Aroma Compounds in Essential Oils: Analyzing Chemical Composition Using Two-Dimensional Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry Combined with Chemometrics
Abstract
:1. Introduction
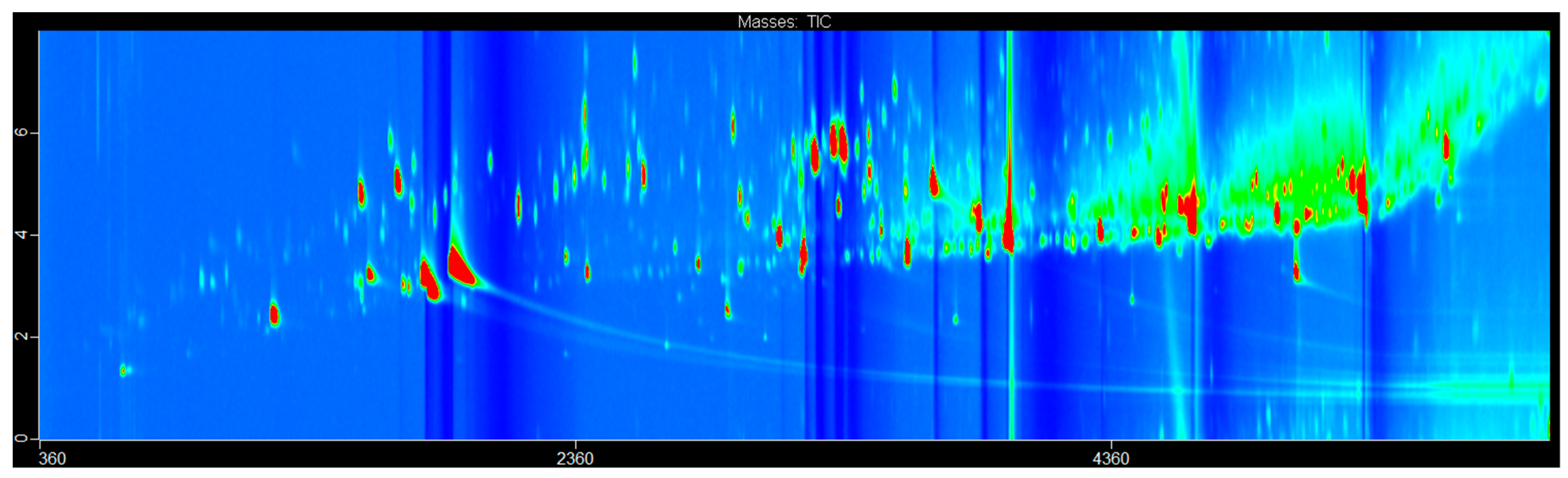
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals, Materials and Samples
3.2. Instrumentation
3.3. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mileva, M.; Kusovski, V.K.; Krastev, D.S.; Dobreva, A.M.; Galabov, A.S. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 11–20. [Google Scholar]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N. Rose flowers—A delicate perfume or a natural healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef]
- Younis, A.; Khan, M.A.; Khan, A.A.; Riaz, A.; Pervez, M.A. Effect of different extraction methods on yield and quality of essential oil from four Rosa species. Floric. Ornam. Biotechnol. 2007, 1, 73–76. [Google Scholar]
- Rusanov, K.; Kovacheva, N.; Atanassov, A.; Atanassov, I. Rosa damascena Mill., the oil-bearing Damask rose: Genetic resources, diversity and perspectives for molecular breeding. Floric. Ornam. Biotechnol. 2009, 3, 14–20. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Chemical composition of essential oil and rose-water extract of Himalayan Musk Rose (Rosa brunonii Lindl.) from Kumaon region of western Himalaya. J. Essent. Oil Res. 2016, 28, 332–338. [Google Scholar] [CrossRef]
- Caissard, J.-C.; Bergougnoux, V.; Martin, M.; Mauriat, M.; Baudino, S. Chemical and histochemical analysis of ‘Quatre Saisons Blanc Mousseux’, a moss rose of the Rosa× damascena group. Ann. Bot. 2006, 97, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Dobreva, A.; Nedeltcheva-Antonova, D.; Nenov, N.; Getchovska, K.; Antonov, L. Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling. Molecules 2021, 26, 4991. [Google Scholar] [CrossRef] [PubMed]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative evaluation of antimicrobial activity and composition of rose oils from various geographic origins, in particular Bulgarian rose oil. Nat. Prod. Commun. 2008, 3, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, M.; Marković, T.; Marković, D.; Calhelha, R.; Fernandes, Â.; Ferreira, I.; Stojković, D.; Ćirić, A.; Glamočlija, J.; Soković, M. Chemical composition and biological properties of Pelargonium graveolens, Leptospermum petersonii and Cymbopogon martinii var. motia essential oils and of Rosa centifolia absolute. J. Serb. Chem. Soc. 2021, 86, 1291–1303. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ansari, S.H.; Mohd, A.; Najmi, A.K. K.Volatile oil composition of Rosa damascena Mill. (Rosaceae). J. Pharmacogn. Phytochem. 2014, 2, 177–181. [Google Scholar]
- Antonelli, A.; Fabbri, C.; Giorgioni, M.E.; Bazzocchi, R. Characterization of 24 old garden roses from their volatile compositions. J. Agric. Food Chem. 1997, 45, 4435–4439. [Google Scholar] [CrossRef]
- Gochev, V.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.; Dobreva, A. Chemical composition and antimicrobial activity of historical rose oil from Bulgaria. J. Essent. Oil-Bear. Plants 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Marney, L.C.; Siegler, W.C.; Parsons, B.A.; Hoggard, J.C.; Wright, B.W.; Synovec, R.E. Tile-based Fisher-ratio software for improved feature selection analysis of comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry data. Talanta 2013, 115, 887–895. [Google Scholar] [CrossRef]
