Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control
Abstract
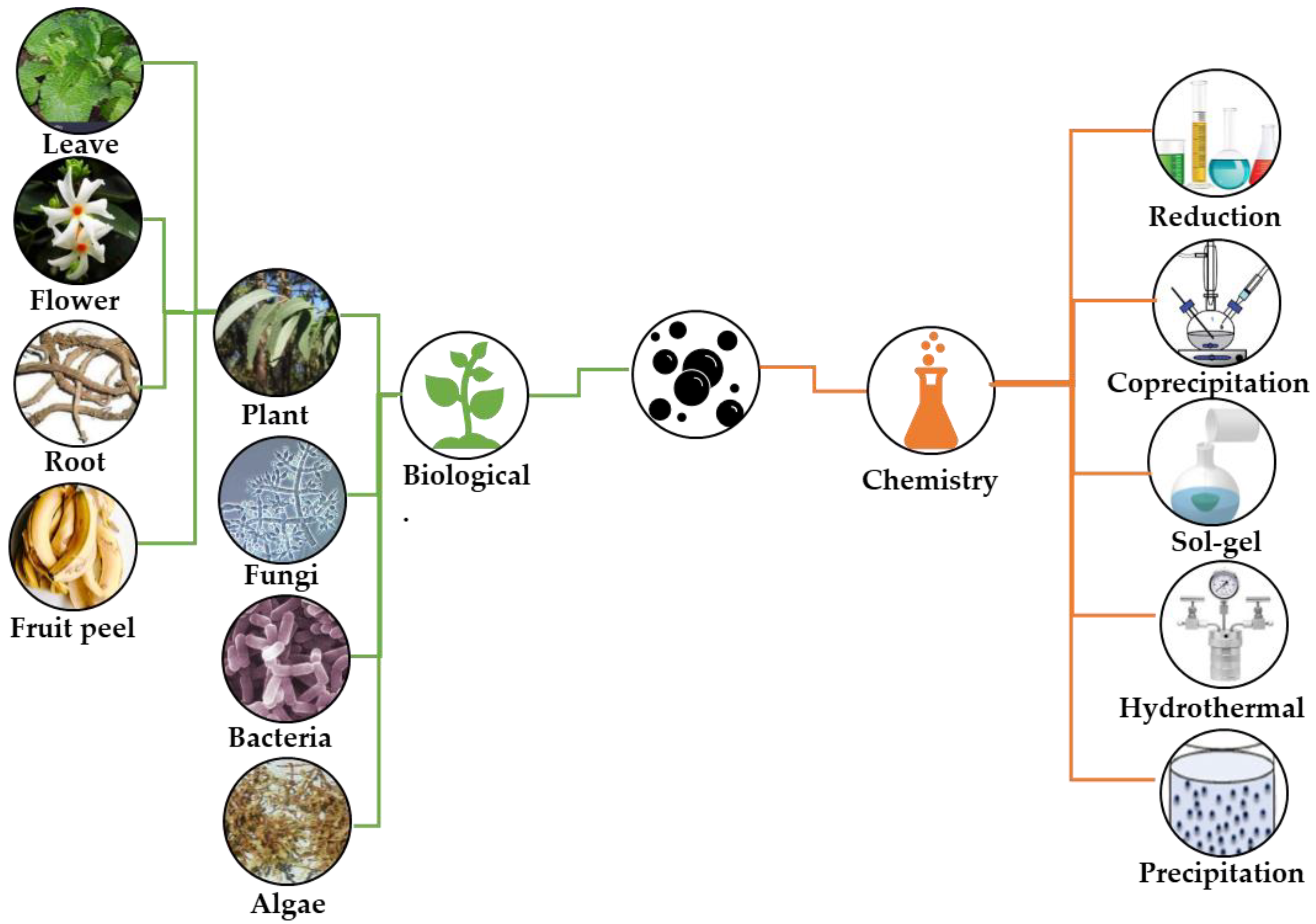
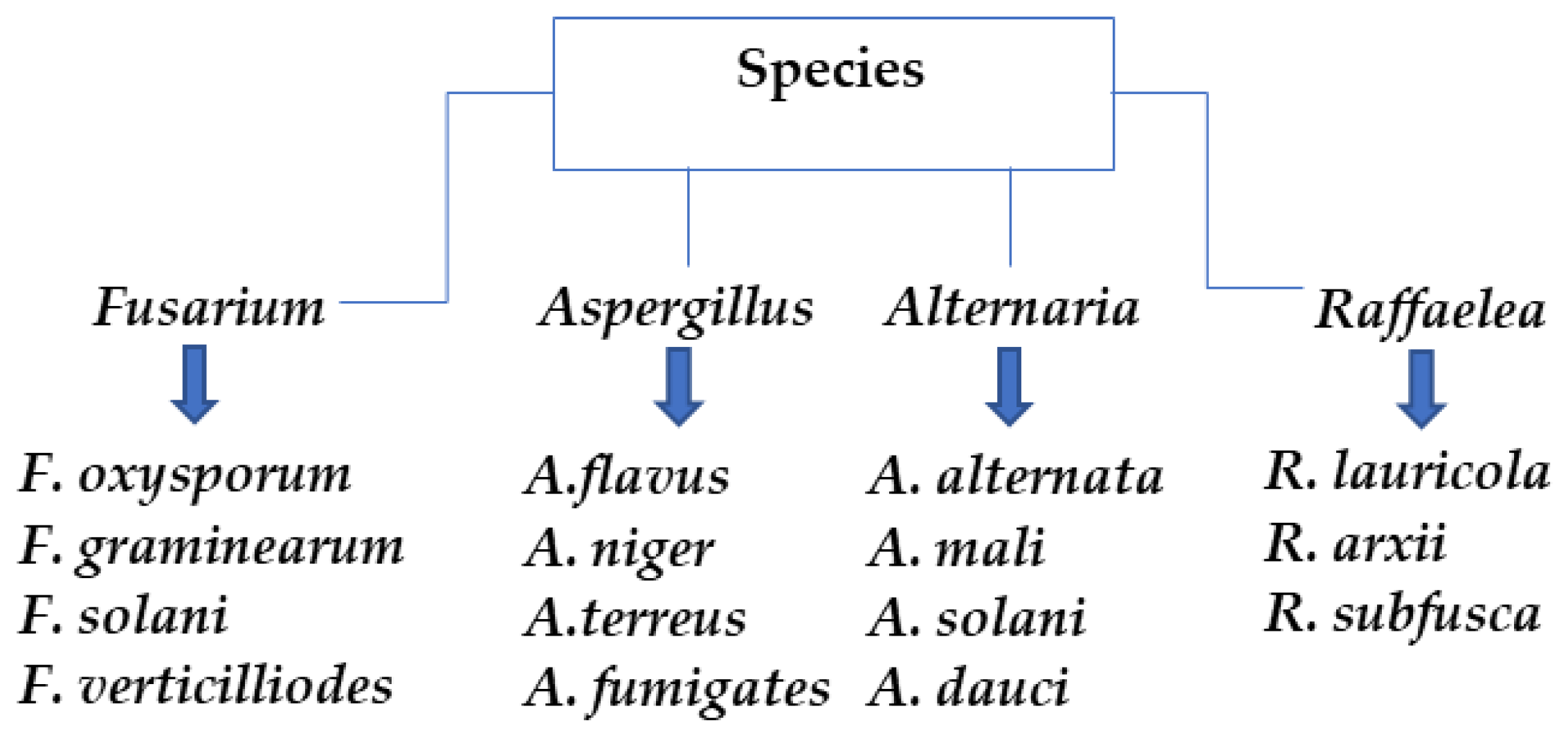
1. Introduction
2. Antifungal Properties of Mono-Metal Oxide Nanoparticles
2.1. Zinc Oxide Nanoparticles
2.2. Copper Oxide Nanoparticles
2.3. Iron oxide Nanoparticles
2.4. Magnesium Oxide Nanoparticles
2.5. Titanium Oxide Nanoparticles
2.6. Other Types of Mono-Metal Oxide Nanoparticles
3. Antifungal Properties of Bi-Metal and Tri-Metal Oxide Nanoparticles
3.1. Bi-Metal Oxide Nanoparticles
