Effect of Glutamic Acid and 6-benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry
Abstract
1. Introduction
2. Results
2.1. Number of Buds and Fruit Quality
2.2. Nonenzymatic Antioxidants in Fruits
2.3. Nonenzymatic Antioxidants in Leaves
2.4. Photosynthetic Pigments
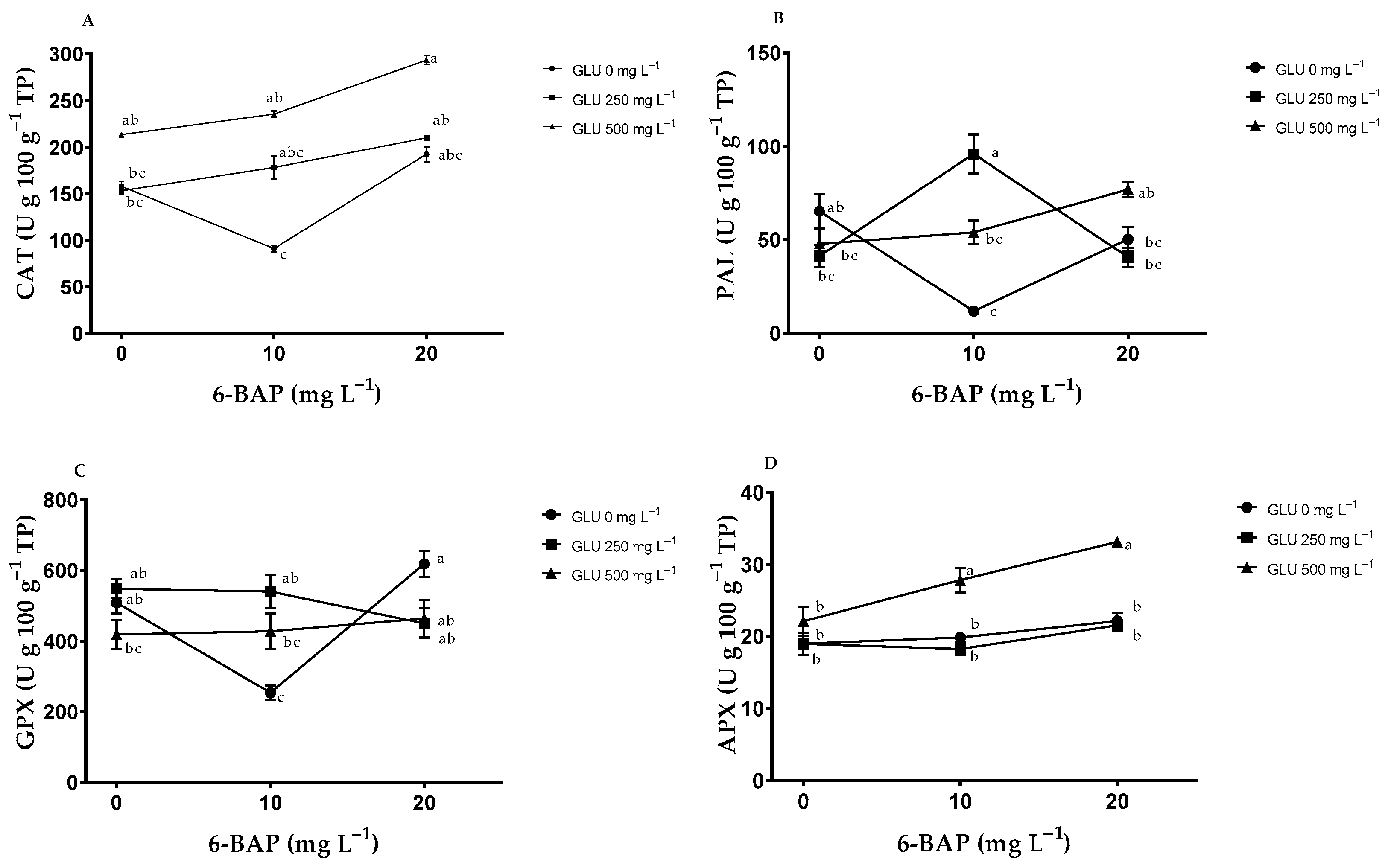
2.5. Enzymatic Antioxidants in Fruits
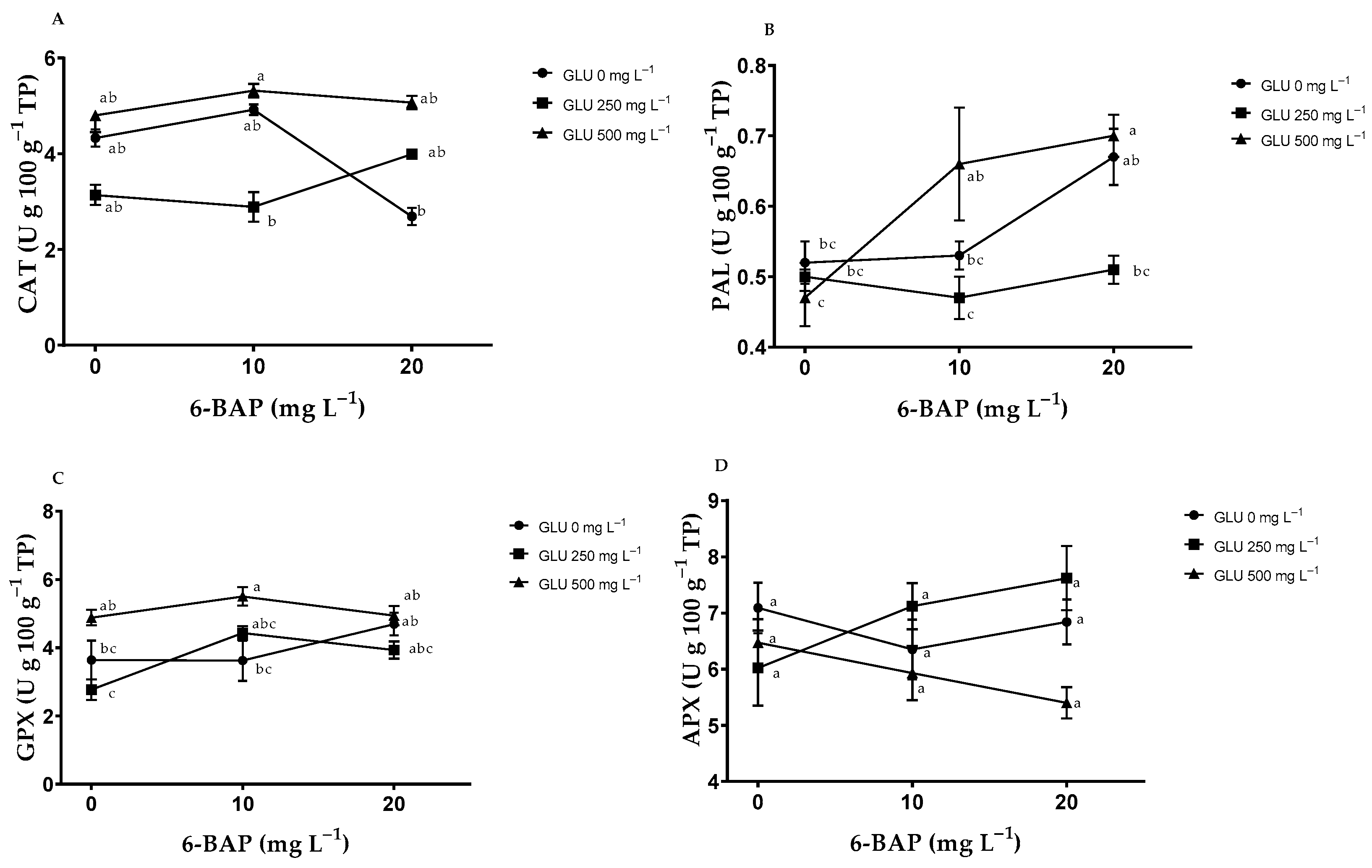
2.6. Enzymatic Antioxidants in Leaves
3. Discussion
3.1. Number of Buds and Fruit Quality
3.2. Nonenzymatic Antioxidants
3.3. Photosynthetic Pigments
3.4. Enzymatic Antioxidants
4. Materials and Methods
4.1. Study Area
4.2. Vegetal Material
4.3. Experimental Design and Treatments
4.4. Fruit Quality
Titrimetric Methods
4.5. Sample Preparation for Biochemical Analysis.
4.5.1. Nonenzymatic Antioxidants
4.5.2. Enzymatic Antioxidants
4.6. Photosynthetic Pigments
4.7. Chemical Reagents
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loera-Alvarado, M. Aspersión de thidiazuron y ácido giberélico combinado con poda sobre fenología del arándano (Vaccinium spp.). Agro Product. 2017, 10, 21–27. [Google Scholar]
- Sarkar, D.; Kar, S.K.; Chattopadhyay, A.; Shikha; Rakshit, A.; Tripathi, V.K.; Dubey, P.K.; Abhilash, P.C. Low Input Sustainable Agriculture: A Viable Climate-Smart Option for Boosting Food Production in a Warming World. Ecol. Indic. 2020, 115, 106412. [Google Scholar] [CrossRef]
- Del Buono, D. Can Biostimulants Be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic Physiological Phenotyping of Drought-Stressed Pepper Plants Treated with “Productivity-Enhancing” and “Survivability-Enhancing” Biostimulants. Front. Plant Sci. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- European Union (EU). Regulation of the European Parliament and of the Council Laying Down Rules on the Making Available on the Market of EU Fertilizing Products ond Amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No. 2003/2003, 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2019:170:TOC (accessed on 1 June 2023).
- Diario Oficial de la Federación (DOF). NORMA Oficial Mexicana NOM-182-SSA1-2010, Etiquetado de Nutrientes Vegetales. Available online: https://www.dof.gob.mx/normasOficiales/4371/salud1a1.htm#:~:text=1.1%20Esta%20norma%20establece%20las,regladores%20de%20crecimiento%20tipo%203 (accessed on 1 June 2023).
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a Plant Biostimulant To Improve Maize (Zea mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef] [PubMed]
- Albarracín, S.L.; Baldeón, M.E.; Sangronis, E.; Cucufate Petruschina, A.; Reyes, F.G.R. L-Glutamato: Un aminoácido clave para las funciones sensoriales y metabólicas. Arch. Latinoam. Nutr. 2016, 66, 101–112. [Google Scholar] [PubMed]
- Hassan, N.M.K.; Marzouk, N.M.; Fawzy, Z.F.; Saleh, S.A. Effect of Bio-Stimulants Foliar Applications on Growth, Yield, and Product Quality of Two Cassava Cultivars. Bull. Natl. Res. Cent. 2020, 44, 59. [Google Scholar] [CrossRef]
- Kong, D.; Ju, C.; Parihar, A.; Kim, S.; Cho, D.; Kwak, J.M. Arabidopsis Glutamate Receptor Homolog3.5 Modulates Cytosolic Ca2+ Level to Counteract Effect of Abscisic Acid in Seed Germination. Plant Physiol. 2015, 167, 1630–1642. [Google Scholar] [CrossRef]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef]