- Parsons, B.A.; Pinkerton, D.K.; Wright, B.W.; Synovec, R.E. Chemical characterization of the acid alteration of diesel fuel: Non-targeted analysis by two-dimensional gas chromatography coupled with time-of-flight mass spectrometry with tile-based Fisher ratio and combinatorial threshold determination. J. Chromatogr. A 2016, 1440, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinklein, T.J.; Jiang, J.; Synovec, R.E. Profiling Olefins in Gasoline by Bromination Using GC× GC-TOFMS Followed by Discovery-Based Comparative Analysis. Anal. Chem. 2022, 94, 9407–9414. [Google Scholar] [CrossRef]
- Trinklein, T.J.; Synovec, R.E. Simulating comprehensive two-dimensional gas chromatography mass spectrometry data with realistic run-to-run shifting to evaluate the robustness of tile-based Fisher ratio analysis. J. Chromatogr. A 2022, 1677, 463321. [Google Scholar] [CrossRef]
- Cain, C.; Synovec, R. New Perspectives on Comparative Analysis for Comprehensive Two-Dimensional Gas Chromatography. LC GC N. Am. 2022, 40, 364–367. [Google Scholar] [CrossRef]
- Jimenez, A.C.; Heist, C.A.; Navaei, M.; Yeago, C.; Roy, K. Longitudinal two-dimensional gas chromatography mass spectrometry as a non-destructive at-line monitoring tool during cell manufacturing identifies volatile features correlative to cell product quality. Cytotherapy 2022, 24, 1136–1147. [Google Scholar] [CrossRef]
- Schöneich, S.; Ochoa, G.S.; Monzón, C.M.; Synovec, R.E. Minimum variance optimized Fisher ratio analysis of comprehensive two-dimensional gas chromatography/mass spectrometry data: Study of the pacu fish metabolome. J. Chromatogr. A 2022, 1667, 462868. [Google Scholar] [CrossRef] [PubMed]
- Pesesse, R.; Stefanuto, P.-H.; Schleich, F.; Louis, R.; Focant, J.-F. Multimodal chemometric approach for the analysis of human exhaled breath in lung cancer patients by TD-GC× GC-TOFMS. J. Chromatogr. B 2019, 1114, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Mikaliunaite, L.; Synovec, R.E. Computational method for untargeted determination of cycling yeast metabolites using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Talanta 2022, 244, 123396. [Google Scholar] [CrossRef]
- Sudol, P.E.; Galletta, M.; Tranchida, P.Q.; Zoccali, M.; Mondello, L.; Synovec, R.E. Untargeted profiling and differentiation of geographical variants of wine samples using headspace solid-phase microextraction flow-modulated comprehensive two-dimensional gas chromatography with the support of tile-based Fisher ratio analysis. J. Chromatogr. A 2022, 1662, 462735. [Google Scholar] [CrossRef]
- Zou, Y.; Gaida, M.; Franchina, F.A.; Stefanuto, P.-H.; Focant, J.-F. Distinguishing between Decaffeinated and Regular Coffee by HS-SPME-GC× GC-TOFMS, Chemometrics, and Machine Learning. Molecules 2022, 27, 1806. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.T.; Beaux, M.F.; Freye, C.E. Evaluation of Different Getter Substrates Using Two-Dimensional Gas Chromatography with Time of Flight Mass Spectrometry. J. Chromatogr. A 2023, 1689, 463760. [Google Scholar] [CrossRef] [PubMed]
- Mazur, D.; Sosnova, A.; Latkin, T.; Artaev, B.; Siek, K.; Koluntaev, D.; Lebedev, A. Application of clusterization algorithms for analysis of semivolatile pollutants in Arkhangelsk snow. Anal. Bioanal. Chem. 2023, 415, 2587–2599. [Google Scholar] [CrossRef]
