A First Approach for the Micropropagation of the Edible and Medicinal Halophyte Inula crithmoides L.
Abstract
1. Introduction
2. Results
2.1. Explant Surface Sterilization and Establishment
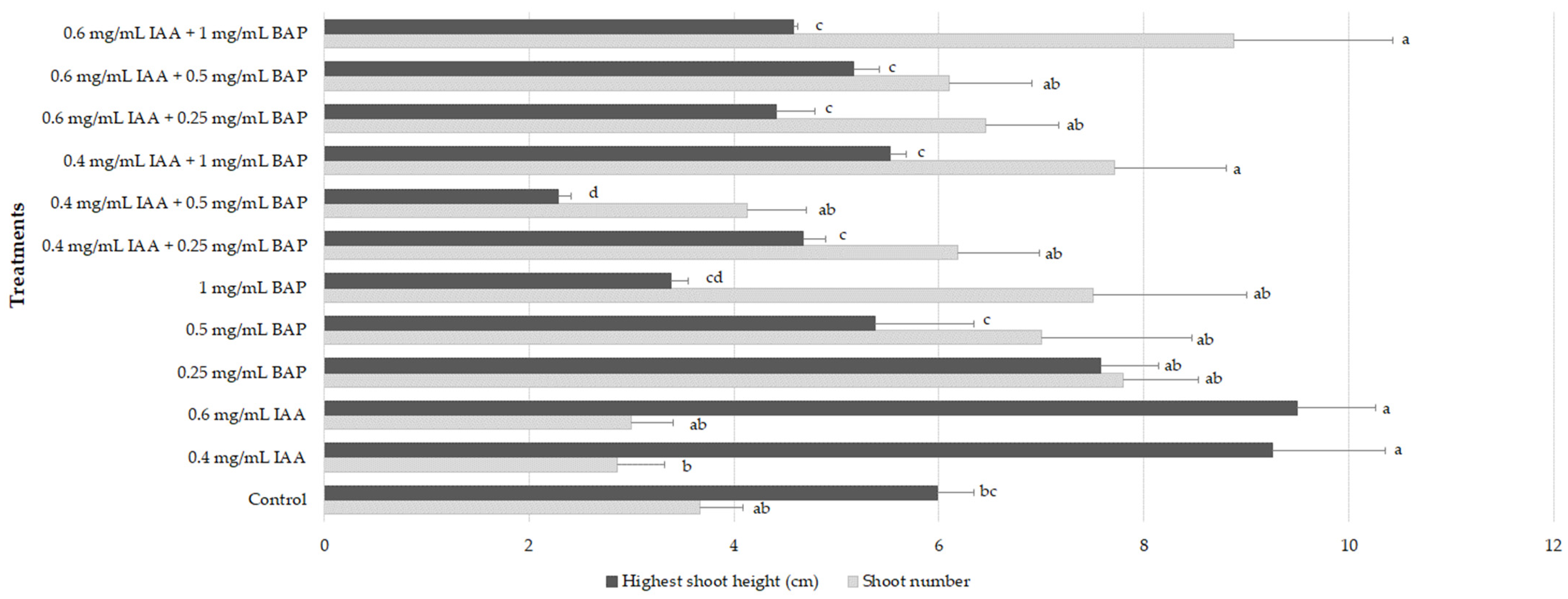
2.2. Shoot Multiplication
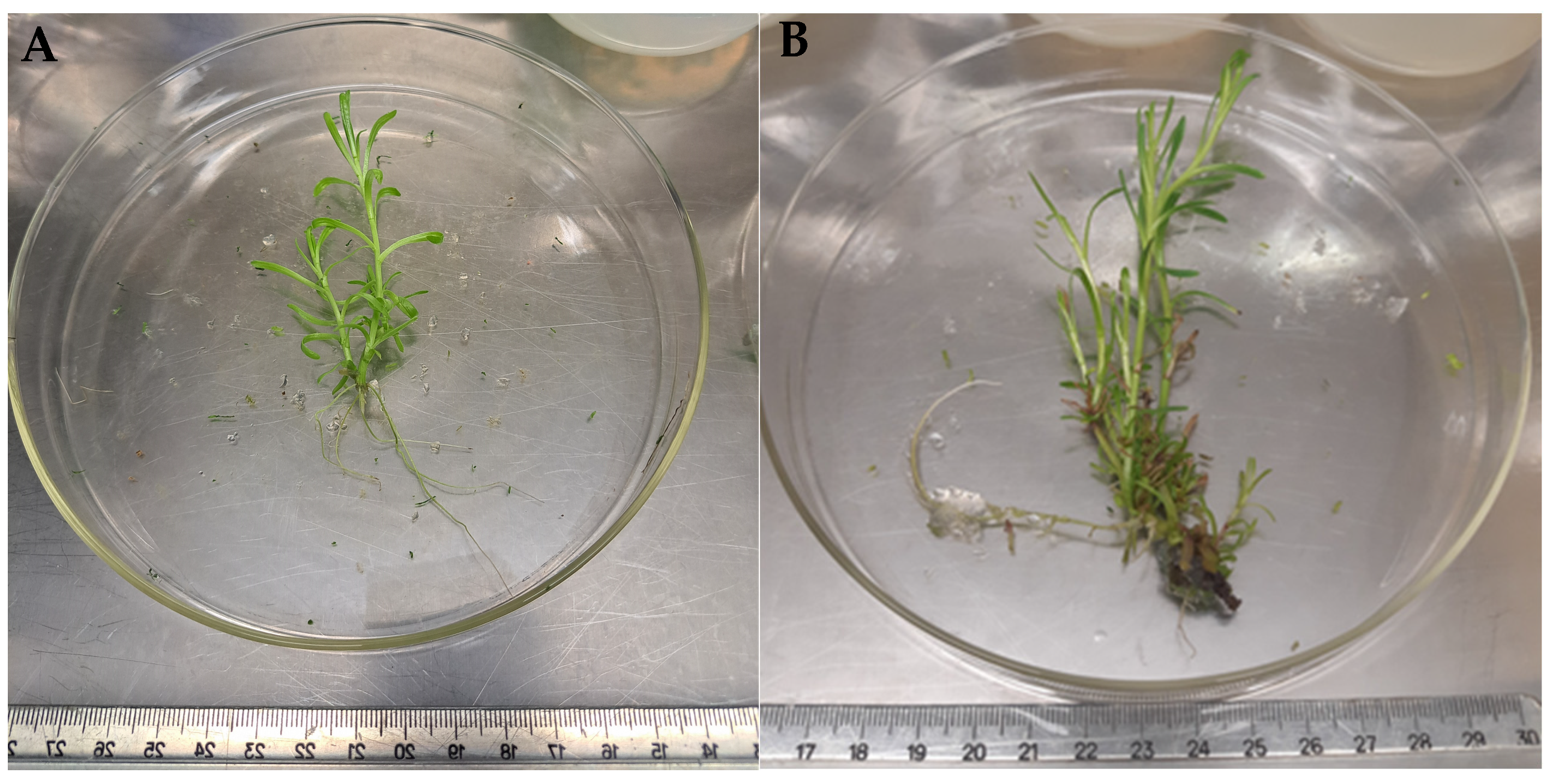
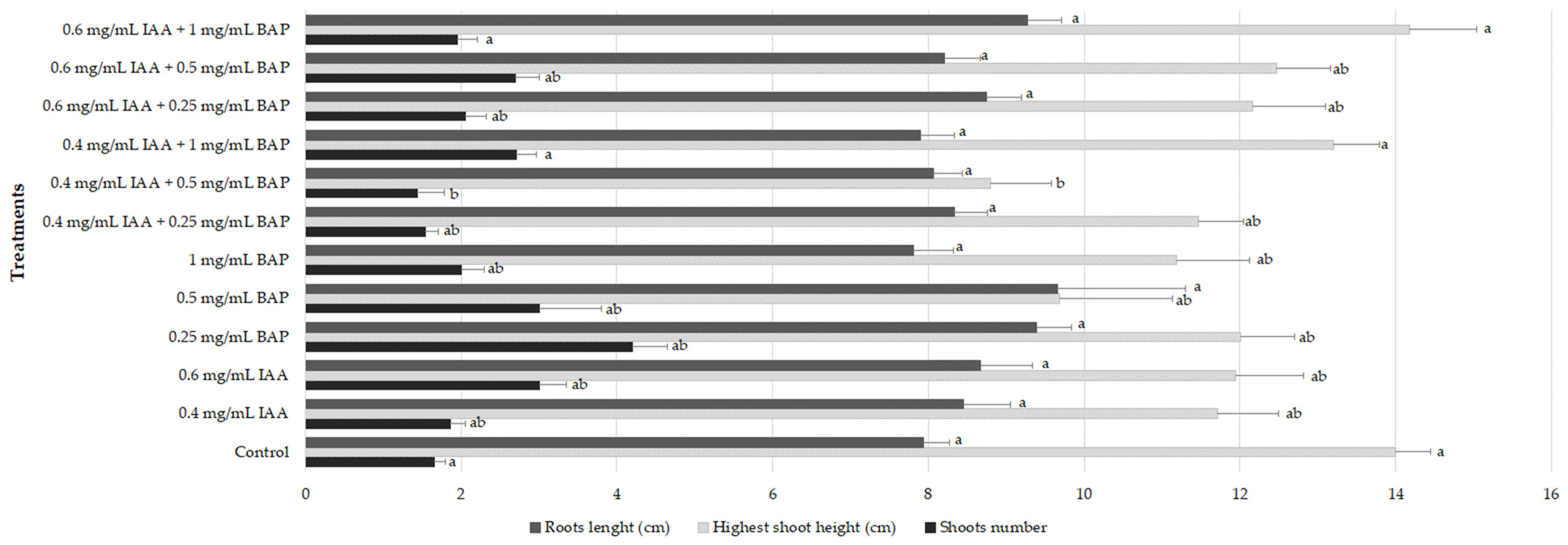
2.3. Rooting
2.4. Acclimatization
3. Discussion
4. Materials and Methods
4.1. Plant Material and Explant Preparation and Disinfection
4.2. Establishment
4.3. Multiplication and Rooting
4.4. Acclimatization
4.5. Statistical Analyses
5. Conclusions
List of Abbreviations
| ABA | Abscisic acid |
| BAP | 6-Benzylaminopurine |
| IAA | Indole-3-acetic acid |
| KIN | Kinetin |
| MS | Murashige and Skoog |
| NAA | 1-Naphthaleneacetic acid |
| PGRs | Plant growth regulators |
| PTC | Plant tissue culture |
| STN | Shoot-tip necrosis |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Bostan, N.; Maria, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plant. Res. 2011, 5, 7108–7118. [Google Scholar]
- Menezes-Benavente, L.; Teixeira, F.K.; Kamei, C.L.A.; Margis-Pinheiro, M. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci. 2004, 166, 323–331. [Google Scholar] [CrossRef]
- Ben Amor, N.; Jiménez, A.; Megdiche, W.; Lundqvist, M.; Sevilla, F.; Abdelly, C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006, 126, 446–457. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Whid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological insights into halophyte bioactive extract action on anti-inflammatory, pain relief and antibiotics-type mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]
- eHALOPH. Available online: https://ehaloph.uc.pt/showplant/11358 (accessed on 9 January 2023).
- Zurayk, R.A.; Baalbaki, R. Inula crithmoides: A candidate plant for saline agriculture. Arid Soil Res. Rehabil. 1996, 10, 213–223. [Google Scholar] [CrossRef]