3.2. Tri-Metal Oxide Nanoparticles
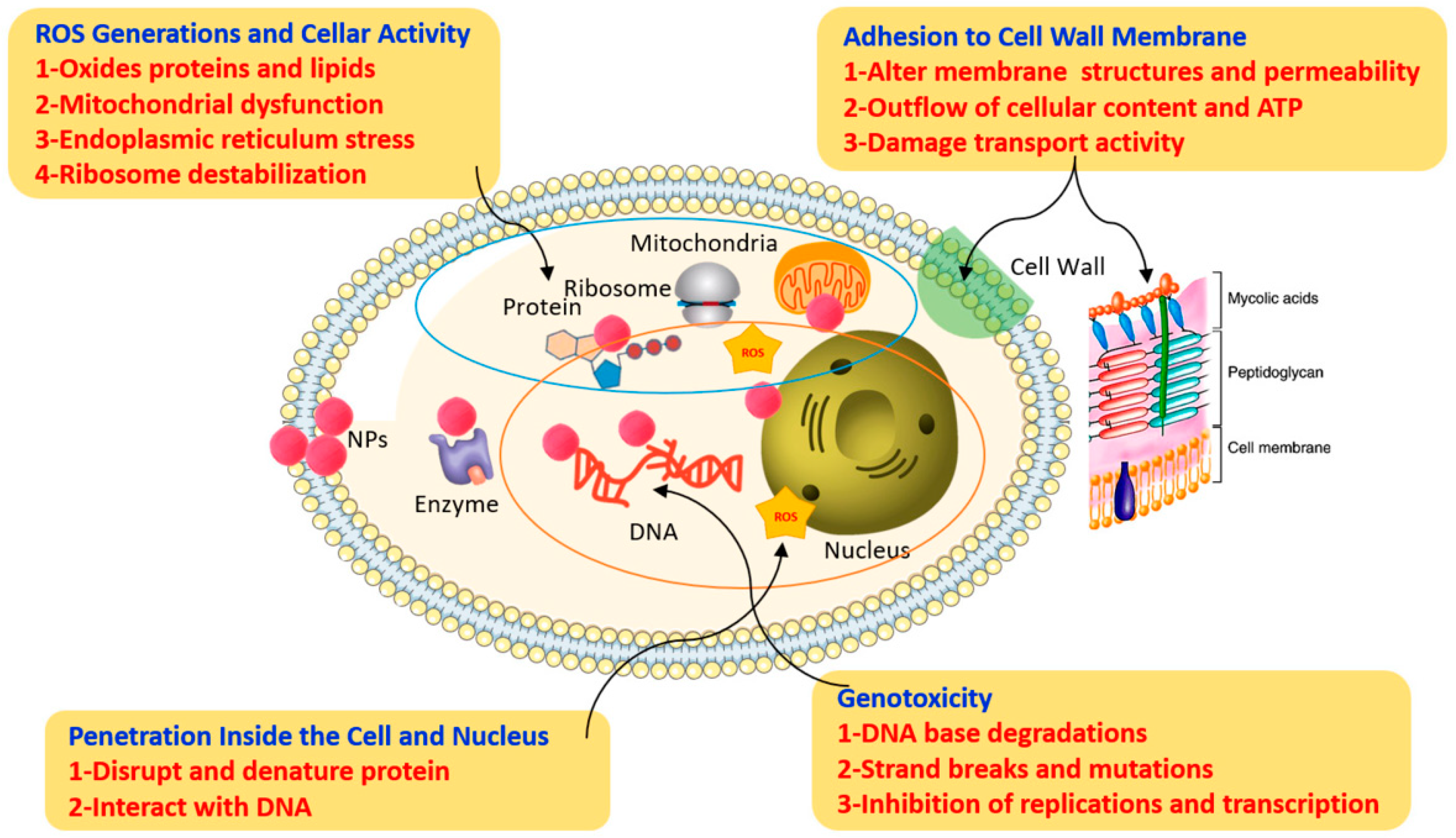
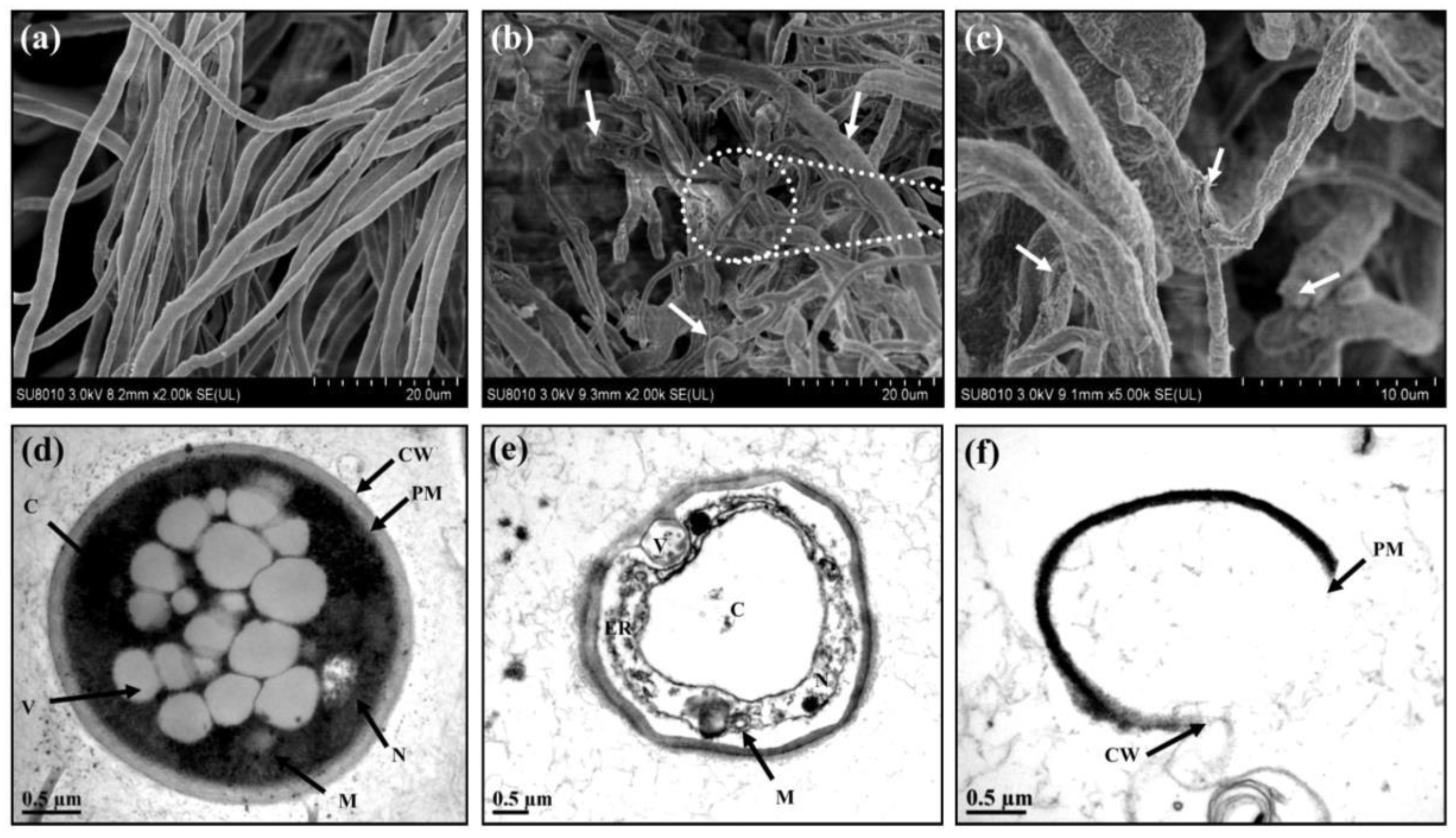
4. Challenges
- Potential ecological effects: Engineered metal oxide nanoparticles, like any other chemical product, may pose environmental dangers through the leakage of nanoparticles into soil or water, impacting non-target organisms. Before these particles are widely used in agriculture or other industries, their possible environmental implications must be studied.
- Inadequate efficacy: While designed metal oxide nanoparticles may have powerful antifungal characteristics, their effectiveness may vary depending on the type of fungus and environmental factors such as humidity, temperature, and pH. More research is needed to enhance their effectiveness against a variety of fungal infections.
- Inadequate standardization: There are no defined techniques for the synthesis, characterization, and testing of tailored metal oxide nanoparticles as fungicides. The absence of uniformity makes comparing the results of different studies and drawing conclusions about their efficacy and safety difficult.
