A High-Continuity Genome Assembly of Chinese Flowering Cabbage (Brassica rapa var. parachinensis) Provides New Insights into Brassica Genome Structure Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and Sequencing
2.3. Genome Size Estimation Based on NGS Sequencing Data
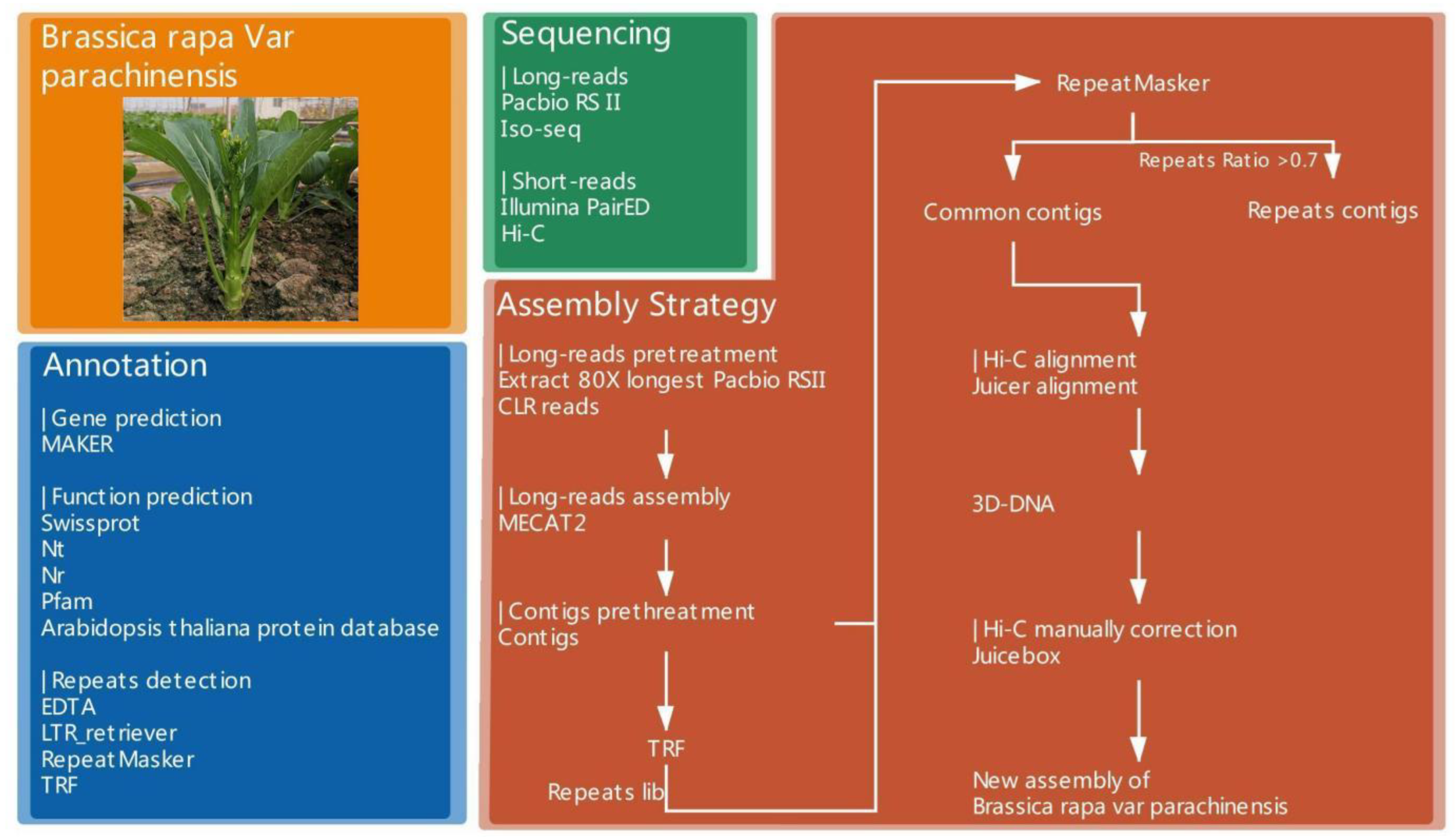
2.4. De Novo Assembly of the Chinese Flowering Cabbage Genome
2.5. Hi-C Library Preparation and Data Analysis
2.6. Single Molecule RNA Sequencing (Iso-Seq) Experiment and Data Analysis
2.7. Repetitive Element Annotation and Construction of Circos Picture
2.8. Protein Coding Gene Prediction
2.9. Phylogenetic Analysis
2.10. Structural Variants Analysis
3. Results
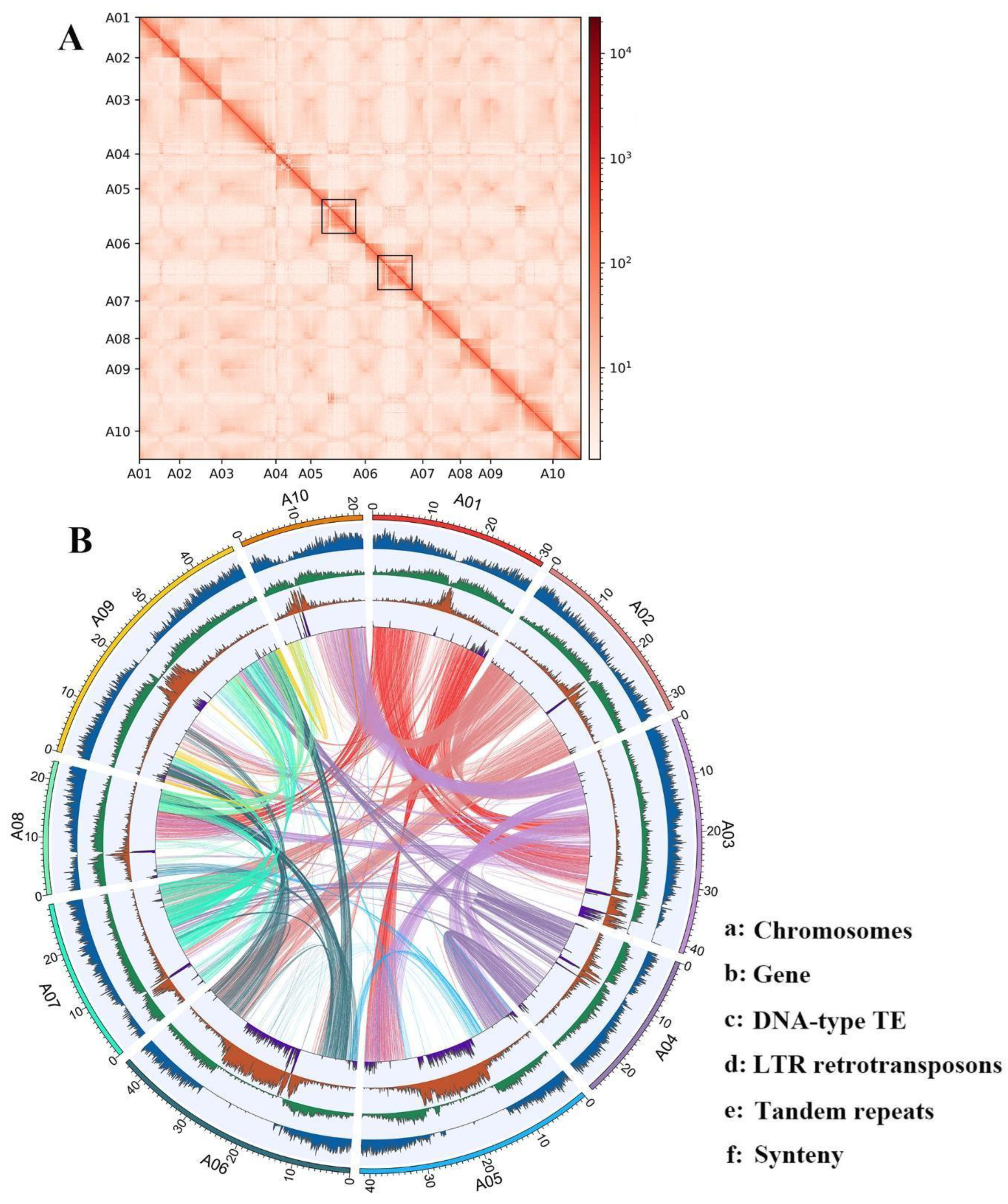
3.1. A Highly Continuous Genome Assembly of Chinese Flowering Cabbage (B. rapa var. parachinensis)
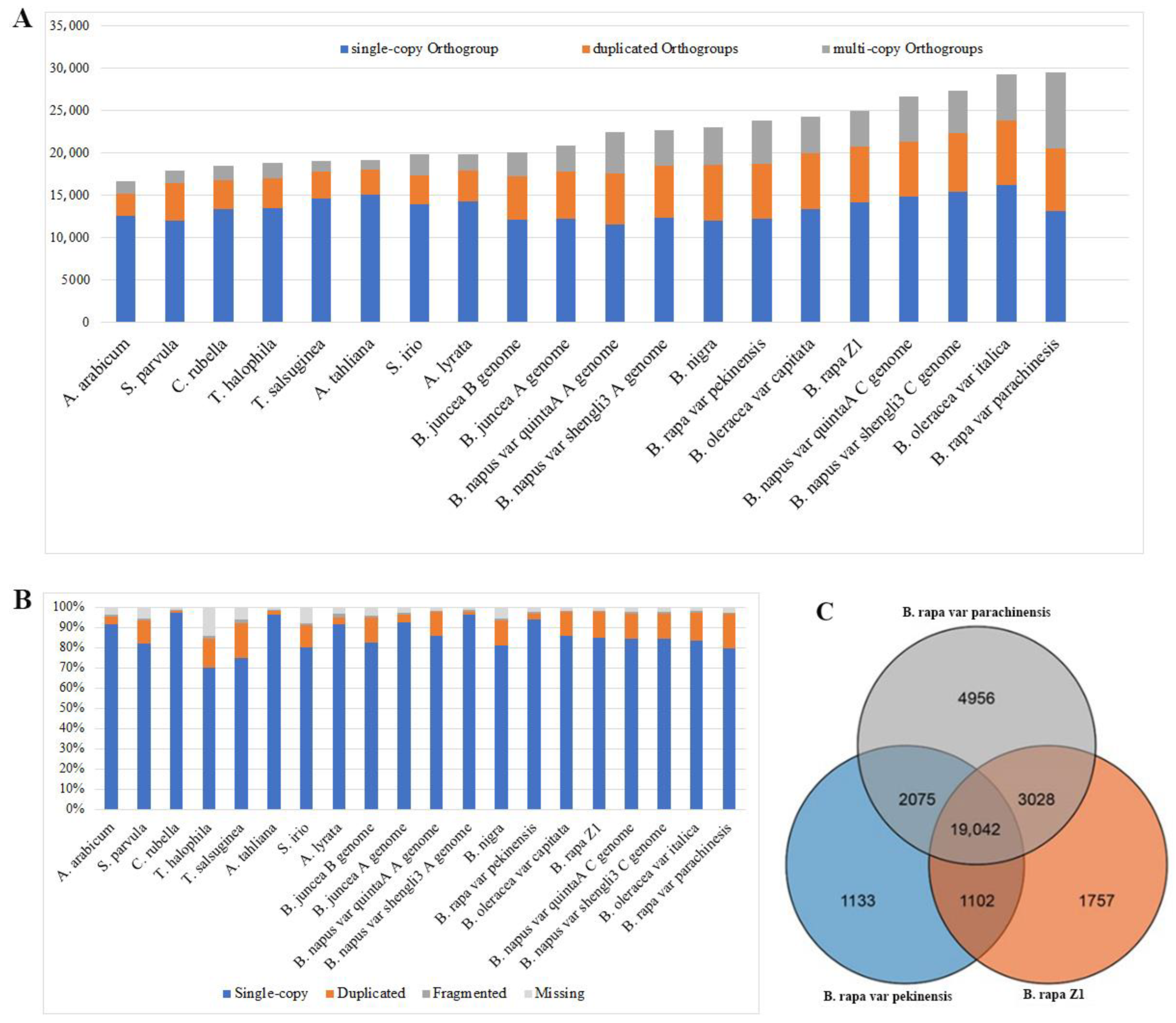
3.2. Gene Duplication Analysis across 20 Eudicot Genomes Reveals the Current B. rapa var. parachinensis Genome Is among the Most High-Quality Assemblies of Brassica Genomes
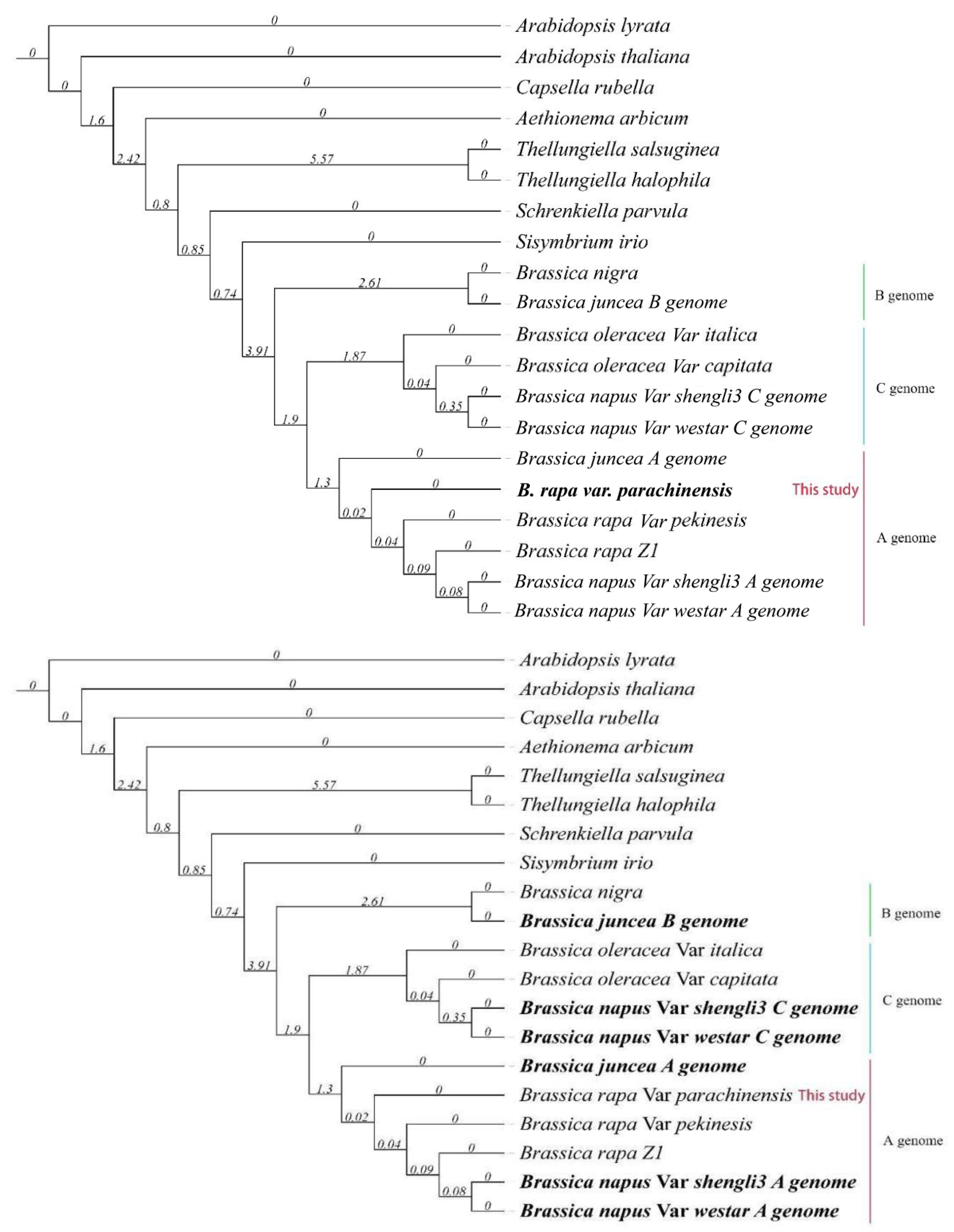
3.3. Phylogenetic Analysis of a Collection of Brassica Genomes Reveals That the Chinese Flowering Cabbage Has a Closer Evolutionary Relationship with the Diploid Progenitor of the Allotetraploid Species, B. Juncea
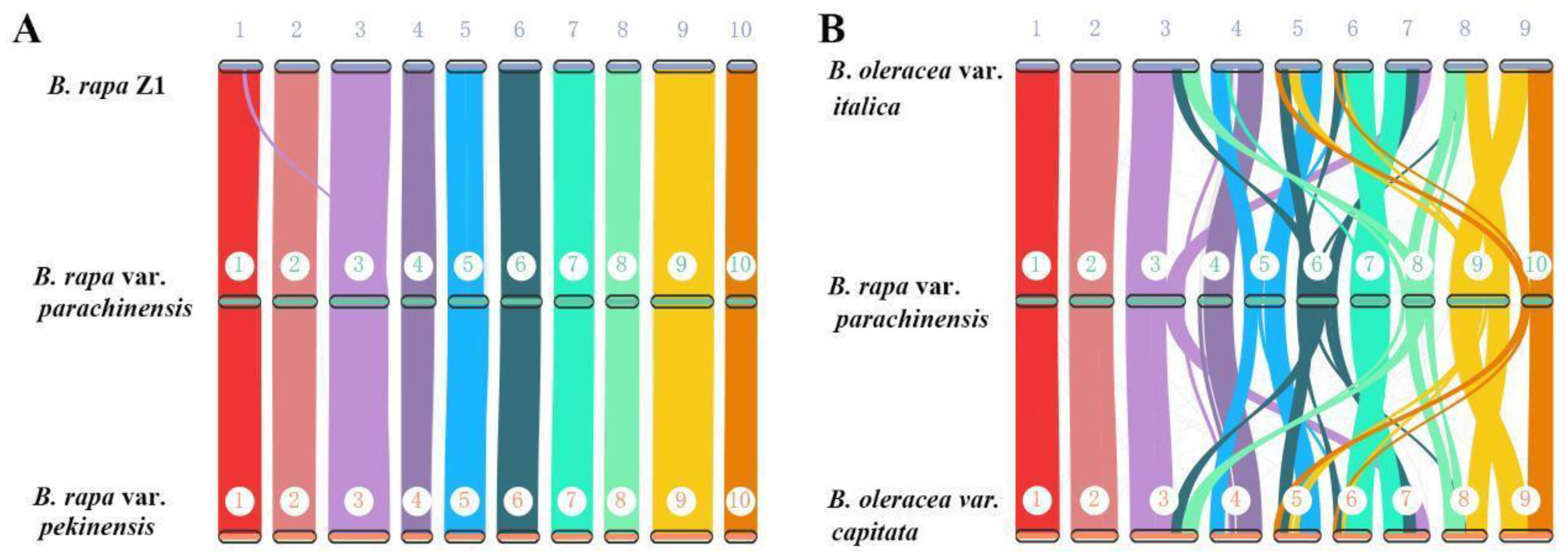
3.4. Extensive Chromosomal Arrangements between Brassica Species
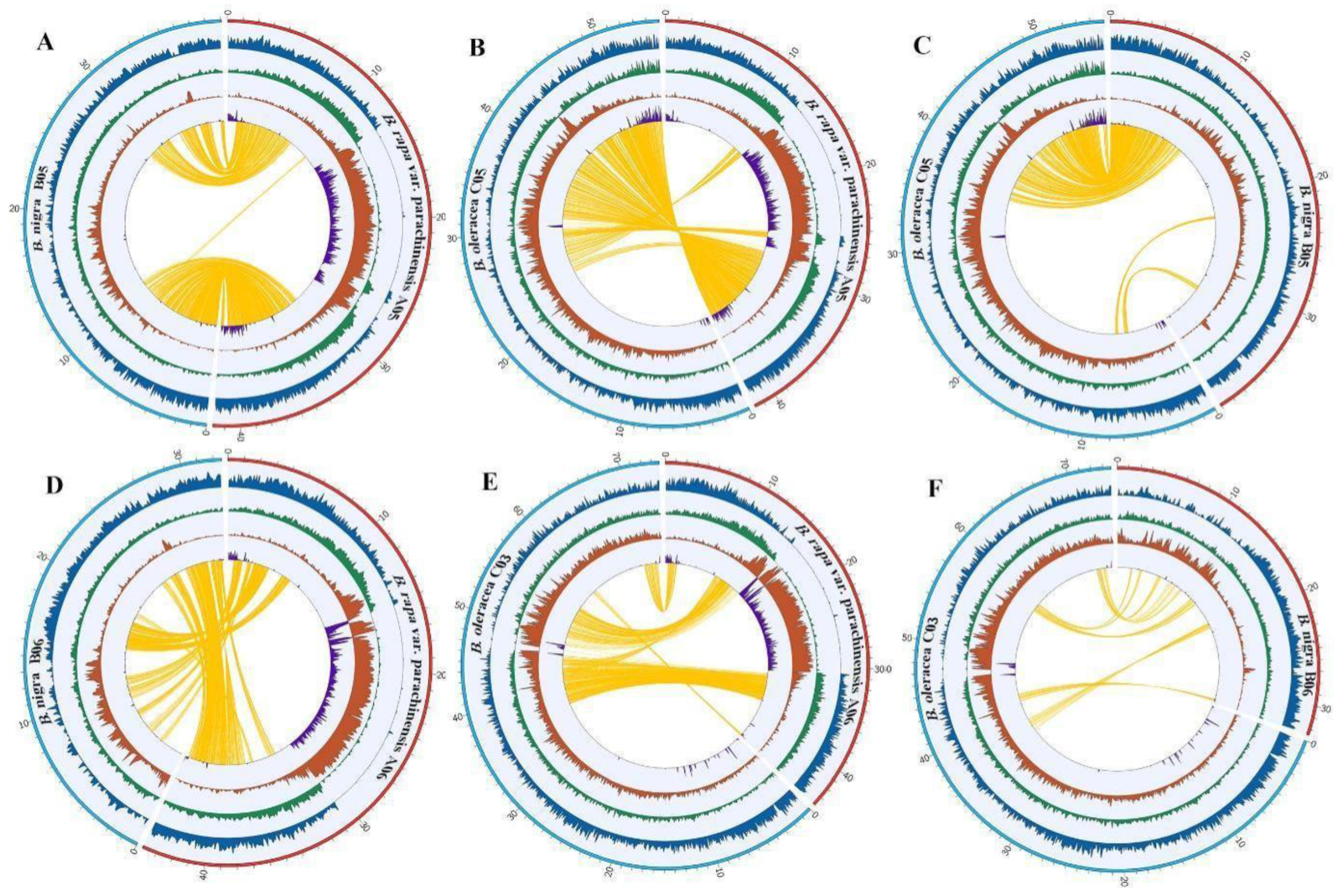
3.5. Genome Structure Evolution in Brassica: Insight from Pericentromeric Regions
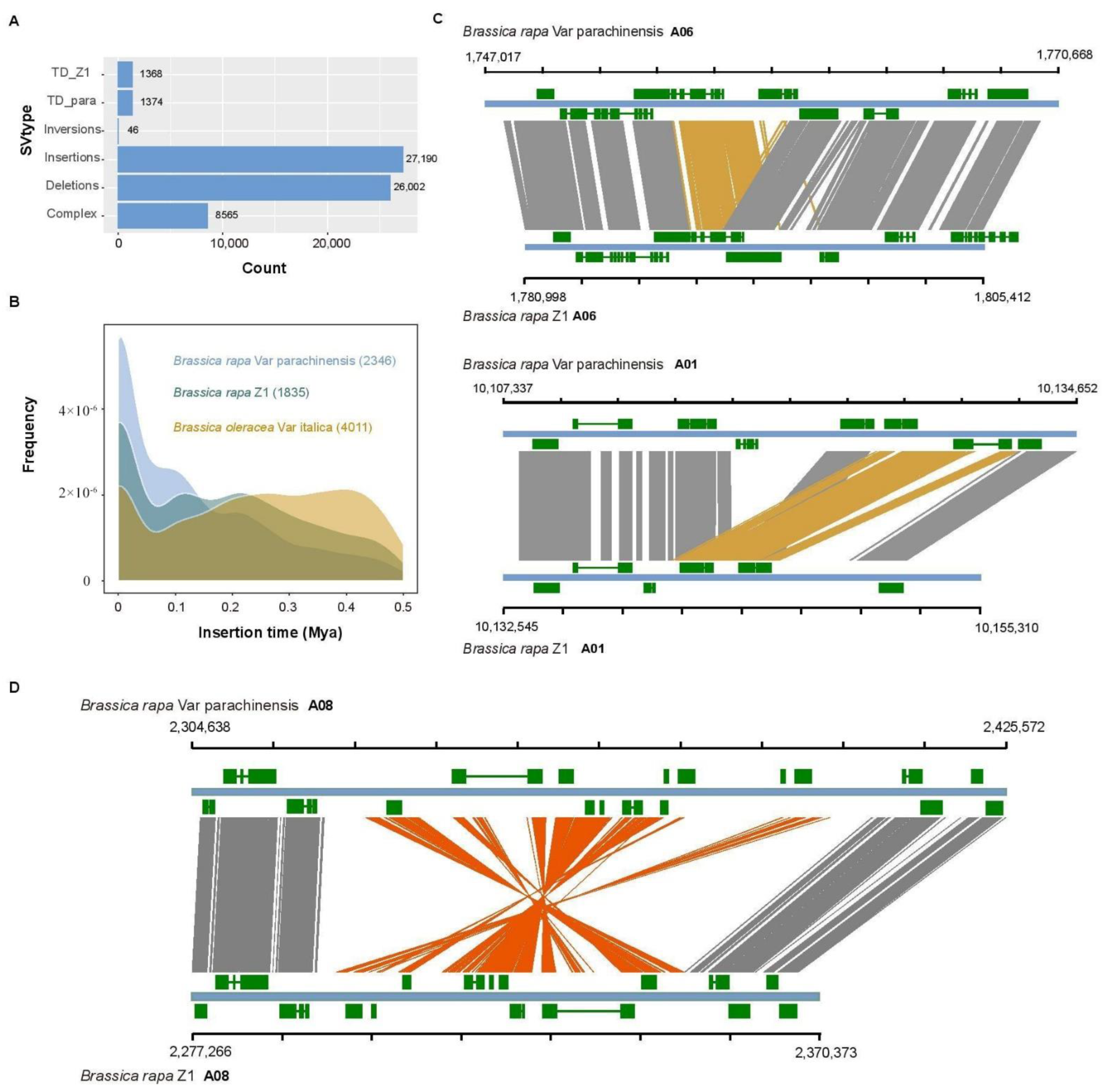
3.6. Structural Variants in Brassica Genomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lysak, M.A.; Koch, M.A.; Pecinka, A.; Schubert, I. Chromosome Triplication Found across the Tribe Brassiceae. Genome Res. 2005, 15, 516–525. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome Parallel Selection Is Associated with Morphotype Diversification and Convergent Crop Domestication in Brassica Rapa and Brassica Oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef]
