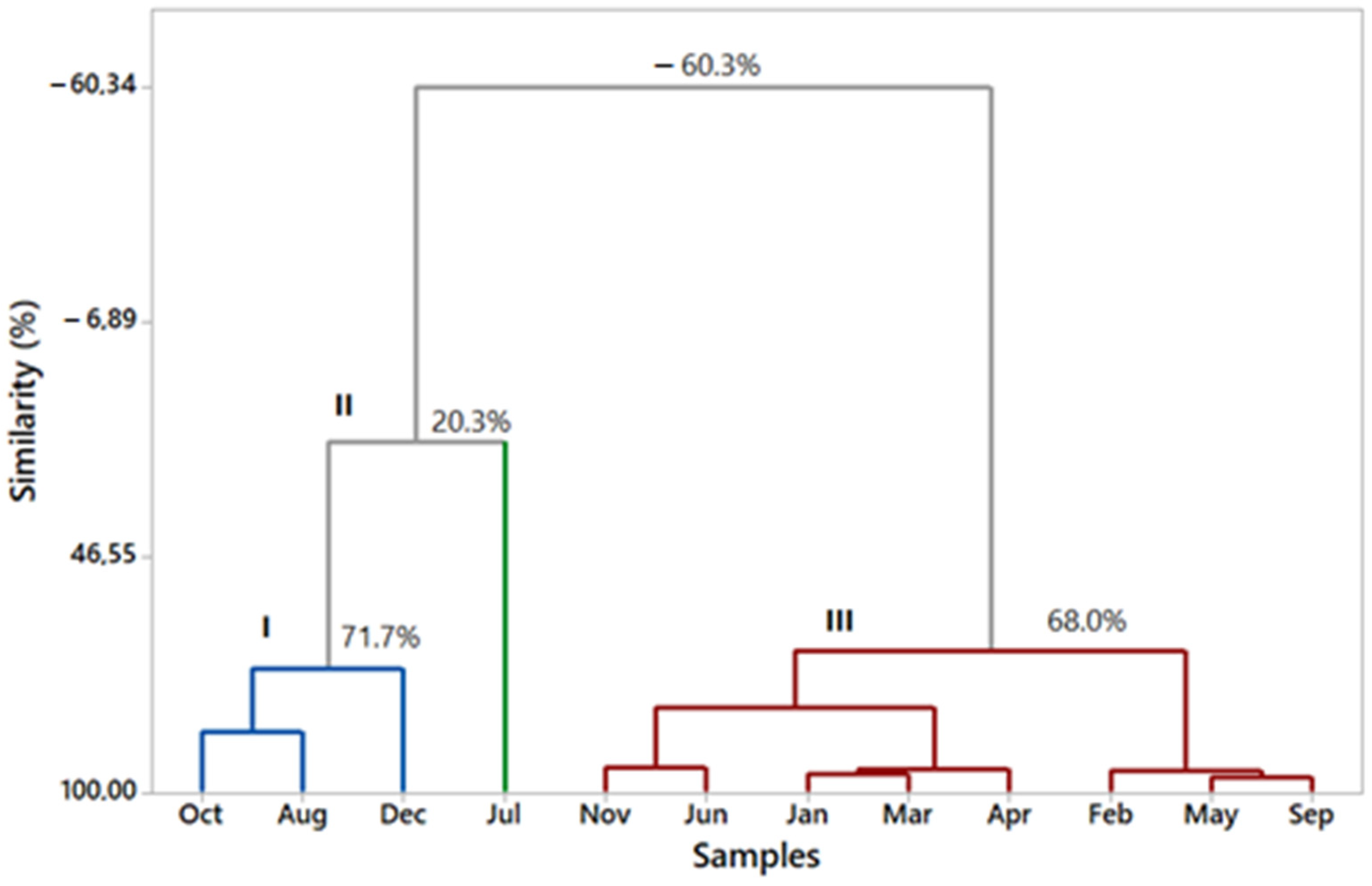
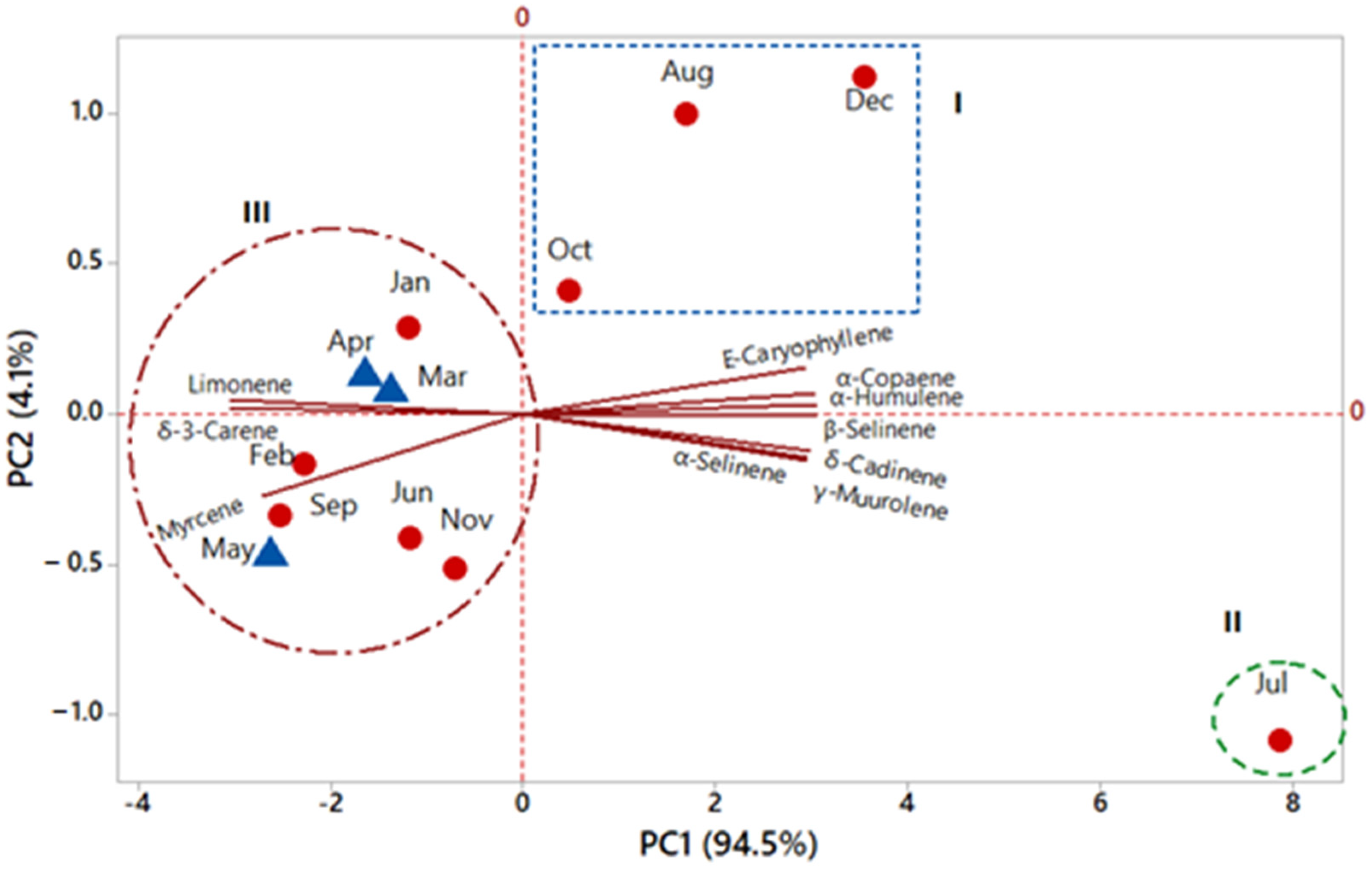
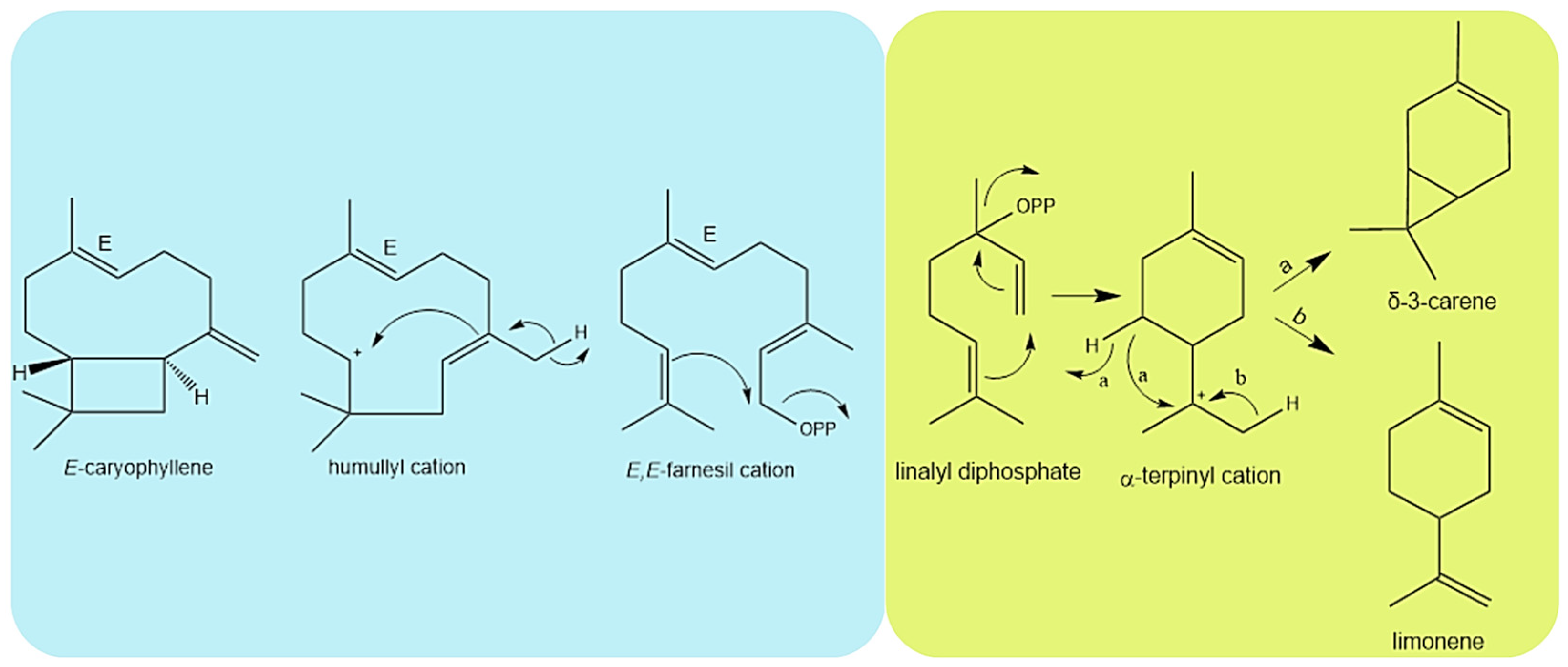
2.2. Seasonal Effects in Essential Oil Chemical Composition
| RI(C) | RI(L) | Month | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential Oil Yield | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 | 0.6 | 0.1 | 0.3 | 0.7 | ||
| Constituents c | (%) * | |||||||||||||
| 929 | 932 a | α-Pinene | 0.78 | 0.91 | 0.39 | 0.55 | 1.55 | 0.79 | 1.59 | 1.61 | 1.64 | 0.60 | 0.44 | 1.41 |
| 946 | 946 a | Camphene | 0.09 | 0.11 | ||||||||||
| 987 | 988 a | Myrcene | 1.44 | 2.67 | 0.56 | 1.78 | 2.47 | 2.03 | 2.07 | 2.79 | 2.58 | 0.42 | 0.84 | 2.63 |
| 1009 | 1008 a | δ-3-Carene | 26.40 | 29.05 | 19.45 | 30.66 | 32.84 | 29.32 | 32.47 | 31.64 | 31.32 | 8.70 | 25.60 | 33.16 |
| 1018 | 1014 a | α-Terpinene | 0.18 | |||||||||||
| 1023 | 1020 a | p-Cymene | 0.18 | 0.03 | 0.25 | 0.28 | 0.18 | 0.11 | 0.02 | |||||
| 1029 | 1024 a | Limonene | 44.05 | 43.28 | 29.56 | 49.27 | 52.80 | 51.99 | 49.81 | 56.24 | 45.20 | 11.42 | 35.58 | 53.64 |
| 1043 | 1044 a | (E)-β-Ocimene | 1.05 | 0.14 | 0.66 | 0.09 | 0.20 | 0.23 | 0.12 | 1.06 | 0.19 | 0.25 | ||