- Resistance risk: As with most antifungal drugs, repeated use of tailored metal oxide nanoparticles as fungicides may result in the formation of resistant fungal strains. Strategies must be devised to reduce the possibility of resistance development while also extending the usefulness of these nanoparticles.
- Concerns about toxicity: If engineered metal oxide nanoparticles penetrate the food chain or are swallowed directly, they may be harmful to humans and animals. Before these particles are widely used, their toxicity must be thoroughly investigated.
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: A review. Sci. Total Environ. 2019, 650, 2863–2879. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef]
- Pujari, J.D.; Yakkundimath, R.; Byadgi, A.S. Image processing based detection of fungal diseases in plants. Procedia Comput. Sci. 2015, 46, 1802–1808. [Google Scholar] [CrossRef]
- Aslam, S.; Tahir, A.; Aslam, M.F.; Alam, M.W.; Shedayi, A.A.; Sadia, S. Recent advances in molecular techniques for the identification of phytopathogenic fungi–a mini review. J. Plant Interact. 2017, 12, 493–504. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S.; et al. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Pacheco-Esteva, M.C.; Soto-Castro, D.; Vásquez-López, A.; Lima, N.B.; Tovar-Pedraza, J.M. First report of Colletotrichum chrysophilum causing papaya anthracnose in Mexico. Plant Dis. 2022, 106, 3213. [Google Scholar] [CrossRef]
- Vásquez-López, A.; Palacios-Torres, R.E.; Camacho-Tapia, M.; Granados-Echegoyen, C.; Lima, N.B.; Vera-Reyes, I.; Tovar-Pedraza, J.M.; Leyva-Mir, S.G. Colletotrichum brevisporum and C. musicola causing leaf anthracnose of taro (Colocasia esculenta) in Mexico. Plant Dis. 2019, 103, 2963. [Google Scholar] [CrossRef]
- Savci, S. Investigation of effect of chemical fertilizers on environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Rahman, K.M.A.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.D.M.; Kupper, K.C.; Augusto, F.; De Carvalho, J.E.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Santamarina, M.P.; Ibáñez, M.D.; Marqués, M.; Roselló, J.; Giménez, S.; Blázquez, M.A. Bioactivity of essential oils in phytopathogenic and post-harvest fungi control. Nat. Prod. Res. 2017, 31, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Prem Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, P. Applications of chitosan and chitosan based metallic nanoparticles in agrosciences-A review. Int. J. Biol. Macromol. 2021, 166, 1554–1569. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.A.; Kalia, M.; Rai, M. Trichoderma: An eco-friendly source of nanomaterials for sustainable agroecosystems. J. Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Paraguay-Delgado, F.; Hermida-Montero, L.A.; Morales-Mendoza, J.E.; Durán-Barradas, Z.; Mtz-Enriquez, A.I.; Pariona, N. Photocatalytic properties of Cu-containing ZnO nanoparticles and their antifungal activity against agriculture-pathogenic fungus. RSC Adv. 2022, 12, 9898–9908. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, J.; Le, T. Zinc oxide nanoparticle as a novel class of antifungal agents: Current advances and future perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Volova, T.; Prudnikova, S.V.; Satish, S.; Prasad, N. Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ. Toxicol. Pharmacol. 2017, 53, 10–17. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Guha, S.; Mehta, S.; Husen, A. Potential applications of engineered nanoparticles in plant disease management: A critical update. Chemosphere 2022, 295, 133798. [Google Scholar] [CrossRef]
- Avila-Quezada, G.D.; Golinska, P.; Rai, M. Engineered nanomaterials in plant diseases: Can we combat phytopathogens? Appl. Microbiol. Biotechnol. 2022, 106, 117–129. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A.; Fang, X. Potential of metal and metal oxide nanoparticles in plant disease diagnostics and management: Recent advances and challenges. Chemosphere 2022, 297, 134114. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Hazarika, A.; Yadav, M.; Yadav, D.K.; Yadav, H.S. An overview of the role of nanoparticles in sustainable agriculture. Biocatal. Agric. Biotechnol. 2022, 43, 102399. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah; et al. Nano-enabled products: Challenges and opportunities for sustainable agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid antifungals for control of plant diseases: Current status and future perspectives. J. Fungi 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Mondaca, F.; Mtz-Enriquez, A.I.; Pariona, N. Copper-based nanostructures for plant disease management. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–201. [Google Scholar]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Herrera-Rivera, M.R.; Rojas-Chávez, H.; Cruz-Martínez, H.; Medina, D.I. Recent Advances in ZnO-Based Carbon Monoxide Sensors: Role of Doping. Sensors 2021, 21, 4425. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.M.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O.E. Needle-and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- Sardar, M.; Ahmed, W.; Al Ayoubi, S.; Nisa, S.; Bibi, Y.; Sabir, M.; Khan, M.M.; Ahmed, W.; Qayyum, A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2022, 29, 88–95. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Murali, M.; Ansari, M.A.; Udayashankar, A.C.; Alzohairy, M.A.; Almatroudi, A.; Niranjana, S.R. Biofabrication of zinc oxide nanoparticles from Melia azedarach and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J. Biol. Sci. 2020, 27, 1923–1930. [Google Scholar] [CrossRef]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111656. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against postharvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc nanoparticles: Mode of action and efficacy against boscalid-resistant Alternaria alternata isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Salem, N.M.; Awwad, A.M. Green synthesis and characterization of ZnO nanoparticles using Solanum rantonnetii leaves aqueous extract and antifungal activity evaluation. Chem. Int. 2022, 8, 12–17. [Google Scholar]
- Bayat, M.; Chudinova, E.; Zargar, M.; Lyashko, M.; Louis, K.; Adenew, F.K. Phyto-assisted green synthesis of zinc oxide nanoparticles and its antibacterial and antifungal activity. Res. Crops 2019, 20, 725–730. [Google Scholar]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Karkhane, M.; Lashgarian, H.E.; Mirzaei, S.Z.; Ghaffarizadeh, A.; Sepahvand, A.; Marzban, A. Antifungal, antioxidant and photocatalytic activities of zinc nanoparticles synthesized by Sargassum vulgare extract. Biocatal. Agric. Biotechnol. 2020, 29, 101791. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Kolahalam, L.A.; Prasad, K.R.S.; Krishna, P.M.; Supraja, N. Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles: Synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese hamster ovary (CHO) cell lines. Heliyon 2021, 7, e07265. [Google Scholar] [CrossRef]
- Issam, N.; Naceur, D.; Nechi, G.; Maatalah, S.; Zribi, K.; Mhadhbi, H. Green synthesised ZnO nanoparticles mediated by Olea europaea leaf extract and their antifungal activity against Botrytis cinerea infecting faba bean plants. Arch. Phytopathol. Plant Protec. 2021, 54, 1083–1105. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hu, C.; Ren, Y.; Lu, Y.; Song, Y.; Ji, Y.; He, J. Green synthesis of zinc oxide nanoparticles using Cinnamomum camphora (L.) Presl leaf extracts and its antifungal activity. J. Environ. Chem. Eng. 2021, 9, 106659. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-mediated ZnO nanoparticles: A green tool for controlling soil-borne pathogens in cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Ramalingam, G.; Vo, D.V.N.; Priya, P.M.; Soto-Moscoso, M. Green synthesis of zinc oxide nanoparticles by Justicia adhatoda leaves and their antimicrobial activity. Chem. Eng. Technol. 2021, 44, 551–558. [Google Scholar] [CrossRef]
- Alhazmi, N.M.; Sharaf, E.M. Fungicidal activity of zinc oxide nanoparticles against azole-resistant Aspergillus flavus isolated from yellow and white maize. Molecules 2023, 28, 711. [Google Scholar] [CrossRef]
- Veronica, H.; Hidayati, R.Z.; Pristya, N.; Maslikah, S.I.; Lestari, S.R. Exploring the potential of ZnO nanoparticle antifungal with biostabilizers bidara leaves (Ziziphus spina-christi L.). AIP Conf. Proc. 2021, 2353, 30025. [Google Scholar]
- Anjali, K.P.; Sangeetha, B.M.; Raghunathan, R.; Devi, G.; Dutta, S. Seaweed-mediated fabrication of zinc oxide nanoparticles and their antibacterial, antifungal and anticancer applications. ChemistrySelect 2021, 6, 647–656. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, R.; Singh, R.R.; Kumari, A. Evaluation of biogenic zinc oxide nanoparticles from Tinospora cordifolia stem extract for photocatalytic, anti-microbial, and antifungal activities. Mater. Chem. Phys. 2023, 297, 127382. [Google Scholar] [CrossRef]
- T-Thienprasert, N.P.; T-Thienprasert, J.; Ruangtong, J.; Jaithon, T.; Huehne, P.S.; Piasai, O. Large Scale Synthesis of Green Synthesized Zinc Oxide Nanoparticles from Banana Peel Extracts and Their Inhibitory Effects against Colletotrichum sp., Isolate KUFC 021, Causal Agent of Anthracnose on Dendrobium Orchid. J. Nanomater. 2021, 2021, 5625199. [Google Scholar] [CrossRef]