- Wang, W.; Guan, R.; Liu, X.; Zhang, H.; Song, B.; Xu, Q.; Fan, G.; Chen, W.; Wu, X.; Liu, X.; et al. Chromosome Level Comparative Analysis of Brassica Genomes. Plant Mol. Biol. 2019, 99, 237–249. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. Author Correction: The Genome Sequence of Allopolyploid Brassica Juncea and Analysis of Differential Homoeolog Gene Expression Influencing Selection. Nat. Genet. 2018, 50, 1616. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The Genome of the Mesopolyploid Crop Species Brassica Rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.P.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica Oleracea Genome Reveals the Asymmetrical Evolution of Polyploid Genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]
- Parkin, I.A.P.; Koh, C.; Tang, H.; Robinson, S.J.; Kagale, S.; Clarke, W.E.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L.; et al. Transcriptome and Methylome Profiling Reveals Relics of Genome Dominance in the Mesopolyploid Brassica Oleracea. Genome Biol. 2014, 15, R77. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Plant Genetics. Early Allopolyploid Evolution in the Post-Neolithic Brassica Napus Oilseed Genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Bayer, P.E.; Hurgobin, B.; Golicz, A.A.; Chan, C.-K.K.; Yuan, Y.; Lee, H.; Renton, M.; Meng, J.; Li, R.; Long, Y.; et al. Assembly and Comparison of Two Closely Related Brassica Napus Genomes. Plant Biotechnol. J. 2017, 15, 1602–1610. [Google Scholar] [CrossRef]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The High-Quality Genome of Brassica Napus Cultivar “ZS11” Reveals the Introgression History in Semi-Winter Morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Belser, C.; Istace, B.; Denis, E.; Dubarry, M.; Baurens, F.-C.; Falentin, C.; Genete, M.; Berrabah, W.; Chèvre, A.-M.; Delourme, R.; et al. Chromosome-Scale Assemblies of Plant Genomes Using Nanopore Long Reads and Optical Maps. Nat Plants 2018, 4, 879–887. [Google Scholar] [CrossRef]