- Mazher, A.A.M.; Zaghloul, S.M.; Mahmoud, S.A.; Siam, H.S. Stimulatory Effect of Kinetin, Ascorbic Acid and Glutamic Acid on Growth and Chemical Constituents of Codiaeum variegatum L. Plants. Am.-Eurasian J. Agric. Environ. Sci. 2011, 6, 318–323. [Google Scholar]
- Serna-Rodríguez, J.R.; Castro-Brindis, R.; Colinas-León, M.T.; Sahagún-Castellanos, J.; Rodríguez-Pérez, J.E. Foliar application of glutamic acid to tomato plants (Lycopersicon esculentum Mill.). Rev. Chapingo Ser. Hortic. 2011, 17, 9–13. [Google Scholar] [CrossRef]
- Soberanes-Pérez, A.; Calderón-Zavala, G.; López-Jiménez, A.; Alvarado-Raya, H.E. Biorreguladores para la producción de higo bajo condiciones de invernadero. Rev. Fitotec. Mex. 2020, 43, 61. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.-H.; Obermeyer, G.; Feijó, J.A. Glutamate Receptor-like Genes Form Ca2+ Channels in Pollen Tubes and Are Regulated by Pistil D-Serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef]
- Wudick, M.M.; Portes, M.T.; Michard, E.; Rosas-Santiago, P.; Lizzio, M.A.; Nunes, C.O.; Campos, C.; Santa Cruz Damineli, D.; Carvalho, J.C.; Lima, P.T.; et al. CORNICHON Sorting and Regulation of GLR Channels Underlie Pollen Tube Ca2+ Homeostasis. Science 2018, 360, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lv, D.G.; Qin, S.J.; Yang, L.; Ma, H.Y.; Liu, G.C. Changes in Photosynthesis, Fluorescence and Nitrogen Metabolism of Hawthorn (Crataegus pinnatifida) in Response to Exogenous Glutamic Acid. Photosynthetica 2010, 48, 339–347. [Google Scholar] [CrossRef]
- El-Shiekh, A.F.; Umaharan, P. Effect of Gibberellic Acid, Glutamic Acid and Pollen Grains Extract on Yield, Quality and Marketa-Bility of “khalas” Date Palm Fruits. Acta Hortic. 2014, 93–97. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Wu, H.-Y.; Juo, Y.-A.; Hsieh, M.-H. Exogenous Glutamate Rapidly Induces the Expression of Genes Involved in Metabolism and Defense Responses in Rice Roots. BMC Genom. 2017, 18, 186. [Google Scholar] [CrossRef]
- Li, Z.-G.; Ye, X.-Y.; Qiu, X.-M. Glutamate Signaling Enhances the Heat Tolerance of Maize Seedlings by Plant Glutamate Receptor-like Channels-Mediated Calcium Signaling. Protoplasma 2019, 256, 1165–1169. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 Mediate Cold Acclimation-Induced Chilling Tolerance by Regulating Apoplastic H2O2 Production and Redox Homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Yoshida, R.; Mori, I.C.; Kamizono, N.; Shichiri, Y.; Shimatani, T.; Miyata, F.; Honda, K.; Iwai, S. Glutamate Functions in Stomatal Closure in Arabidopsis and Fava Bean. J. Plant Res. 2016, 129, 39–49. [Google Scholar] [CrossRef]
- Cortleven, A.; Ehret, S.; Schmülling, T.; Johansson, H. Ethylene-Independent Promotion of Photomorphogenesis in the Dark by Cytokinin Requires COP1 and the CDD Complex. J. Exp. Bot. 2019, 70, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key Players in the Modulation of Heavy Metal Stress Tolerance in Plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Duarte, E. Regeneración de yemas adventicias en segmentos de hojas y entrenudos de Balfourodendron riedelianum (Engl.) Engl. Colomb. For. 2022, 25, 67–76. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xing, L.; Zhang, S.; Zhao, C.; Han, M. Effect of Exogenous 6-Benzylaminopurine (6-BA) on Branch Type, Floral Induction and Initiation and Related Gene Expression in ‘Fuji’ Apple (Malus domestica Borkh). Plant Growth Regul. 2016, 79, 65–70. [Google Scholar] [CrossRef]