- Suba, S.; Vijayakumar, S.; Vidhya, E.; Punitha, V.N.; Nilavukkarasi, M. Microbial mediated synthesis of ZnO nanoparticles derived from Lactobacillus spp: Characterizations, antimicrobial and biocompatibility efficiencies. Sens. Int. 2021, 2, 100104. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.S.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Film. 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Wani, A.H.; Shah, M.A. Unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J. Appl. Pharm. Sci. 2012, 2, 40–44. [Google Scholar]
- Kamal, A.; Saba, M.; Kamal, A.; Batool, M.; Asif, M.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Habib, D.; Ahmad, S. Bioinspired green synthesis of bimetallic iron and zinc oxide nanoparticles using mushroom extract and use against Aspergillus niger; the most devastating fungi of the green world. Catalysts 2023, 13, 400. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef]
- De la Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018, 2018, 3498527. [Google Scholar] [CrossRef]
- Erazo, A.; Mosquera, S.A.; Rodríguez-Paéz, J.E. Synthesis of ZnO nanoparticles with different morphology: Study of their antifungal effect on strains of Aspergillus niger and Botrytis cinerea. Mater. Chem. Phys. 2019, 234, 172–184. [Google Scholar] [CrossRef]
- Bhargav, P.K.; Murthy, K.S.R.; Kaur, K.; Goyat, M.S.; Pandey, J.K.; Dubey, S.; Pant, C. Influence of Al and Al-Cu dual doping on structural, optical, wetting and anti-fungal properties of ZnO nanoparticles. Mater. Res. Innov. 2020, 24, 385–394. [Google Scholar] [CrossRef]
- Mosquera-Sánchez, L.P.; Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Guerra–Sierra, B.E.; Munoz-Florez, J.E.; Rodríguez-Páez, J.E. Antifungal effect of zinc oxide nanoparticles (ZnO-NPs) on Colletotrichum sp., causal agent of anthracnose in coffee crops. Biocatal. Agric. Biotechnol. 2020, 25, 101579. [Google Scholar] [CrossRef]
- Wani, A.H.; Amin, M.; Shahnaz, M.; Shah, M.A. Antimycotic activity of nanoparticles of MgO, FeO and ZnO on some pathogenic fungi. Int. J. Manuf. Mater. Mech. Eng. 2012, 2, 59–70. [Google Scholar] [CrossRef]
- Pariona, N.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A.; Mtz-Enriquez, A.I. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Appl. Nanosci. 2020, 10, 435–443. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Ghosh, S.; Gryzenhout, M.; Conradie, J. One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci. Rep. 2021, 11, 8305. [Google Scholar] [CrossRef] [PubMed]
- Pariona, N.; Cereceda, S.B.; Mondaca, F.; Carrión, G.; Mtz-Enriquez, A.I. Antifungal activity and degradation of methylene blue by ZnO, Cu, and Cu2O/Cu nanoparticles, a comparative study. Mater. Lett. 2021, 301, 130182. [Google Scholar] [CrossRef]
- González-Merino, A.M.; Hernández-Juárez, A.; Betancourt-Galindo, R.; Ochoa-Fuentes, Y.M.; Valdez-Aguilar, L.A.; Limón-Corona, M.L. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum-Solanum lycopersicum pathosystem under controlled conditions. J. Phytopathol. 2021, 169, 533–544. [Google Scholar] [CrossRef]
- Hermida-Montero, L.A.; Paraguay-Delgado, F.; Cruz, L.F.; Carrillo, D.; Mtz-Enriquez, A.I.; Pariona, N. The role of coating and size of ZnO nanoparticles on the antifungal activity against Raffaelea species. Mater. Lett. 2021, 301, 130314. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 2013, 26, 913–924. [Google Scholar] [CrossRef]
- Yehia, R.S.; Ahmed, O.F. In vitro study of the antifungal efficacy of zinc oxide nanoparticles against Fusarium oxysporum and Penicilium expansum. Afr. J. Microbiol. Res. 2013, 19, 1917–1923. [Google Scholar]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; White, J.C. Effect of metalloid and metal oxide nanoparticles on Fusarium wilt of watermelon. Plant Dis. 2021, 105, 1394–1401. [Google Scholar] [CrossRef]
- Vera-Reyes, I.; Esparza-Arredondo, I.J.E.; Lira-Saldivar, R.H.; Granados-Echegoyen, C.A.; Alvarez-Roman, R.; Vásquez-López, A.; Díaz-Barriga Castro, E. In vitro antimicrobial effect of metallic nanoparticles on phytopathogenic strains of crop plants. J. Phytopathol. 2019, 167, 461–469. [Google Scholar] [CrossRef]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B Biol. 2021, 219, 112206. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Siddiqui, Z.A. Role of zinc oxide nanoparticles in the management of disease complex of beetroot (Beta vulgaris L.) caused by Pectobacterium betavasculorum, Meloidogyne incognita and Rhizoctonia solani. Hortic. Environ. Biotechnol. 2021, 62, 225–241. [Google Scholar] [CrossRef]
- Ahamad, L.; Siddiqui, Z.A. Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Exp. Parasitol. 2021, 230, 108176. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Siddiqui, Z.A. Zinc oxide nanoparticles affect growth, photosynthetic pigments, proline content and bacterial and fungal diseases of tomato. Arch. Phytopathol. Plant Protec. 2021, 54, 1519–1538. [Google Scholar] [CrossRef]