- D’Agostino, G.; Badalamenti, N.; Franco, P.; Bruno, M.; Gallo, G. The chemical composition of the flowers essential oil of Inula crithmoides (Asteraceae) growing in aeolian islands, Sicily (Italy) and its biocide properties on microorganisms affecting historical art crafts. Nat. Prod. Res. 2022, 36, 2993–3001. [Google Scholar] [CrossRef]
- Miara, M.D.; Souidi, Z.; Benhanifa, K.; Daikh, A.; Hammou, M.A.; Moumenine, A.; Sabi, I.H. Diversity, natural habitats, ethnobotany and conservation of the flora of the Macta marches (North-West Algeria). Int. J. Environ. Stud. 2021, 78, 817–837. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.; Fraternale, D.; Genovese, S.; Curini, M.; Ricci, D. Composition and Antioxidant Activity of Inula crithmoides Essential Oil Grown in Central Italy (Marche Region). Nat. Prod. Commun. 2010, 5, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Gama, F.; Castañeda-Loaiza, V.; Costa, C.; Schüler, L.M.; Santos, T.; Salazar, M.; Nunes, C.; Cruz, R.M.S.; Varela, J.; et al. Nutritional and functional evaluation of Inula crithmoides and Mesembryanthemum nodiflorum grown in different salinities for human consumption. Molecules 2021, 26, 4543. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Moraes, R.M.; Cerdeira, A.L.; Lourenço, M.V. Using micropropagation to develop medicinal plants into crops. Molecules 2021, 26, 1752. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Akin, B. Tissue culture techniques of medicinal and aromatic plants: History, cultivation and micropropagation. J. Sci. Rep.-A 2020, 45, 253–266. [Google Scholar]
- Trejgell, A.; Kaminska, M.; Lisowska, K.; Tretyn, A. Micropropagation of Inula germanica L. from the seedlings explants. Not. Bot. Horti Agrobot. 2018, 46, 52–57. [Google Scholar] [CrossRef]
- Jabeen, N.; Shawl, A.S.; Dar, G.H.; Jan, A.; Sultan, P. Micropropagation of Inula racemosa Hook.f. a valuable medicinal plant. Int. J. Bot. 2007, 3, 296–301. [Google Scholar] [CrossRef]