| 1055 | 1054 a | γ-Terpinene | 0.27 | 0.20 | 0.27 | 0.30 | 0.08 | 0.14 | 0.22 | 0.14 | 0.32 | |||
| 1079 | 1085 a | p-Mentha-2,4(8)-diene | 0.05 | 0.05 | 0.06 | 0.06 | ||||||||
| 1084 | 1086 a | Terpinolene | 0.49 | 0.67 | 0.27 | 0.54 | 0.59 | 0.71 | 0.41 | 0.43 | 0.66 | 0.39 | 0.48 | 0.82 |
| 1089 | 1089 a | p-Cymenene | 0.16 | 0.13 | 0.15 | 0.14 | 0.12 | 0.10 | 0.05 | 0.09 | 0.16 | |||
| 1098 | 1095 a | Linalool | 0.30 | 0.55 | 0.45 | 0.13 | 0.12 | 0.08 | 0.10 | 0.17 | ||||
| 1112 | 1113 b | 4,8-Dimethyl-(E)-nona-1,3,7-triene | 0.24 | 0.32 | 0.56 | 0.42 | 0.03 | 0.14 | 0.20 | 0.18 | 0.19 | 0.08 | ||
| 1193 | 1186 a | α-Terpineol | 0.09 | 0.14 | 0.03 | 0.03 | 0.06 | 0.06 | 0.13 | |||||
| 1197 | 1200 a | trans-Dihydrocarvone | 0.15 | 0.14 | 0.08 | 0.21 | 0.44 | |||||||
| 1217 | 1215 a | trans-Carveol | 0.26 | 0.15 | 0.27 | 0.17 | 0.06 | 0.06 | 0.12 | 0.03 | 0.18 | 0.30 | 0.09 | |
| 1229 | 1226 a | neoiso-Dihydrocarveol | 0.19 | |||||||||||
| 1242 | 1239 a | Carvone | 0.01 | 0.04 | ||||||||||
| 1374 | 1374 a | α-Copaene | 3.59 | 2.76 | 6.27 | 2.43 | 1.50 | 2.14 | 2.17 | 1.19 | 2.33 | 8.32 | 4.70 | 1.11 |
| 1388 | 1389 a | β-Elemene | 0.05 | 0.01 | 0.02 | 0.07 | ||||||||