- Mangena, P. Evolving Role of Synthetic Cytokinin 6-Benzyl Adenine for Drought Stress Tolerance in Soybean (Glycine max L. Merr.). Front. Sustain. Food Syst. 2022, 6, 01–14. [Google Scholar] [CrossRef]
- Ramy, G.E.-K.; Atef, M.K.N.; Ahmed, A.A.E.-S. The Role of Benzyl Amino Purine and Kinetin in Enhancing the Growth and Flowering of Three Gaillardia Varieties. Alex. J. Agric. Sci. 2019, 64, 277–288. [Google Scholar] [CrossRef]
- Burke, J.J. 6-Benzyladenine Enhancements of Cotton Yields. J. Cotton Sci. 2013, 17, 245–252. [Google Scholar]
- Yang, D.Q.; Luo, Y.L.; Dong, W.H.; Yin, Y.P.; Li, Y.; Wang, Z.L. Response of Photosystem II Performance and Antioxidant Enzyme Activities in Stay-Green Wheat to Cytokinin. Photosynthetica 2018, 56, 567–577. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.W.; Ren, T.; Li, P.F.; Liu, Q.X.; Li, X.K. Effects of Exogenous Cytokinin on Photosynthesis, Senescence, and Yield Performance of Inferior Rice Tillers Grown under Different Nitrogen Regimes. Photosynthetica 2020, 58, 137–145. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.L.; Wang, D.Y.; Huang, J.X.; Liu, Y.B.; Zhu, M.; Li, F.H. Mitigative Effect of 6-Benzyladenine on Photosynthetic Capacity and Leaf Ultrastructure of Maize Seedlings under Waterlogging Stress. Photosynthetica 2022, 60, 389–399. [Google Scholar] [CrossRef]
- Grimes, S.J.; Phillips, T.D.; Hahn, V.; Capezzone, F.; Graeff-Hönninger, S. Growth, Yield Performance and Quality Parameters of Three Early Flowering Chia (Salvia hispanica L.) Genotypes Cultivated in Southwestern Germany. Agriculture 2018, 8, 154. [Google Scholar] [CrossRef]
- Yang, H.; Tian, T.; Wu, D.; Guo, D.; Lu, J. Prevention and Treatment Effects of Edible Berries for Three Deadly Diseases: Cardiovascular Disease, Cancer and Diabetes. Crit. Rev. Food Sci. Nutr. 2019, 59, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wuyang, H.; Zheng, Y.; Dajing, L.; Yanhong, M.; Jianzhong, Z.; Zhongquan, S. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.-C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild Blueberry Proanthocyanidins Shape Distinct Gut Microbiota Profile and Influence Glucose Homeostasis and Intestinal Phenotypes in High-Fat High-Sucrose Fed Mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Heiss, C.; Williams, C.; Rodriguez-Mateos, A. Blueberries and Cardiovascular Disease Prevention. Food Funct. 2019, 10, 7621–7633. [Google Scholar] [CrossRef]
- Gill, K.; Kumar, P.; Negi, S.; Sharma, R.; Joshi, A.K.; Suprun, I.I.; Al-Nakib, E.A. Physiological Perspective of Plant Growth Regulators in Flowering, Fruit Setting and Ripening Process in Citrus. Sci. Hortic. 2023, 309, 111628. [Google Scholar] [CrossRef]
- Milyaev, A.; Kofler, J.; Klaiber, I.; Czemmel, S.; Pfannstiel, J.; Flachowsky, H.; Stefanelli, D.; Hanke, M.-V.; Wünsche, J.-N. Toward Systematic Understanding of Flower Bud Induction in Apple: A Multi-Omics Approach. Front. Plant. Sci. 2021, 12, 604810. [Google Scholar] [CrossRef]