- Titaley, I.A.; Ogba, O.M.; Chibwe, L.; Hoh, E.; Cheong, P.H.-Y.; Simonich, S.L.M. Automating data analysis for two-dimensional gas chromatography/time-of-flight mass spectrometry non-targeted analysis of comparative samples. J. Chromatogr. A 2018, 1541, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Differentiation of wines according to grape variety using multivariate analysis of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection data. Food Chem. 2013, 141, 3897–3905. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.; Morrison, P.; Marriott, P. Orthogonality considerations in comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2005, 1071, 47–53. [Google Scholar] [CrossRef]
- Cordero, C.; Rubiolo, P.; Cobelli, L.; Stani, G.; Miliazza, A.; Giardina, M.; Firor, R.; Bicchi, C. Potential of the reversed-inject differential flow modulator for comprehensive two-dimensional gas chromatography in the quantitative profiling and fingerprinting of essential oils of different complexity. J. Chromatogr. A 2015, 1417, 79–95. [Google Scholar] [CrossRef]
- Dimandja, J.M.D.; Stanfill, S.B.; Grainger, J.; Patterson Jr, D.G. Application of comprehensive two-dimensional gas chromatography (GC× GC) to the qualitative analysis of essential oils. J. High Resolut. Chromatogr. 2000, 23, 208–214. [Google Scholar] [CrossRef]
- Marriott, P.; Shellie, R.; Fergeus, J.; Ong, R.; Morrison, P. High resolution essential oil analysis by using comprehensive gas chromatographic methodology. Flavour Fragr. J. 2000, 15, 225–239. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P. Opportunities for ultra-high resolution analysis of essential oils using comprehensive two-dimensional gas chromatography: A review. Flavour Fragr. J. 2003, 18, 179–191. [Google Scholar] [CrossRef]
- Gomes da Silva, M.; Cardeal, Z.; Marriott, P. Comprehensive Two-Dimensional Gas Chromatography: Application to Aroma and Essential Oil Analysis. ACS Publications: Washington, DC, USA, 2008. [Google Scholar]
- Zhu, S.; Lu, X.; Xing, J.; Zhang, S.; Kong, H.; Xu, G.; Wu, C. Comparison of comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry and gas chromatography–mass spectrometry for the analysis of tobacco essential oils. Anal. Chim. Acta 2005, 545, 224–231. [Google Scholar] [CrossRef]
- Li, J.; Qian, C.; Duan, T.; Cai, T.; Xiang, Z. Determination of the volatiles in Rosa chinensis cultivars by comprehensive two-dimensional gas chromatography (GC× GC) and quadrupole time-of-flight (QTOF) mass spectrometry (MS). Anal. Lett. 2021, 54, 573–589. [Google Scholar] [CrossRef]
- Filippi, J.-J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2013, 1288, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ghosh, D.; Chaudhary, N.; Chanotiya, C.S. Rainfall-induced premature senescence modulates biochemical and essential oils profiles in Pelargonium graveolens L′ Hér. under sub-tropical climate. Ind. Crops Prod. 2022, 178, 114630. [Google Scholar] [CrossRef]
- Nur, Ç. Chemical Fingerprinting of the Geranium (Pelargonium graveolens) Essential Oil by Using FTIR, Raman and GC-MS Techniques. Eur. J. Sci. Technol. 2021, 25, 810–814. [Google Scholar]
- Gupta, R.; Mallavarapu, G.; Banerjee, S.; Kumar, S. Characteristics of an isomenthone-rich somaclonal mutant isolated in a geraniol-rich rose-scented geranium accession of Pelargonium graveolens. Flavour Fragr. J. 2001, 16, 319–324. [Google Scholar] [CrossRef]