- Sun, D.; Wang, C.; Zhang, X.; Zhang, W.; Jiang, H.; Yao, X.; Liu, L.; Wen, Z.; Niu, G.; Shan, X. Draft Genome Sequence of Cauliflower ( L. Var. ) Provides New Insights into the C Genome in Species. Hortic Res 2019, 6, 82. [Google Scholar] [CrossRef]
- Song, J.-M.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R.; et al. Eight High-Quality Genomes Reveal Pan-Genome Architecture and Ecotype Differentiation of Brassica Napus. Nat Plants 2020, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Woody, S.; Greenham, K.; VanBuren, R.; Colle, M.; Edger, P.P.; Sartor, R.; Zheng, Y.; Levendoski, N.; Lim, J.; et al. Genetic and Genomic Resources to Study Natural Variation in. Plant Direct 2020, 4, e00285. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Rahim, M.A.; Zhao, Y.; Yang, S.; Wang, Z.; Su, H.; Li, L.; Niu, L.; Harun-Ur-Rashid, M.; Yuan, Y.; et al. Comparative Transcriptome Analysis of Early- and Late-Bolting Traits in Chinese Cabbage (). Front. Genet. 2021, 12, 590830. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, X.; Wu, J.; Liu, M.; Grob, S.; Cheng, F.; Liang, J.; Cai, C.; Liu, Z.; Liu, B.; et al. Erratum: Author Correction: Improved Reference Genome by Single-Molecule Sequencing and Chromosome Conformation Capture Technologies. Hortic Res 2019, 6, 124. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Zhao, X.; Wang, W.; Zhang, D.; Yu, Y.; Bayer, P.E.; Edwards, D.; Yu, S.; Zhang, F. Assembly of the Non-Heading Pak Choi Genome and Comparison with the Genomes of Heading Chinese Cabbage and the Oilseed Yellow Sarson. Plant Biotechnol. J. 2021, 19, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, C.; Shao, G.; Wu, S.; Liu, P.; Cao, P.; Jiang, P.; Wang, S.; Zhu, H.; Lin, X.; et al. The Reference Genome and Full-Length Transcriptome of Pakchoi Provide Insights into Cuticle Formation and Heat Adaption. Hortic Res 2022, 9, uhac123. [Google Scholar] [CrossRef]