| 1420 | 1417 a | (E)-Caryophyllene | 13.49 | 9.79 | 23.16 | 7.74 | 5.13 | 7.48 | 6.73 | 4.10 | 8.54 | 24.98 | 17.20 | 4.44 |
| 1433 | 1437 a | α-Guaiene | 0.05 | 0.55 | ||||||||||
| 1436 | 1439 a | Aromadendrene | 0.35 | 0.33 | 0.89 | 0.19 | 0.12 | 0.19 | 0.17 | 0.06 | 0.23 | 1.84 | 0.53 | 0.07 |
| 1447 | 1449 a | α-Himachalene | 0.11 | 0.05 | 0.30 | 0.09 | ||||||||
| 1454 | 1452 a | α-Humulene | 1.14 | 0.92 | 2.20 | 0.61 | 0.37 | 0.60 | 0.53 | 0.25 | 0.77 | 3.25 | 1.72 | 0.30 |
| 1458 | 1458 a | allo-Aromadendrene | 0.05 | 0.14 | 0.02 | 0.04 | 0.39 | 0.05 | ||||||
| 1473 | 1478 a | γ-Muurolene | 1.03 | 0.56 | 1.34 | 0.38 | 0.18 | 0.35 | 0.27 | 0.11 | 0.59 | 3.71 | 1.02 | 0.12 |
| 1478 | 1483 a | α-Amorphene | 0.08 | 0.05 | 0.14 | 0.03 | 0.03 | 0.06 | 0.55 | 0.10 | ||||
| 1487 | 1489 a | β-Selinene | 1.70 | 1.39 | 3.37 | 0.90 | 0.53 | 0.86 | 0.70 | 0.27 | 1.02 | 5.13 | 2.08 | 0.3 |
| 1494 | 1498 a | α-Selinene | 1.28 | 1.02 | 2.38 | 0.69 | 0.40 | 0.64 | 0.51 | 0.22 | 0.75 | 6.20 | 1.48 | 0.22 |
| 1496 | 1500 a | α-Muurolene | 0.38 | 0.28 | 0.57 | 0.18 | 0.10 | 0.16 | 0.15 | 0.05 | 0.21 | 2.23 | 0.54 | 0.06 |
| 1499 | 1509 a | α-Bulnesene | 0.20 | |||||||||||
| 1500 | 1495 a | cis-Cadina-1,4-diene | 0.01 | |||||||||||