- Perica, M.C.; Vršek, I.; Mitić, B. In Vitro propagation of Inula verbascifolia (Willd.) Hausskn. subsp. verbascifolia. Plant Biosyst. 2008, 142, 1–4. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J. In Vitro propagation of Inula royleana DC. Acta Soc. Bot. Pol. Pol. 2004, 73, 5–8. [Google Scholar] [CrossRef]
- Custódio, L.; Charles, G.; Magné, C.; Barba-Espín, G.; Piqueras, A.; Hernández, J.A.; Ben Hamed, K.; Castañeda-Loaiza, V.; Fernandes, E.; Rodrigues, M.J. Application of in vitro plant tissue culture techniques to halophyte species: A review. Plants 2023, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Nezami-Alanagh, E.; Barreal, M.E.; Kher, M.M.; Wicaksono, A.; Gulyás, A.; Hidvégi, N.; Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Márton, L.; et al. Shoot tip necrosis of in vitro plant cultures: A reappraisal of possible causes and solutions. Planta 2020, 252, 47. [Google Scholar] [CrossRef]
- Hashim, S.N.; Ghazali, S.Z.; Sidik, N.J.; Chia-Chay, T.; Saleh, A. Surface sterilization method for reducing contamination of Clinacanthus nutans nodal explants intended for in-vitro culture. E3S Web Conf. 2021, 306, 01004. [Google Scholar] [CrossRef]
- Mok, D.W.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Kaloo, Z.A.; Singh, S.; Altaf, T. Micropropagation of medicinally important species of family Asteraceae—A review. J. Recent Sci. Res. 2003, 4, 1296–1303. [Google Scholar]
- Campanoni, P.; Nick, P. Auxin-dependent cell division and cell elongation. 1-naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol. 2005, 137, 939–948. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Tichá, I.; Kadleček, P.; Haisel, D.; Plzáková, Š. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plant. 1999, 42, 481–497. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Hossain, M.M.; Sharma, M.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Acclimatization of in vitro-derived Dendrobium. Hort. Plant J. 2007, 3, 110–124. [Google Scholar] [CrossRef]