- Elmer, W.H.; de la Torre-Roche, R.; Zuverza-Mena, N.; Adisa, I.H.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Influence of Single and Combined Mixtures of Metal Oxide Nanoparticles on Eggplant Growth, Yield, and Verticillium Wilt Severity. Plant Dis. 2021, 105, 1153–1161. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S.A. Comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Elkodous, M.A.; El-Sayyad, G.S. Response Surface Methodology Optimization of Mono-dispersed MgO Nanoparticles Fabricated by Ultrasonic-Assisted Sol–Gel Method for Outstanding Antimicrobial and Antibiofilm Activities. J. Clust Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]
- Jamdagni, P.; Rana, J.S.; Khatri, P.; Nehra, K. Comparative account of antifungal activity of green and chemically synthesized Zinc oxide nanoparticles in combination with agricultural fungicides. Int. J. Nano Dimens. 2018, 9, 198–208. [Google Scholar]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Sierra, B.G.; Muñoz-Florez, J.E.; Erazo-Castillo, L.A.; Rodríguez-Páez, J.E. ZnO-based nanofungicides: Synthesis, characterization and their effect on the coffee fungi Mycena citricolor and Colletotrichum sp. Mater. Sci. Eng. C 2019, 98, 808–825. [Google Scholar] [CrossRef]
- Yadav, A.; Das, S.; Biswas, S.; Yadav, A.; Debnath, N. Effect of synthetic route in particle size distribution of zinc oxide, silver and carbon nanoparticles and its role in controlling phytopathogenic fungus Alternaria solani. Arch. Phytopathol. Plant Prot. 2021, 54, 1675–1688. [Google Scholar] [CrossRef]
- Patino-Portela, M.C.; Arciniegas-Grijalba, P.A.; Mosquera-Sanchez, L.P.; Sierra, B.E.G.; Munoz-Florez, J.E.; Erazo-Castillo, L.A.; Rodriguez-Paez, J.E. Effect of method of synthesis on antifungal ability of ZnO nanoparticles: Chemical route vs. green route. Adv. Nano Res. 2021, 10, 191–210. [Google Scholar]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Hai, N.T.T.; Viet, N.M.; Nguyen, V.H. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ. Res. 2022, 203, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kovačec, E.; Regvar, M.; van Elteren, J.T.; Arčon, I.; Papp, T.; Makovec, D.; Vogel-Mikuš, K. Biotransformation of copper oxide nanoparticles by the pathogenic fungus Botrytis cinerea. Chemosphere 2017, 180, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, D.; Roopan, S.M. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mat. Sci. Eng. C 2017, 80, 38–44. [Google Scholar] [CrossRef]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 2020, 3, 79–90. [Google Scholar] [CrossRef]
- Nagore, P.; Ghotekar, S.; Mane, K.; Ghoti, A.; Bilal, M.; Roy, A. Structural Properties and Antimicrobial Activities of Polyalthia longifolia Leaf Extract-Mediated CuO Nanoparticles. BioNanoSci 2021, 11, 579–589. [Google Scholar] [CrossRef]
- Shammout, M.W.; Awwad, A.M. A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chem. Int. 2021, 7, 71–78. [Google Scholar]
- Hao, Y.; Cao, X.; Ma, C.; Zhang, Z.; Zhao, N.; Ali, A.; Hou, T.; Xiang, Z.; Zhuang, J.; Wu, S.; et al. Potential applications and antifungal activities of engineered nanomaterials against gray mold disease agent Botrytis cinerea on rose petals. Front. Plant Sci. 2017, 8, 1332. [Google Scholar] [CrossRef]
- Hao, Y.; Fang, P.; Ma, C.; White, J.C.; Xiang, Z.; Wang, H.; Zhang, Z.; Rui, Y.; Xing, B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ. Res. 2019, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Borgatta, J.; Ma, C.; Elmer, W.; Hamers, R.J.; White, J.C. Copper nanomaterial morphology and composition control foliar transfer through the cuticle and mediate resistance to root fungal disease in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2020, 68, 11327–11338. [Google Scholar] [CrossRef]
- Elmer, W.H.; Zuverza-Mena, N.; Triplett, L.R.; Roberts, E.L.; Silady, R.A.; White, J.C. Foliar application of copper oxide nanoparticles suppresses fusarium wilt development on chrysanthemum. Environ. Sci. Technol. 2021, 55, 10805–10810. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Mag. Mag. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sangaiya, P.; Jayaprakash, R. A review on iron oxide nanoparticles and their biomedical applications. J. Supercon. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Cross, K.M.; Lu, Y.; Zheng, T.; Zhan, J.; McPherson, G.; John, V. Water decontamination using iron and iron oxide nanoparticles. In Nanotechnology Applications for Clean Water; Savage, N., Duncan, J., Sustich, R., Diallo, M., Street, A., Eds.; William Andrew Inc.: New York, NY, USA, 2009; pp. 347–364. [Google Scholar]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287. [Google Scholar] [CrossRef]