| 1503 | 1505 a | (E,E)-α-Farnesene | 0.05 | 0.10 | 0.02 | 0.05 | 0.01 | |||||||
| 1511 | 1513 a | γ-Cadinene | 0.34 | 0.29 | 0.68 | 0.15 | 0.08 | 0.15 | 0.13 | 0.04 | 0.19 | 1.69 | 0.48 | 0.05 |
| 1516 | 1513 a | δ-Cadinene | 1.28 | 1.05 | 2.5 | 0.66 | 0.64 | 0.49 | 0.17 | 0.80 | 6.24 | 1.92 | 0.27 | |
| 1516 | 1511 a | δ-Amorphene | 0.38 | |||||||||||
| 1519 | 1521 a | trans-Calamenene | 0.03 | 0.26 | ||||||||||
| 1521 | 1528 a | Zonarene | 0.18 | 0.04 | ||||||||||
| 1530 | 1533 a | trans-Cadina-1,4-diene | 0.11 | 0.12 | 0.23 | 0.05 | 0.03 | 0.05 | 0.08 | 0.48 | 0.17 | |||
| 1534 | 1537 a | α-Cadinene | 0.11 | 0.08 | 0.19 | 0.03 | 0.04 | 0.53 | 0.13 | |||||
| 1538 | 1545 a | Selina-4(15),7(11)-diene | 0.31 | |||||||||||
| 1539 | 1544 a | α-Calacorene | 0.11 | 0.06 | 0.18 | 0.02 | 0.03 | 1.12 | 0.17 | |||||
| 1559 | 1559 a | Germacrene B | 0.09 | |||||||||||
| 1559 | 1561 a | (E)-Nerolidol | 0.04 | 0.04 | ||||||||||
| 1572 | 1570 a | Caryophyllene alcohol d | 0.42 | 0.34 | 0.72 | 0.09 | 0.05 | 0.09 | 0.53 | 0.02 | ||||
| 1579 | 1582 a | Caryophyllene oxide | 0.11 | 0.26 | 0.37 | 0.07 | 0.01 | 0.05 | 0.09 | 0.01 | 0.05 | 2.32 | 0.25 | |