- Agustí, M.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C. Advances in Citrus Flowering: A Review. Front. Plant. Sci. 2022, 13, 868831. [Google Scholar] [CrossRef]
- Ávila, J.; Salvo, S.; Muñoz, C. Comparison of Linear Regression Models Considering Heteroscedasticity of Fruits and Flower Buds of Highbush Blueberry Cultivated in Chile. Sci. Hortic. 2013, 151, 57–62. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Park, H.-G.; Kim, S.-M.; Park, J.-I.; Lee, E.-J.; Kim, H.L.; Kim, J.G. Flower and Leaf Bud Density Manipulation Affects Fruit Set, Leaf-to-Fruit Ratio, and Yield in Southern Highbush ‘Misty’ Blueberry. Sci. Hortic. 2021, 290, 110530. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Sadak, M.S.; Saudy, H.S. Stimulation Effects of Glutamic and 5-Aminolevulinic Acids on Photosynthetic Pigments, Physio-Biochemical Constituents, Antioxidant Activity and Yield of Peanut. Gesunde Pflanz. 2022, 74, 1–10. [Google Scholar] [CrossRef]
- Farid, M.; Farid, S.; Zubair, M.; Ghani, M.A.; Rizwan, M.; Ishaq, H.K.; Alkahtani, S.; Abdel-Daim, M.M.; Ali, S. Glutamic Acid-Assisted Phytomanagement of Chromium Contaminated Soil by Sunflower (Helianthus annuus L.): Morphophysiological and Biochemical Alterations. Front. Plant. Sci. 2020, 11, 1297. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, S.; Wang, L.; Liang, X.; Wang, X.; Wu, H.; Zou, H.; Zhang, C.; Wang, M. Foliar Spraying of 6-Benzylaminopurine Promotes Growth and Flavonoid Accumulation in Mulberry (Morus alba L.). J. Plant Growth Regul. 2022, 41, 2232–2245. [Google Scholar] [CrossRef]
- Valverde-Miranda, D.; Díaz-Pérez, M.; Gómez-Galán, M.; Callejón-Ferre, Á.-J. Total Soluble Solids and Dry Matter of Cucumber as Indicators of Shelf Life. Postharvest Biol. Technol. 2021, 180, 111603. [Google Scholar] [CrossRef]
- Ariza Flores, R.; Barrios Ayala, A.; Herrera García, M.; Barbosa Moreno, F.; Michel Aceves, A.; Otero Sánchez, M.A.; Alia Tejacal, I. Fitohormonas y bioestimulantes para la floración, producción y calidad de lima mexicana de invierno. Rev. Mex. Cienc. Agrícolas 2015, 6, 1653–1666. [Google Scholar] [CrossRef][Green Version]
- Madrid, M.; Beaudry, R. Small fruits: Raspberries, blackberries, blueberries. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut: Produce; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–346. ISBN 978-0-12-804599-2. [Google Scholar]
- FAO Protocolo de Calidad Para Arándanos Frescos. Boletin Oficial No. 31.163. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC071758 (accessed on 4 November 2022).
- Canli, F.; Pektas, M. Improving Fruit Size and Quality of Low Yielding and Small Fruited Pear Cultivars with Benzyladenine and Gibberellin Applications. Eur. J. Hortic. Sci. 2015, 80, 103–108. [Google Scholar] [CrossRef]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Magazin, N.; Miodragović, M.; Popara, G. Bioregulators Can Improve Fruit Size, Yield and Plant Growth of Northern Highbush Blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2018, 235, 214–220. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Jäger, A.K.; Johnson, S.D.; Van Staden, J. Influence of Plant Growth Regulators on Flowering, Fruiting, Seed Oil Content and Oil Quality of Jatropha Curcas. S. Afr. J. Bot. 2010, 76, 440–446. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.; Alberdi, M.; Mora, M.L.; Ulloa-Inostroza, E.M.; Ribera-Fonseca, A.E. Impact of Cold-Storage and UV-C Irradiation Postharvest Treatments on Quality and Antioxidant Properties of Fruits from Blueberry Cultivars Grown in Southern Chile. J. Soil Sci. Plant Nutr. 2020, 20, 1751–1758. [Google Scholar] [CrossRef]