- Koka, J.A.; Wani, A.H.; Bhat, M.Y. Evaluation of antifungal activity of Magnesium oxide (MgO) and Iron oxide (FeO) nanoparticles on rot causing fungi. J. Drug Deliv. Ther. 2019, 9, 173–292. [Google Scholar]
- Bilesky-Jose, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fernandes Fraceto, L.; De Lima, R. Biogenic α-Fe2O3 nanoparticles enhance the biological activity of trichoderma against the plant pathogen Sclerotinia sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Photo catalytic, antimicrobial and antifungal activity of biogenic iron oxide nanoparticles synthesised using Aegle marmelos extracts. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1738–1744. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Lu, M.; Lu, S.; Li, Z.; Ding, W. Comparative study on the fungicidal activity of metallic MgO nanoparticles and macroscale MgO against soilborne fungal phytopathogens. Front. Microbiol. 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, I.; Takehara, Y.; Ota, M.; Imada, K.; Sasaki, K.; Kajihara, H.; Sakai, S.; Jogaiah, S.; Ito, S.I. Magnesium oxide induces immunity against Fusarium wilt by triggering the jasmonic acid signaling pathway in tomato. J. Biotechnol. 2021, 325, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Bala, A.; Singh, H.; Ahuja, R.; Kumar, A. Development of MgO-sepoilite nanocomposites against phytopathogenic fungi of rice (Oryzae sativa): A green approach. ACS Omega 2020, 5, 13557–13565. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Mozammel, M.; Khosroushahi, A.Y. Antifungal effects of ZnO, TiO2 and ZnO-TiO2 nanostructures on Aspergillus flavus. Pestic. Biochem. Phy. 2021, 176, 104869. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.U.R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Derbalah, A.; Elsharkawy, M.M.; Hamza, A.; El-Shaer, A. Resistance induction in cucumber and direct antifungal activity of zirconium oxide nanoparticles against Rhizoctonia solani. Pestic. Biochem. Phys. 2019, 157, 230–236. [Google Scholar] [CrossRef]
- Ahmed, T.; Ren, H.; Noman, M.; Shahid, M.; Liu, M.; Ali, M.A.; Zhang, J.; Tian, Y.; Qi, X.; Li, B. Green synthesis and characterization of zirconium oxide nanoparticles by using a native Enterobacter sp. and its antifungal activity against bayberry twig blight disease pathogen Pestalotiopsis versicolor. NanoImpact 2021, 21, 100281. [Google Scholar] [CrossRef]
- Joshi, N.C.; Chaudhary, N.; Rai, N. Medicinal plant leaves extract based synthesis, characterisations and antimicrobial activities of ZrO2 nanoparticles (ZrO2 NPs). Bio. Nano Sci. 2021, 11, 497–505. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]
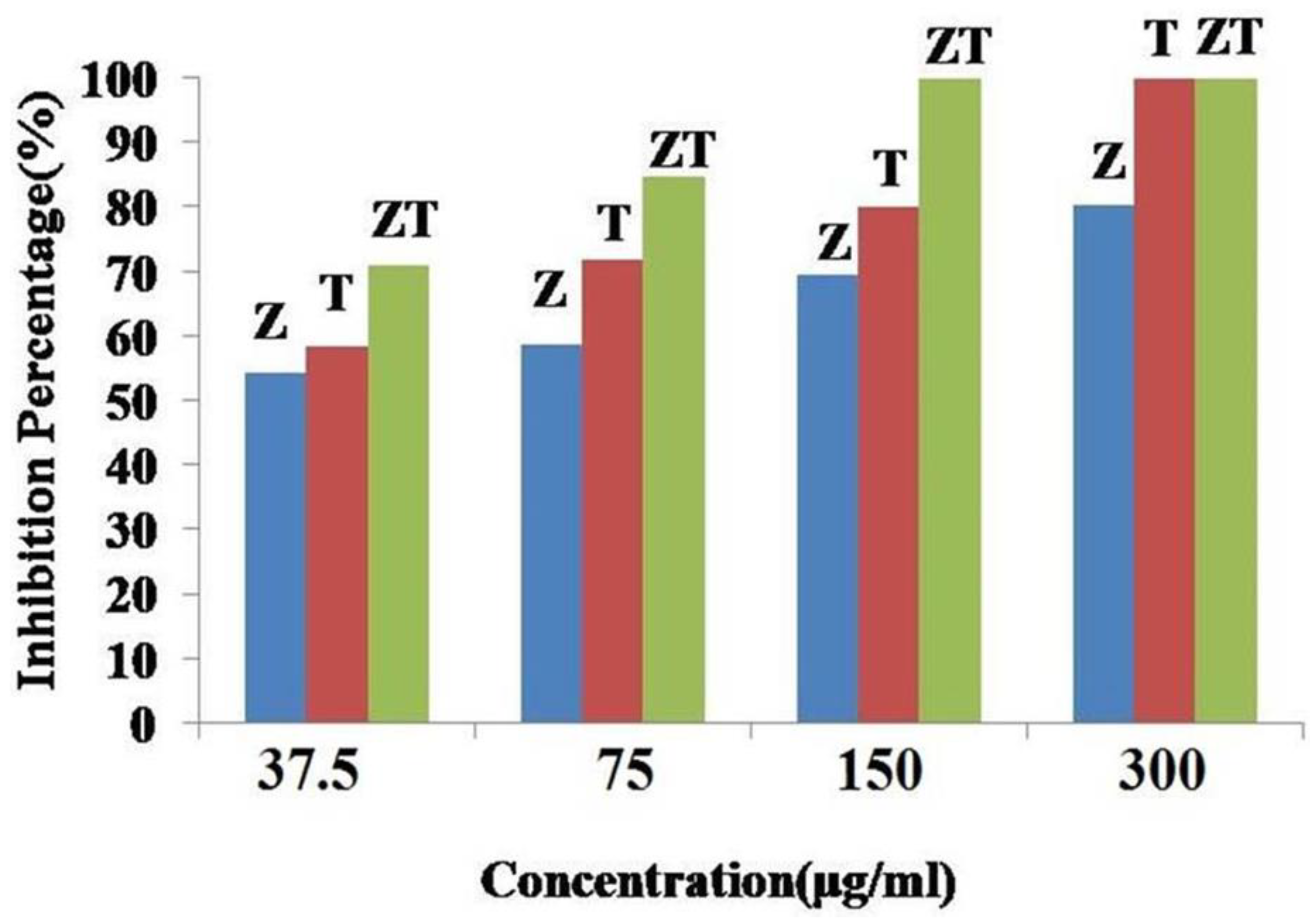
- Uyen, V.N.; Anh, N.P.; Van, N.T.; Tri, N.; Minh, N.V.; Huy, N.N.; Ha, H.K. Characteristics and antifungal activity of CuO-ZnO nanocomposites synthesised by the sol-gel technique. Vietnam J. Sci. Technol. 2020, 62, 17–22. [Google Scholar] [CrossRef]
- Najibi Ilkhechi, N.; Mozammel, M.; Khosroushahi, A.Y. Antifungal effects of ZnO-TiO2/Au nanostructures on Aspergillus flavus. J. Aust. Ceram. Soc. 2021, 57, 793–802. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, D.; Kumar, A.; Ala’a, H.; Pathania, D.; Naushad, M.; Mola, G.T. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Tellez-Cruz, M.M.; Solorza-Feria, O.; Calaminici, P.; Medina, D.I. Catalytic activity trends from pure Pd nanoclusters to M@PdPt (M=Co, Ni, and Cu) core-shell nanoclusters for the oxygen reduction reaction: A first-principles analysis. Int. J. Hydrogen Energy 2021, 45, 13738. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Trimetallic nanoparticles: Greener synthesis and their applications. Nanomaterials 2020, 10, 1784. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Luna, A.R.; Vásquez-López, A.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; Cruz-Martínez, H.; Medina, D.I. Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants 2023, 12, 2461. https://doi.org/10.3390/plants12132461
Cruz-Luna AR, Vásquez-López A, Rojas-Chávez H, Valdés-Madrigal MA, Cruz-Martínez H, Medina DI. Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants. 2023; 12(13):2461. https://doi.org/10.3390/plants12132461
Chicago/Turabian StyleCruz-Luna, Aida R., Alfonso Vásquez-López, Hugo Rojas-Chávez, Manuel A. Valdés-Madrigal, Heriberto Cruz-Martínez, and Dora I. Medina. 2023. "Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control" Plants 12, no. 13: 2461. https://doi.org/10.3390/plants12132461
APA StyleCruz-Luna, A. R., Vásquez-López, A., Rojas-Chávez, H., Valdés-Madrigal, M. A., Cruz-Martínez, H., & Medina, D. I. (2023). Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants, 12(13), 2461. https://doi.org/10.3390/plants12132461