- Tan, X.-L.; Fan, Z.-Q.; Kuang, J.-F.; Lu, W.-J.; Reiter, R.J.; Lakshmanan, P.; Su, X.-G.; Zhou, J.; Chen, J.-Y.; Shan, W. Melatonin Delays Leaf Senescence of Chinese Flowering Cabbage by Suppressing ABFs-Mediated Abscisic Acid Biosynthesis and Chlorophyll Degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Xiao, X.-M.; Xu, Y.-M.; Zeng, Z.-X.; Tan, X.-L.; Liu, Z.-L.; Chen, J.-W.; Su, X.-G.; Chen, J.-Y. Activation of the Transcription of by a BrTCP21 Transcription Factor Is Associated with Gibberellin-Delayed Leaf Senescence in Chinese Flowering Cabbage during Storage. Int. J. Mol. Sci. 2019, 20, 3860. [Google Scholar] [CrossRef]
- Kamran, M.; Xie, K.; Sun, J.; Wang, D.; Shi, C.; Lu, Y.; Gu, W.; Xu, P. Modulation of Growth Performance and Coordinated Induction of Ascorbate-Glutathione and Methylglyoxal Detoxification Systems by Salicylic Acid Mitigates Salt Toxicity in Choysum (Brassica Parachinensis L.). Ecotoxicol. Environ. Saf. 2020, 188, 109877. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Liu, F.; Wu, J.; Zou, J.; Xiao, X.; Zhao, F.; Zhu, B. HTQC: A Fast Quality Control Toolkit for Illumina Sequencing Data. BMC Bioinformatics 2013, 14, 33. [Google Scholar] [CrossRef]
- Xiao, C.-L.; Chen, Y.; Xie, S.-Q.; Chen, K.-N.; Wang, Y.; Han, Y.; Luo, F.; Xie, Z. MECAT: Fast Mapping, Error Correction, and de Novo Assembly for Single-Molecule Sequencing Reads. Nat. Methods 2017, 14, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS One 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem Repeats Finder: A Program to Analyze DNA Sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [PubMed]