| 1629 | 1630 a | γ-Eudesmol | 0.07 | 0.18 | 0.04 | 0.02 | 1.46 | 0.18 | ||||||
| 1652 | 1649 a | β-Eudesmol | 0.30 | |||||||||||
| 1653 | 1652 a | α-Cadinol | 0.08 | 0.22 | 0.03 | 1.14 | 0.18 | |||||||
| 1656 | 1658 a | Selin-11-en-4α-ol | 0.03 | 0.08 | ||||||||||
| Monoterpene hydrocarbons | 73.32 | 78.03 | 50.52 | 83.98 | 90.76 | 85.39 | 87.10 | 93.35 | 83.12 | 21.85 | 63.4 | 92.31 | ||
| Oxygenated monoterpenes | 0.26 | 0.54 | 0.82 | 0.91 | 0.09 | 0.23 | 0.48 | 0.11 | 0.42 | 0.21 | 1.06 | 0.26 | ||
| Sesquiterpene hydrocarbons | 25.35 | 18.85 | 44.61 | 14.01 | 8.82 | 13.44 | 11.88 | 6.46 | 15.75 | 67.82 | 32.76 | 6.95 | ||
| Oxygenated sesquiterpenes | 0.53 | 0.82 | 1.57 | 0.23 | 0.01 | 0.12 | 0.09 | 0.01 | 0.14 | 5.75 | 0.65 | 0.02 | ||
| Others | 0.24 | 0.32 | 0.56 | 0.42 | 0.03 | 0.14 | 0.20 | 0.00 | 0.18 | 0.00 | 0.19 | 0.08 | ||
| Total identified (%) | 99.70 | 98.56 | 98.08 | 99.55 | 99.71 | 99.32 | 99.75 | 99.93 | 99.61 | 95.63 | 98.06 | 99.62 | ||
2.3. Antioxidant Capacity
3. Materials and Methods
3.1. Plant Material and Climatic Data
3.2. Extraction and Essential Oil Composition
3.3. Antioxidant Evaluation
3.3.1. β-Carotene/Linoleic Acid Assay
3.3.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations List