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential Health Benefits of Anthocyanins in Oxidative Stress Related Disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Ling, D.Z.; Jian, L.L.; Hou, B.C. Effects of glutamic acid and TDZ (Thidiazuron) on the fruit coloration and quality of Litchi chinensis Sonn. J. Trop. Subtrop. Bot. 2012, 20, 382–387. [Google Scholar]
- Wang, L.; Wang, Z.H.; Li, Z.Q.; Zhu, Y.N. Promotion of L-Glutamic Acid on Anthocyanin Accumulation of Fuji Apples. J. Fruit Sci. 2006, 23, 157–160. [Google Scholar]
- Li, B.; Zhang, X.; Duan, R.; Han, C.; Yang, J.; Wang, L.; Wang, S.; Su, Y.; Wang, L.; Dong, Y.; et al. Genomic Analysis of the Glutathione S-Transferase Family in Pear (Pyrus communis) and Functional Identification of PcGST57 in Anthocyanin Accumulation. Int. J. Mol. Sci. 2022, 23, 746. [Google Scholar] [CrossRef]
- Han Jian, N.A.U.; Shang Gaopan, N.A.U.; Zhang Binbin, J.A.A.S. Effects of foliar spraying of L-glutamic acid and rhamnose solution on changes of pigment content and physiological properties in leaves of red-leaf peach in summer. J. Nanjing Agric. Univ. 2012, 35, 19–24. [Google Scholar]
- Amin, A.A.; Gharib, F.A.E.; El-Awadi, M.; Rashad, E.-S.M. Physiological Response of Onion Plants to Foliar Application of Putrescine and Glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Cui, Y.; De Guo, L.; Xing Ming, H.; Wen, D. Changes in Flavonoids Concentration of Hawthorn (Crataegus pinnatifida) in Response to Exogenous Amino Acids. J. Hortic. For. 2015, 7, 193–199. [Google Scholar] [CrossRef][Green Version]
- Chen, B.; Yang, H. 6-Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. J. Sci. Food Agric. 2013, 93, 1915–1921. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of Exogenous Application of Salt Stress and Glutamic Acid on Lettuce (Lactuca sativa L.). Sci. Hortic. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Fardus, J.; Hossain, M.S.; Fujita, M. Modulation of the Antioxidant Defense System by Exogenous L-Glutamic Acid Application Enhances Salt Tolerance in Lentil (Lens culinaris Medik.). Biomolecules 2021, 11, 587. [Google Scholar] [CrossRef]
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of Oxidative Stress and Antioxidative Defense Mechanisms in Gossypium hirsutum L. in Response to Extreme Abiotic Conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Yao, X.; Zhu, Z.; Xu, S.; Zha, D. Exogenous 6-Benzylaminopurine Confers Tolerance to Low Temperature by Amelioration of Oxidative Damage in Eggplant (Solanum melongena L.) Seedlings. Braz. J. Bot. 2016, 39, 409–416. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Paul Bolwell, G. Reactive Oxygen Species and Their Role in Plant Defence and Cell Wall Metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Fardus, J.; Hossain, M.S.; Fujita, M. Potential Role of L-Glutamic Acid in Mitigating Cadmium Toxicity in Lentil (Lens culinaris Medik.) through Modulating the Antioxidant Defence System and Nutrient Homeostasis. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12485. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and Seed Application of Amino Acids Affects the Antioxidant Metabolism of the Soybean Crop. Front. Plant. Sci. 2017, 8, 327. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effect of Selenium Application on Phenylalanine Ammonia-Lyase (PAL) Activity, Phenol Leakage and Total Phenolic Content in Garlic (Allium sativum L.) under NaCl Stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- QiaoZhen, L.; Ben, X.; YanLi, S.; WeiRong, X.; HongJun, D. Effects of exogenous 6-BA on anthocyanin content and expression of related genes in grape berry. Nat. Sci. Ed. 2019, 47, 112–125. [Google Scholar]
- Chen, J.-Y.; Wen, P.-F.; Kong, W.-F.; Pan, Q.-H.; Zhan, J.-C.; Li, J.-M.; Wan, S.-B.; Huang, W.-D. Effect of Salicylic Acid on Phenylpropanoids and Phenylalanine Ammonia-Lyase in Harvested Grape Berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Capocasa, F.; Scalzo, J.; Mezzetti, B.; Battino, M. Combining Quality and Antioxidant Attributes in the Strawberry: The Role of Genotype. Food Chem. 2008, 111, 872–878. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Daruwala, R.; Wang, Y.; Eck, P.K.; Song, J.; Koh, W.S.; Levine, M. Vitamina C: De las acciones moleculares a la ingesta óptima. In Manual de Antioxidantes; Oregon State University: Corvallis, OR, USA, 2001; ISBN 978-0-429-20758-7. [Google Scholar]
- Yu, Z.; Dahlgren, R.A. Evaluation of Methods for Measuring Polyphenols in Conifer Foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and Growth-Promoting Effect of Selenium on Senescing Lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf Senescence and Lipid Peroxidation: Effects of Some Phytohormones and Scavengers of Free Radicals and Singlet Oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzym. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Lojkowska, E.; Chinou, I. Effect of L-Phenylalanine on PAL Activity and Production of Naphthoquinone Pigments in Suspension Cultures of Arnebia Euchroma (Royle) Johnst. Vitr. Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 273–280. ISBN 978-1-60761-702-0. [Google Scholar]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Munira, S.; Hossain, M.; Zakaria, M.; Ahmed, J.; Islam, M. Evaluation of Potato Varieties against Salinity Stress in Bangladesh. Int. J. Plant Soil Sci. 2015, 6, 73–81. [Google Scholar] [CrossRef]
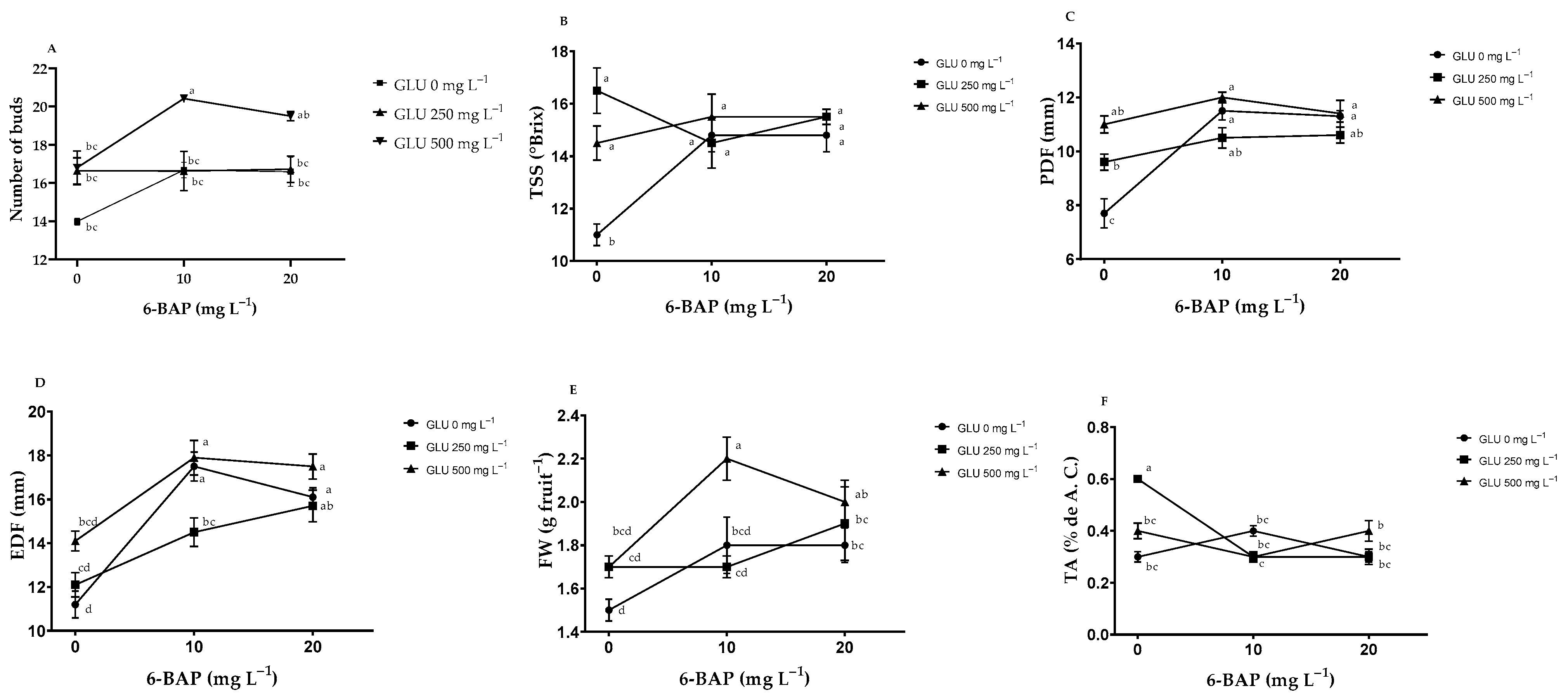
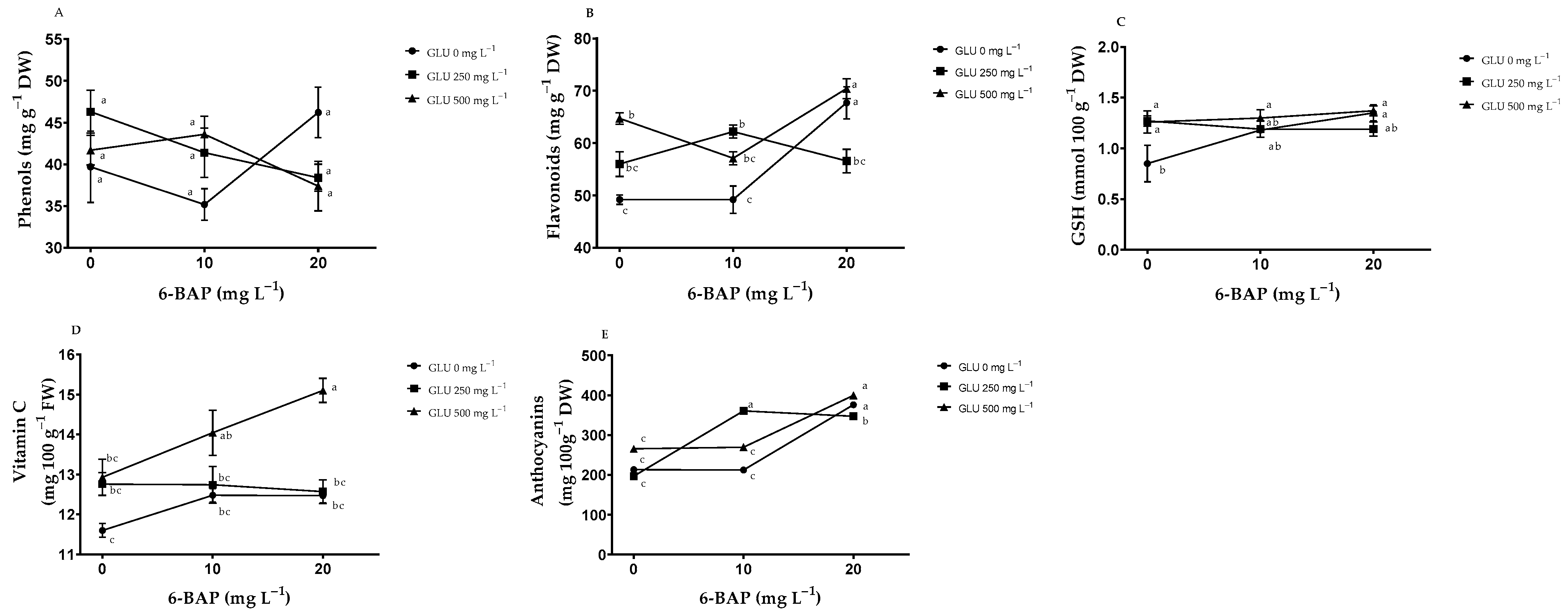
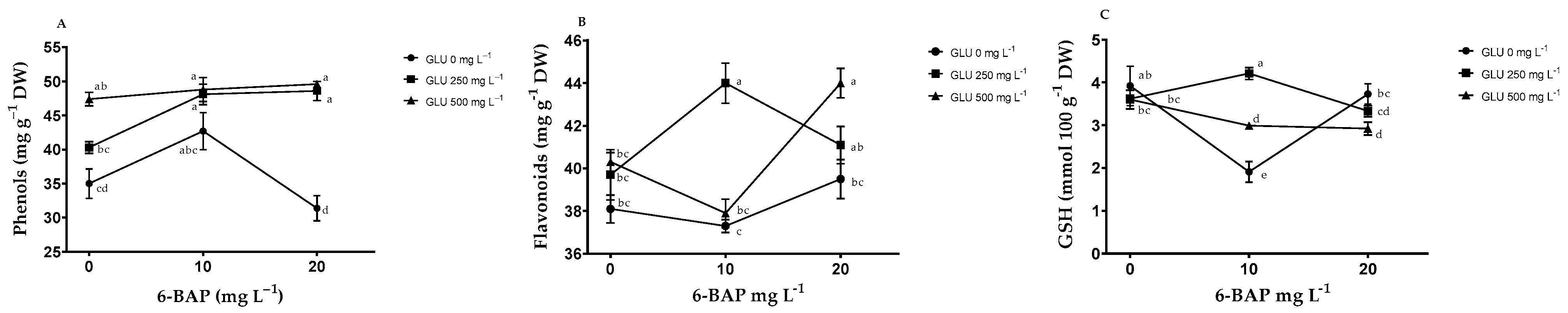
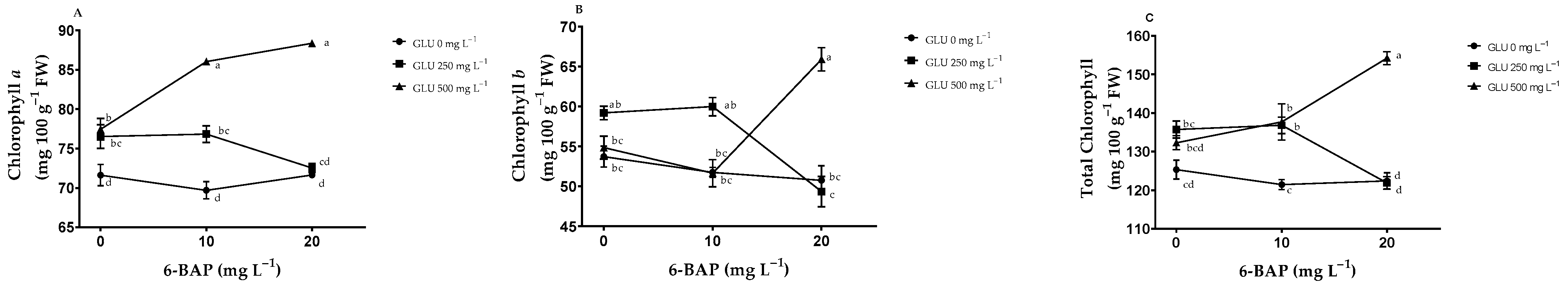
| Treatments | NB | TSS (°Brix) | PDF (mm) | EDF (mm) | FW (g) | TA (% de A. C.) | |
|---|---|---|---|---|---|---|---|
| GLU | 0 | 15.40 ± 0.41 b | 13.50 ±0.61b | 10.17±0.56 b | 14.93 ± 0.87 b | 1.69 ± 0.04 b | 0.32 ± 0.01 b |
| 250 | 16.70 ± 0.43 b | 15.17 ±0.47a | 10.22 ±0.20 b | 14.06 ± 0.56 b | 1.73 ± 0.04 b | 0.40 ± 0.04 a | |
| 500 | 18.90 ± 0.54 a | 15.50 ±0.37a | 11.45 ±0.22 a | 16.48 ± 0.61 a | 1.95 ± 0.06 a | 0.37 ± 0.03 ab | |