- Yang, X.; Liu, H.; Ma, Z.; Zou, Y.; Zou, M.; Mao, Y.; Li, X.; Wang, H.; Chen, T.; Wang, W.; et al. Chromosome-Level Genome Assembly of Triplophysa Tibetana, a Fish Adapted to the Harsh High-Altitude Environment of the Tibetan Plateau. Mol. Ecol. Resour. 2019, 19, 1027–1036. [Google Scholar] [CrossRef]
- Durand, N.C.; Shamim, M.S.; Machol, I.; Rao, S.S.P.; Huntley, M.H.; Lander, E.S.; Aiden, E.L. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst 2016, 3, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, O.; Batra, S.S.; Omer, A.D.; Nyquist, S.K.; Hoeger, M.; Durand, N.C.; Shamim, M.S.; Machol, I.; Lander, E.S.; Aiden, A.P.; et al. De Novo Assembly of the Genome Using Hi-C Yields Chromosome-Length Scaffolds. Science 2017, 356, 92–95. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Cai, C.; Wang, X.; Liu, B.; Wu, J.; Liang, J.; Cui, Y.; Cheng, F.; Wang, X. Brassica Rapa Genome 2.0: A Reference Upgrade through Sequence Re-Assembly and Gene Re-Annotation. Mol. Plant 2017, 10, 649–651. [Google Scholar] [CrossRef]
- Vasimuddin, M.; Misra, S.; Li, H.; Aluru, S. Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems. In Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 20–24 May 2019. [Google Scholar]
- Wolff, J.; Bhardwaj, V.; Nothjunge, S.; Richard, G.; Renschler, G.; Gilsbach, R.; Manke, T.; Backofen, R.; Ramírez, F.; Grüning, B.A. Galaxy HiCExplorer: A Web Server for Reproducible Hi-C Data Analysis, Quality Control and Visualization. Nucleic Acids Res. 2018, 46, W11–W16. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Su, W.; Liao, Y.; Chougule, K.; Agda, J.R.A.; Hellinga, A.J.; Lugo, C.S.B.; Elliott, T.A.; Ware, D.; Peterson, T.; et al. Author Correction: Benchmarking Transposable Element Annotation Methods for Creation of a Streamlined, Comprehensive Pipeline. Genome Biol. 2022, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Korf, I.; Robb, S.M.C.; Parra, G.; Ross, E.; Moore, B.; Holt, C.; Sánchez Alvarado, A.; Yandell, M. MAKER: An Easy-to-Use