| GC/MS | Gas chromatography-mass spectrometry |
| GC-FID | Gas chromatography–flame ionization detector |
| HCA | Hierarchical cluster analysis |
| PCA | Principal component analysis |
| R | Pearson’s correlation coefficient |
| RI(C) | Calculated Retention Index |
| RI(L) | Literature Retention Index |
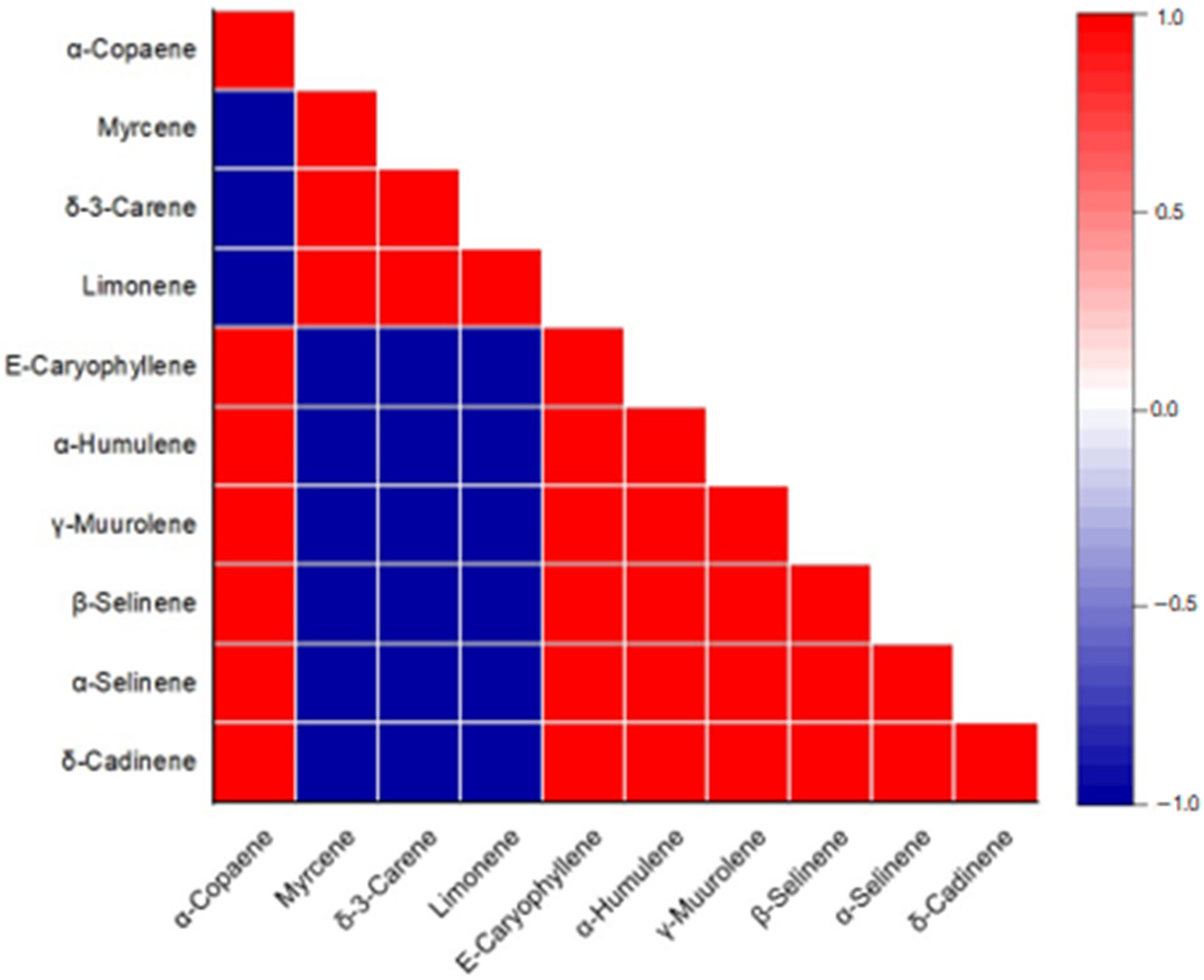
Appendix A
| Myrcene | δ-3-Carene | Limonene | α-Copaene | (E)-Caryophyllene | α-Humulene | γ-Muurolene | β-Selinene | α-Selinene | |
|---|---|---|---|---|---|---|---|---|---|
| δ-3-carene | 0.84 | ||||||||
| limonene | 0.84 | 0.96 | |||||||
| α-copaene | −0.91 | −0.97 | −0.98 | ||||||
| (E)-caryophyllene | −0.93 | −0.93 | −0.95 | 0.98 | |||||
| α-humulene | −0.89 | −0.98 | −0.99 | 0.99 | 0.97 | ||||
| γ-muurolene | −0.77 | −0.97 | −0.96 | 0.93 | 0.87 | 0.95 | |||
| β-selinene | −0.86 | −0.99 | −0.99 | 0.99 | 0.96 | 0.99 | 0.96 | ||
| α-selinene | −0.76 | −0.97 | −0.96 | 0.93 | 0.86 | 0.94 | 0.99 | 0.96 | |
| δ-cadinene | −0.79 | −0.98 | −0.97 | 0.95 | 0.89 | 0.96 | 0.99 | 0.97 | 0.99 |
References
- Kew Science. Anacardiaceae R.Br. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002342-2 (accessed on 26 May 2023).