- El-Din, W.M.S.; Mokhtar, N.A.Y.O. Effect of irrigation scheduling and some antitranspirants on water relations and productivity of Mentha varidis L. Am.-Euras. J. Agric. Environ. Sci. 2020, 20, 367–390. [Google Scholar]


| Treatments | Rooting (%) | Callus (%) | |
|---|---|---|---|
| IAA (mg/L) | BAP (mg/L) | ||
| 0 | 0 | 100 | 0 |
| 0.4 | 0 | 100 | 0 |
| 0.6 | 0 | 100 | 0 |
| 0 | 0.25 | 100 | 0 |
| 0 | 0.5 | 50 | 75 |
| 0 | 1 | 100 | 100 |
| 0.4 | 0.25 | 41 | 100 |
| 0.4 | 0.5 | 13 | 100 |
| 0.4 | 1 | 76 | 100 |
| 0.6 | 0.25 | 64 | 100 |
| 0.6 | 0.5 | 70 | 100 |
| 0.6 | 1 | 88 | 100 |
| Treatments | Shoot-Tip Necrosis (%) | |
|---|---|---|
| IAA (mg/L) | BAP (mg/L) | |
| 0 | 0 | 58 |
| 0.4 | 0 | 0 |
| 0.6 | 0 | 30 |
| 0 | 0.25 | 6 |
| 0 | 0.5 | 0 |
| 0 | 1 | 0 |
| 0.4 | 0.25 | 18 |
| 0.4 | 0.5 | 22 |
| 0.4 | 1 | 41 |
| 0.6 | 0.25 | 5 |
| 0.6 | 0.5 | 19 |
| 0.6 | 1 | 33 |
| Treatment | Survival (%) | Plant Height (cm) |
|---|---|---|
| Control | 9.80 | - |
| Paraffin | 83.3 | 18.3 ± 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.J.; Castañeda-Loaiza, V.; Fernandes, E.; Custódio, L. A First Approach for the Micropropagation of the Edible and Medicinal Halophyte Inula crithmoides L. Plants 2023, 12, 2366. https://doi.org/10.3390/plants12122366
Rodrigues MJ, Castañeda-Loaiza V, Fernandes E, Custódio L. A First Approach for the Micropropagation of the Edible and Medicinal Halophyte Inula crithmoides L. Plants. 2023; 12(12):2366. https://doi.org/10.3390/plants12122366
Chicago/Turabian StyleRodrigues, Maria João, Viana Castañeda-Loaiza, Eliana Fernandes, and Luísa Custódio. 2023. "A First Approach for the Micropropagation of the Edible and Medicinal Halophyte Inula crithmoides L." Plants 12, no. 12: 2366. https://doi.org/10.3390/plants12122366
APA StyleRodrigues, M. J., Castañeda-Loaiza, V., Fernandes, E., & Custódio, L. (2023). A First Approach for the Micropropagation of the Edible and Medicinal Halophyte Inula crithmoides L. Plants, 12(12), 2366. https://doi.org/10.3390/plants12122366