Annotation Pipeline Designed for Emerging Model Organism Genomes. Genome Res. 2008, 18, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Kent, W.J.; Smit, A.; Zhang, Z.; Baertsch, R.; Hardison, R.C.; Haussler, D.; Miller, W. Human-Mouse Alignments with BLASTZ. Genome Res. 2003, 13, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Baertsch, R.; Hinrichs, A.; Miller, W.; Haussler, D. Evolution’s Cauldron: Duplication, Deletion, and Rearrangement in the Mouse and Human Genomes. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 11484–11489. [Google Scholar] [CrossRef]
- Harris, R.S. Improved Pairwise Alignment of Genomic DNA; Pennsylvania State University: University Park, PA, USA, 2007. [Google Scholar]
- Liao, Y.; Zhang, X.; Chakraborty, M.; Emerson, J.J. Topologically Associating Domains and Their Role in the Evolution of Genome Structure and Function in. Genome Res. 2021, 31, 397–410. [Google Scholar] [CrossRef]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative Evolutionary Analysis of Chalcone Synthase and Alcohol Dehydrogenase Loci in Arabidopsis, Arabis, and Related Genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving Fundamental Biases in Whole Genome Comparisons Dramatically Improves Orthogroup Inference Accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.C.; Yu, Y.; Copetti, D.; Zwickl, D.J.; Zhang, L.; Zhang, C.; Chougule, K.; Gao, D.; Iwata, A.; Goicoechea, J.L.; et al. Publisher Correction: Genomes of 13 Domesticated and Wild Rice Relatives Highlight Genetic Conservation, Turnover and Innovation across the Genus Oryza. Nat. Genet. 2018, 50, 1618. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, X.; Li, B.; Liu, T.; Chen, J.; Bai, Z.; Wang, M.; Shi, J.; Walling, J.G.; Wing, R.A.; et al. Comparison of Oryza Sativa and Oryza Brachyantha Genomes Reveals Selection-Driven Gene Escape from the Centromeric Regions. Plant Cell 2018, 30, 1729–1744. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.R.; Chebotarov, D.; Duitama, J.; Smith, S.; De la Hoz, J.F.; Mohiyuddin, M.; Wing, R.A.; McNally, K.L.; Tatarinova, T.; Grigoriev, A.; et al. Structural Variants in 3000 Rice Genomes. Genome Res. 2019, 29, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.L.; Schneider, A.M.; Lu, Y.; Niranjan, T.; Shen, P.; Robinson, M.A.; Steranka, J.P.; Valle, D.; Civin, C.I.; Wang, T.; et al. Mobile Interspersed Repeats Are Major Structural Variants in the Human Genome. Cell 2010, 141, 1171–1182. [Google Scholar] [CrossRef]
- Audano, P.A.; Sulovari, A.; Graves-Lindsay, T.A.; Cantsilieris, S.; Sorensen, M.; Welch, A.E.; Dougherty, M.L.; Nelson, B.J.; Shah, A.; Dutcher, S.K.; et al. Characterizing the Major Structural Variant Alleles of the Human Genome. Cell 2019, 176, 663–675.e19. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gracz-Bernaciak, J.; Żywicki, M.; Karłowski, W.; Twardowski, T.; Tyczewska, A. Identification of Structural Variants in Two Novel Genomes of Maize Inbred Lines Possibly Related to Glyphosate Tolerance. Plants 2020, 9, 523. [Google Scholar] [CrossRef]
- Voichek, Y.; Weigel, D. Identifying Genetic Variants Underlying Phenotypic Variation in Plants without Complete Genomes. Nat. Genet. 2020, 52, 534–540. [Google Scholar] [CrossRef]
- Cai, X.; Chang, L.; Zhang, T.; Chen, H.; Zhang, L.; Lin, R.; Liang, J.; Wu, J.; Freeling, M.; Wang, X. Impacts of Allopolyploidization and Structural Variation on Intraspecific Diversification in Brassica Rapa. Genome Biol. 2021, 22, 166. [Google Scholar] [CrossRef]
| Number | Size | Sequence Coverage (X) | Percentage (%) | |
|---|---|---|---|---|
| Estimate of genome size | 515 Mb | |||
| PacBio reads | 4,448,280 | 113,068 Mb | 219.31 | |
| PacBio reads N50 | 28,414 b | |||
| 80X PacBio reads N50 | 43,902 b | |||
| Illumina reads | 322,016,292 | 42,330 Mb | 82.10 | |