| ANOVA | <0.0001 | 0.0021 | <0.0001 | 0.0002 | <0.0001 | 0.0366 | |
| 6-BAP | 0 | 15.46± 0.47 b | 14.00±0.77 a | 9.43± 0.46 b | 12.45 ± 0.46 b | 1.64 ± 0.03 b | 0.43 ± 0.03 a |
| 10 | 17.60 ± 0.63 a | 14.92 ±0.45 a | 11.32 ±0.25 a | 16.62 ± 0.59 a | 1.72 ± 0.07 a | 0.30 ± 0.02 b | |
| 20 | 17.90 ± 0.51 a | 15.25 ±0.25 a | 11.18 ±0.20 a | 16.39 ± 0.39 a | 1.87 ± 0.04 a | 0.36 ± 0.03 ab | |
| ANOVA | 0.0016 | 0.0748 | <0.0001 | <0.0001 | <0.0001 | 0.0016 | |
| GLU*6-BAP | ANOVA | 0.0082 | 0.0250 | 0.0002 | 0.0427 | 0.0006 | 0.0007 |
| CV | 7.35 | 9.01 | 6.52 | 8.09 | 18.21 | 16.31 | |
| Treatments | Phenols (mg g−1 DW) | Flavonoids (mg g−1 DW) | GSH (mmol 100 g−1 DW) | Vitamin C (mg 100 g−1 FW) | Anthocyanins (mg 100 g−1 DW) | |
|---|---|---|---|---|---|---|
| GLU | 0 | 40.35 ± 8.13 a | 55.38 ± 10.31 b | 1.13 ± 0.31 b | 12.16 ± 0.17 b | 267.15 ± 18.58 b |
| 250 | 42.04 ± 6.08 a | 58.25 ± 5.07 b | 1.22 ± 0.07 ab | 12.69 ± 0.18 b | 301.68 ± 17.88 a | |
| 500 | 40.91 ± 5.56 a | 64.08 ± 6.39 a | 1.31 ± 0.15 a | 14.18 ± 0.44 a | 311.59 ± 16.67 a | |
| ANOVA | 0.7392 | <0.0001 | 0.0188 | <0.0001 | 0.006 | |
| 6-BAP | 0 | 42.58 ± 6.93 a | 56.63 ± 7.34 b | 1.13 ± 0.33 b | 12.42 ± 0.27 b | 225.22 ± 7.87 c |
| 10 | 40.07 ± 6.15 a | 56.17 ± 6.69 b | 1.22 ± 0.08 ab | 13.09 ± 0.32 b | 281.08 ± 13.09 b | |
| 20 | 40.64 ± 6.78 a | 64.91 ± 8.02 a | 1.30 ± 0.10 a | 13.52 ± 0.53 a | 374.11 ± 5.97 a | |
| ANOVA | 0.4956 | <0.0001 | 0.0266 | 0.0039 | <0.0001 | |
| GLU*6-BAP | ANOVA | 0.148 | <0.0001 | 0.009 | 0.0096 | <0.0001 |
| CV | 14.65 | 7.51 | 13.87 | 4.62 | 12.65 | |
| Treatments | Phenols (mg g−1 DW) | Flavonoids (mg g−1 DW) | GSH (mmol 100 g−1 DW) | |
|---|---|---|---|---|
| GLU | 0 | 36.36 ± 6.77 b | 38.32 ± 1.67 b | 3.19 ± 0.98 b |
| 250 | 45.64 ± 4.72 a | 41.58 ± 2.79 a | 3.72 ± 0.39 a | |
| 500 | 48.61 ± 2.64 a | 40.74 ± 2.88 a | 3.17 ± 0.35 b | |
| ANOVA | <0.0001 | 0.1639 | <0.0001 | |
| 6-BAP | 0 | 40.9 ± 6.05 b | 39.08 ± 2.03 b | 3.71 ± 0.31 a |
| 10 | 46.52 ± 5.10 a | 40.01 ± 3.31 ab | 3.04 ± 0.98 c | |
| 20 | 43.18 ± 9.10 b | 41.54 ± 2.54 a | 3.33 ± 0.38 b | |
| ANOVA | 0.0008 | 0.132 | <0.0001 | |
| GLU*6-BAP | ANOVA | 0.0009 | <0.0001 | <0.0001 |
| CV | 8.48 | 4.57 | 6.62 | |
| Treatments | Chlorophyll a (mg 100 g−1 FW) | Chlorophyll b (mg 100 g−1 FW) | Total Chlorophyll (mg 100 g−1 FW) | |
|---|---|---|---|---|
| GLU | 0 | 70.99 ± 2.32 c | 52.07 ± 3.05 b | 123.06 ± 2.15 c |
| 250 | 75.29 ± 3.07 b | 56.18 ± 5.75 a | 131.47 ± 3.11 b | |
| 500 | 83.95 ± 5,15 a | 57.46 ± 2.27 a | 141.41 ± 5.99 a | |
| ANOVA | <0.0001 | 0.0068 | <0.0001 | |
| 6-BAP | 0 | 75.19 ± 3.95 b | 55.92 ± 3.52 a | 131.11 ± 2.64 a |
| 10 | 77.52 ± 7.16 a | 54.46 ± 5.09 a | 131.98 ± 3.58 a | |
| 20 | 77.51 ± 8 a | 55.33 ± 6.54 a | 132.85 ± 6.16 a | |
| ANOVA | 0.0084 | 0.6781 | 0.6814 | |
| GLU*6-BAP | ANOVA | <0.0001 | <0.0001 | <0.0001 |
| CV | 2.89 | 8.24 | 4.08 | |
| Treatments | CAT (U g 100 g−1 TP) | PAL (U g 100 g−1 TP) | GPX (U g 100 g−1 TP) | APX (U g 100 g−1 TP) | |
|---|---|---|---|---|---|
| GLU | 0 | 3.98 ± 1.40 b | 0.57 ± 0.10 a | 3.98 ± 1.18 b | 6.76 ± 1.10 ab |
| 250 | 3.34 ± 1.36 b | 0.49 ± 0.06 b | 3.71 ± 0.86 b | 6.92 ± 1.35 a | |
| 500 | 5.06 ± 0.91 a | 0.61 ± 0.16 a | 5.1 ± 0.61 a | 5.93 ± 0.95 b | |