- Correia, S.D.J.; David, J.P.; David, J.M. Metabólitos secundários de espécies de Anacardiaceae. Quim. Nova 2006, 29, 1287–1300. [Google Scholar] [CrossRef]
- Dos Santos, A.C.A.; Rossato, M.; Agostini, F.; Serafini, L.A.; dos Santos, P.L.; Molon, R.; Dellacassa, E.; Moyna, P. Chemical composition of the essential oils from leaves and fruits of Schinus molle L. and Schinus terebinthifolius Raddi from southern Brazil. J. Essent. Oil Bear. Plants 2009, 12, 16–25. [Google Scholar] [CrossRef]
- Tlili, N.; Yahia, Y.; Feriani, A.; Labidi, A.; Ghazouani, L.; Nasri, N.; Saadaoui, E.; Khaldi, A. Schinus terebinthifolius vs. Schinus molle: A comparative study of the effect of species and location on the phytochemical content of fruits. Ind. Crops Prod. 2018, 122, 559–565. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; Abd El-Ghffar, E.A.; Eldahshan, O.A.; Singab, A.N.B. The genus Schinus (Anacardiaceae): A review on phytochemicals and biological aspects. Nat. Prod. Res. 2022, 36, 4833–4851. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Orth, A.I. Caracterização funcional do sistema reprodutivo da aroeira-vermelha (Schinus terebinthifolius Raddi), em Florianópolis-SC, Brasil. Rev. Bras. Frutic. 2004, 26, 198–201. [Google Scholar] [CrossRef]
- Williams, D.A.; Overholt, W.A.; Cuda, J.P.; Hughes, C.R. Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Mol. Ecol. 2005, 14, 3643–3656. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.P.M.M.; Oliveira, T.K.B.; do Ó, N.P.R.; Sarmento, D.A.; Gadelha, N.C. Schinus yerebinthifolius Raddi (sroeira) e Suas propriedades na medicina popular. Rev. Verde Agroecol. Desenvolv. Sustentável 2015, 10, 23. [Google Scholar] [CrossRef]
- Penzeys. Pink Peppercorns. Available online: https://www.penzeys.com/online-catalog/pink-peppercorns/c-24/p-1410/pd-s (accessed on 20 February 2023).
- dōTERRA. Pink Pepper Oil Schinus molle. Available online: https://www.doterra.com/US/en/p/doterra-pink-pepper (accessed on 26 May 2023).
- Medeiros, K.C.P.; Monteiro, J.C.; Diniz, M.F.F.M.; Medeiros, I.A.; Silva, B.A.; Piuvezam, M.R. Effect of the activity of the Brazilian polyherbal formulation: Eucalyptus globulus Labill, Peltodon radicans Pohl and Schinus terebinthifolius Radd in inflammatory models. Rev. Bras. Farmacogn. 2007, 17, 23–28. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Demuner, A.J.; Clemente, A.D.; de Paula, V.F.; Ismail, F.M.D. Seasonal variation in the composition of volatile oils from Schinus terebinthifolius Raddi. Quim. Nova 2007, 30, 1959–1965. [Google Scholar] [CrossRef]
- de Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445. [Google Scholar] [CrossRef]
- Velázquez, E.; Tournier, H.; Mordujovich de Buschiazzo, P.; Saavedra, G.; Schinella, G. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Schmourlo, G.; Mendonça-Filho, R.R.; Alviano, C.S.; Costa, S.S. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J. Ethnopharmacol. 2005, 96, 563–568. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.R.F.; de Souza Luna, J.; dos Santos, A.F.; de Andrade, M.C.C.; Sant’Ana, A.E.G.; Genet, J.-P.; Marquez, B.; Neuville, L.; Moreau, N. Anti-bacterial activity of some Brazilian medicinal plants. J. Ethnopharmacol. 2006, 105, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and antioxidant evaluation of essential oil from Eugenia uniflora L., curzerene-rich, thermally produced in situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.V.L.; de Nazaré S. da Cruz, E.; de Sousa Barroso, A.; Mourão, R.H.V.; Setzer, W.N.; da Silva, J.K.; do Nascimento, W.M.O.; da Costa, J.S.; Figueiredo, P.L.B. Chemometric analysis of the seasonal variation in the essential oil composition of Psidium acutangulum growing in the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104528. [Google Scholar] [CrossRef]
- Ennigrou, A.; Hosni, K.; Casabianca, H.; Vulliet, E.; Smiti, S. Leaf volatile oil constituents of Schinus terebinthifolius and Schinus molle from Tunisia. In Proceedings of the 6th Baltic Conference on Food Science and Technology FOODBALT-2011, Jelgava, Latvia, 5–6 May 2011; pp. 5–6. Available online: https://llufb.llu.lv/conference/foodbalt/2011/FOODBALT-Proceedings-2011-90-92.pdf (accessed on 28 June 2023).