| HiC reads | 441,545,786 | 66,231 Mb | 128.46 | |
| Total reads | 221,630 Mb | 429.89 | ||
| Contigs | 450 | 384 Mb | 74.50 | |
| N50 of contigs | 7.2 Mb | |||
| Longest contig | 19.9 M | |||
| scaffold | 69 | 384 Mb | 74.62 | |
| N50 of scaffold | 32.2 Mb | |||
| Longest scaffold | 47.5 Mb | |||
| GC content | 144.4 Mb | 37.61 | ||
| Total repetitive sequences | 170.3 Mb | 44.26 | ||
| Total protein-coding genes | 47,598 | 47.3 Mb | 12.31 | |
| Average length per gene | 2060 bp | |||
| Average exons per gene | 6.13 | 199 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Jiang, D.; Wang, J.; Liao, Y.; Zhang, T.; Zhang, H.; Dai, X.; Ren, H.; Chen, C.; Zheng, Y. A High-Continuity Genome Assembly of Chinese Flowering Cabbage (Brassica rapa var. parachinensis) Provides New Insights into Brassica Genome Structure Evolution. Plants 2023, 12, 2498. https://doi.org/10.3390/plants12132498
Li G, Jiang D, Wang J, Liao Y, Zhang T, Zhang H, Dai X, Ren H, Chen C, Zheng Y. A High-Continuity Genome Assembly of Chinese Flowering Cabbage (Brassica rapa var. parachinensis) Provides New Insights into Brassica Genome Structure Evolution. Plants. 2023; 12(13):2498. https://doi.org/10.3390/plants12132498
Chicago/Turabian StyleLi, Guangguang, Ding Jiang, Juntao Wang, Yi Liao, Ting Zhang, Hua Zhang, Xiuchun Dai, Hailong Ren, Changming Chen, and Yansong Zheng. 2023. "A High-Continuity Genome Assembly of Chinese Flowering Cabbage (Brassica rapa var. parachinensis) Provides New Insights into Brassica Genome Structure Evolution" Plants 12, no. 13: 2498. https://doi.org/10.3390/plants12132498
APA StyleLi, G., Jiang, D., Wang, J., Liao, Y., Zhang, T., Zhang, H., Dai, X., Ren, H., Chen, C., & Zheng, Y. (2023). A High-Continuity Genome Assembly of Chinese Flowering Cabbage (Brassica rapa var. parachinensis) Provides New Insights into Brassica Genome Structure Evolution. Plants, 12(13), 2498. https://doi.org/10.3390/plants12132498