| ANOVA | 0.0009 | 0.0025 | 0.0001 | 0.0356 | |
| 6-BAP | 0 | 4.09 ± 1.21 a | 0.5 ± 0.07 b | 3.76 ± 1.22 b | 6.53 ± 1.18 a |
| 10 | 4.38 ± 1.55 a | 0.55 ± 0.14 ab | 4.51 ± 1.14 a | 6.47 ± 1.11 a | |
| 20 | 3.92 ± 1.51 a | 0.63 ± 0.11 a | 4.52 ± 0.71 a | 6.62 ± 1.31 a | |
| ANOVA | 0.5495 | 0.0012 | 0.0192 | 0.9229 | |
| GLU*6-BAP | ANOVA | 0.0318 | 0.0272 | 0.058 | 0.0725 |
| CV | 27.81 | 16.04 | 18.76 | 17.4 | |
| Treatments | CAT (U g 100 g−1 TP) | PAL (U g 100 g−1 TP) | GPX (U g 100 g−1 TP) | APX (U g 100 g−1 TP) | |
|---|---|---|---|---|---|
| GLU | 0 | 147.03 ± 11.34 b | 46.93 ± 6.0 a | 460.51 ± 20.86 a | 20.33 ± 0.71 b |
| 250 | 180.42 ± 6.48 b | 51.70 ± 5.74 a | 512.97 ± 11.93 a | 19.59 ± 0.55 b | |
| 500 | 247.42 ± 9.09 a | 46.93 ± 4.13 a | 437.05 ± 5.94 a | 27.69 ± 1.46 a | |
| ANOVA | 0.0001 | 0.2278 | 0.0729 | <0.0001 | |
| 6-BAP | 0 | 174.77 ± 7.37 b | 52.11 ± 5.18 a | 492.11 ± 14.52 a | 20.03 ± 0.90 b |
| 10 | 168.17 ± 15.98 b | 50.10 ± 6.68 a | 407.29 ± 17.51 b | 21.96 ± 1.28 b | |
| 20 | 231.93 ± 11.87 a | 53.14 ± 4.21 a | 511.14 ± 18.57 a | 25.60 ± 1.49 a | |
| ANOVA | 0.0062 | 0.8588 | 0.0071 | <0.0001 | |
| GLU*6-BAP | ANOVA | 0.3328 | <0.0001 | <0.0001 | 0.0067 |
| C.V. | 29.26 | 29.52 | 19.07 | 11.83 | |
| Phenological Stage | mEq L−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CE | pH | NO3− | NH4+ | H2PO4− | SO42− | K+ | Ca2+ | Mg2+ | |
| Vegetative | 1.1–1.2 | 5.0–5.5 | 4 | 5 | 1.5 | 5.5 | 2.5 | 2 | 1.5 |
| Differentiation Flowering | 0.8–0.9 | 5.0–5.5 | 2 | 2 | 1.5 | 5 | 3.5 | 2 | 1.0 |
| Fruit production | 1.1–1.3 | 5.0–5.5 | 3 | 3 | 1.5 | 6 | 4 | 2.25 | 1.25 |
| Treatment | GLU (mg L−1) | 6-BAP (mg L−1) | Keys |
|---|---|---|---|
| T1 * | 0 | 0 | 0–0 mg L−1 |
| T2 | 0 | 10 | 0–10 mg L−1 |
| T3 | 0 | 20 | 0–20 mg L−1 |
| T4 | 250 | 0 | 250–0 mg L−1 |
| T5 | 250 | 10 | 250–10 mg L−1 |
| T6 | 250 | 20 | 250–20 mg L−1 |
| T7 | 500 | 0 | 500–0 mg L−1 |
| T8 | 500 | 10 | 50–10 mg L−1 |
| T9 | 500 | 20 | 500–20 mg L−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-León, M.I.; González-Fuentes, J.A.; Valdez-Aguilar, L.A.; Benavides-Mendoza, A.; Alvarado-Camarillo, D.; Castillo-Chacón, C.E. Effect of Glutamic Acid and 6-benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry. Plants 2023, 12, 2363. https://doi.org/10.3390/plants12122363
Pérez-León MI, González-Fuentes JA, Valdez-Aguilar LA, Benavides-Mendoza A, Alvarado-Camarillo D, Castillo-Chacón CE. Effect of Glutamic Acid and 6-benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry. Plants. 2023; 12(12):2363. https://doi.org/10.3390/plants12122363
Chicago/Turabian StylePérez-León, María Itzel, José Antonio González-Fuentes, Luis Alonso Valdez-Aguilar, Adalberto Benavides-Mendoza, Daniela Alvarado-Camarillo, and Carlos Estuardo Castillo-Chacón. 2023. "Effect of Glutamic Acid and 6-benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry" Plants 12, no. 12: 2363. https://doi.org/10.3390/plants12122363
APA StylePérez-León, M. I., González-Fuentes, J. A., Valdez-Aguilar, L. A., Benavides-Mendoza, A., Alvarado-Camarillo, D., & Castillo-Chacón, C. E. (2023). Effect of Glutamic Acid and 6-benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry. Plants, 12(12), 2363. https://doi.org/10.3390/plants12122363