- Santana, J.S.; Sartorelli, P.; Guadagnin, R.C.; Matsuo, A.L.; Figueiredo, C.R.; Soares, M.G.; da Silva, A.M.; Lago, J.H.G. Essential oils from Schinus terebinthifolius leaves–Chemical composition and in vitro cytotoxicity evaluation. Pharm. Biol. 2012, 50, 1248–1253. [Google Scholar] [CrossRef]
- Santos, M.R.A.; Lima, R.A.; Silva, A.; Lima, D.K.S.; Sallet, L.A.P.; Teixeira, C.A.D.; Facundo, V.A. Composição química e atividade inseticida do óleo essencial de Schinus terebinthifolius Raddi (Anacardiaceae) sobre a broca-do-café (Hypothenemus hampei) ferrari. Rev. Bras. Plantas Med. 2013, 15, 757–762. [Google Scholar] [CrossRef]
- Cole, E.R.; dos Santos, R.B.; Lacerda Júnior, V.; Martins, J.D.L.; Greco, S.J.; Cunha Neto, A. Chemical composition of essential oil from ripe fruit of Schinus terebinthifolius Raddi and evaluation of its activity against wild strains of hospital origin. Braz. J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef]
- da Cruz, E.d.N.S.; Peixoto, L.d.S.; da Costa, J.S.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal variability of a caryophyllane chemotype essential oil of Eugenia patrisii Vahl occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef]
- Frank, L.; Wenig, M.; Ghirardo, A.; Krol, A.; Vlot, A.C.; Schnitzler, J.; Rosenkranz, M. Isoprene and β-caryophyllene confer plant resistance via different plant internal signalling pathways. Plant. Cell Environ. 2021, 44, 1151–1164. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H. Essential oils composition and antioxidant properties of three Thymus species. Evid.-Based Complement. Altern. Med. 2012, 2012, 728065. [Google Scholar] [CrossRef]
- da Silva Mendonça, J.; de Cássia Avellaneda Guimarães, R.; Zorgetto-Pinheiro, V.A.; Di Pietro Fernandes, C.; Marcelino, G.; Bogo, D.; de Cássia Freitas, K.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural antioxidant evaluation: A review of detection methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef] [PubMed]
- Junior, M.R.M.; Silva, T.A.A.R.; Franchi, G.C.; Nowill, A.; Pastore, G.M.; Hyslop, S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chem. 2009, 116, 8–12. [Google Scholar] [CrossRef]
- Emami, S.A.; Abedindo, B.F.; Hassanzadeh-Khayyat, M. Antioxidant activity of the essential oils of different parts of Juniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae). Iran. J. Pharm. Res. 2011, 10, 799–810. [Google Scholar] [PubMed]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- (INMET) Instituto Nacional de Metereologia. Available online: http://www.inmet.gov.br/portal (accessed on 23 July 2022).
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of Citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- de Sousa Peixoto Barros, L.; de Nazaré Santos da Cruz, E.; de Araújo Guimarães, B.; Setzer, W.N.; Veras Mourão, R.H.; do Rosário da Silva, J.K.; Silva da Costa, J.; Baia Figueiredo, P.L. Chemometric analysis of the seasonal variation in the essential oil composition and antioxidant activity of a new geraniol chemotype of Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson from the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104503. [Google Scholar] [CrossRef]

| Parameter | Essential Oil Yield | δ-3-Carene | Limonene | (E)-Caryophyllene |
|---|---|---|---|---|
| Temperature | −0.22 | −0.33 | −0.40 | 0.43 |
| Humidity | 0.19 | 0.32 | 0.40 | −0.37 |
| Insolation | −0.26 | −0.35 | −0.44 | 0.39 |
| Precipitation | 0.43 | 0.46 | 0.56 * | −0.54 |
| Sample | Inhibition (%) |
|---|---|
| October | 26.16 ± 3.69 a,b |
| November | 24.58 ± 3.56 a |
| December | 28.29 ± 1.57 a,b |
| January | 26.00 ± 2.90 a |
| February | 24.90 ± 2.04 a |
| March | 22.77 ± 4.37 a |
| April | 30.65 ± 1.35 a,b,c |
| May | 24.04 ± 2.28 a |
| June | 37.62 ± 2.74 c,d |
| August | 44.15 ±3.05 d |
| September | 34.31 ± 2.29 b,c |
| Trolox | 82.93 ± 1.82 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, B.d.A.; Silva, R.C.; Andrade, E.H.d.A.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach. Plants 2023, 12, 2497. https://doi.org/10.3390/plants12132497
Guimarães BdA, Silva RC, Andrade EHdA, Setzer WN, da Silva JK, Figueiredo PLB. Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach. Plants. 2023; 12(13):2497. https://doi.org/10.3390/plants12132497
Chicago/Turabian StyleGuimarães, Bruna de Araújo, Renata Cunha Silva, Eloisa Helena de Aguiar Andrade, William N. Setzer, Joyce Kelly da Silva, and Pablo Luis B. Figueiredo. 2023. "Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach" Plants 12, no. 13: 2497. https://doi.org/10.3390/plants12132497
APA StyleGuimarães, B. d. A., Silva, R. C., Andrade, E. H. d. A., Setzer, W. N., da Silva, J. K., & Figueiredo, P. L. B. (2023). Seasonality, Composition, and Antioxidant Capacity of Limonene/δ-3-Carene/(E)-Caryophyllene Schinus terebinthifolia Essential Oil Chemotype from the Brazilian Amazon: A Chemometric Approach. Plants, 12(13), 2497. https://doi.org/10.3390/plants12132497