- Möllenbeck, S.; König, T.; Schreier, P.; Schwab, W.; Rajaonarivony, J.; Ranarivelo, L. Chemical composition and analyses of enantiomers of essential oils from Madagascar. Flavour Fragr. J. 1997, 12, 63–69. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography–mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Zhang, H.; Setzer, W.N. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am. J. Essent. Oil. Nat. Prod. 2014, 2, 13–16. [Google Scholar]
- Mnif, W.; Dhifi, W.; Jelali, N.; Baaziz, H.; Hadded, A.; Hamdi, N. Characterization of leaves essential oil of Pelargonium graveolens originating from Tunisia: Chemical composition, antioxidant and biological activities. J. Essent. Oil-Bear. Plants 2011, 14, 761–769. [Google Scholar] [CrossRef]
- Ahamad, J.; Uthirapathy, S. Chemical characterization and antidiabetic activity of essential oils from Pelargonium graveolens leaves. ARO-Sci. J. Koya Univ. 2021, 9, 109–113. [Google Scholar]
- Marinkovic, J.; Markovic, T.; Nikolic, B.; Soldatovic, I.; Ivanov, M.; Ciric, A.; Sokovic, M.; Markovic, D. Antibacterial and Antibiofilm Potential of Leptospermum petersonii FM Bailey, Eucalyptus citriodora Hook., Pelargonium graveolens L’Hér. and Pelargonium roseum (Andrews) DC. Essential Oils Against Selected Dental Isolates. J. Essent. Oil-Bear. Plants 2021, 24, 304–316. [Google Scholar] [CrossRef]
- Shamspur, T.; Mostafavi, A. Chemical composition of the volatile oil of Rosa kazanlik and Rosa gallica from Kerman Province in Iran. J. Essent. Oil-Bear. Plants 2010, 13, 78–84. [Google Scholar] [CrossRef]
- Hosni, K. Rosa× alba: Source of essential minerals and volatile oils. Nat. Prod. Bioprospect. 2011, 1, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Gateva, S.; Jovtchev, G.; Chanev, C.; Georgieva, A.; Stankov, A.; Dobreva, A.; Mileva, M. Assessment of anti-cytotoxic, anti-genotoxic and antioxidant potentials of Bulgarian Rosa alba L. essential Oil. Caryologia 2020, 73. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Khamechian, T.; Fallah, M.H.; Kermani, M.M. Chemical composition and antimicrobial activity of Rosa damascena Mill essential oil. J. Biol. Act. Prod. Nat. 2011, 1, 19–26. [Google Scholar]
- Babu, K.G.; Singh, B.; Joshi, V.P.; Singh, V. Essential oil composition of Damask rose (Rosa damascena Mill.) distilled under different pressures and temperatures. Flavour Fragr. J. 2002, 17, 136–140. [Google Scholar] [CrossRef]
- Mileva, M.; Krumova, E.; Miteva-Staleva, J.; Kostadinova, N.; Dobreva, A.; Galabov, A.S. Chemical compounds, in vitr o antioxidant and antifungal activities of some plant essentia l oils belonging to Rosaceae family. Compt. Rend. Acad. Bulg. Sci. 2014, 67, 1363–1368. [Google Scholar]
- Farooq, A.; Younis, A.; Qasim, M.; RlAZ, A.; Abbas, S.M.; Tariq, U. Gas chromatography analysis of the absolute rose oil from Rosa damascena landraces and scented rose species from Pakistan. Int. J. Agric. Biol. 2012, 14, 714–719. [Google Scholar]
- Kumar, R.; Sharma, S.; Sood, S.; Agnihotri, V.K. Agronomic interventions for the improvement of essential oil content and composition of damask rose (Rosa damascena Mill.) under western Himalayas. Ind. Crops Prod. 2013, 48, 171–177. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Rusanov, K.E.; Kovacheva, N.M.; Atanassov, I.I. Comparative GC/MS analysis of rose flower and distilled oil volatiles of the oil bearing rose Rosa damascena. Biotechnol. Biotechnol. Equip. 2011, 25, 2210–2216. [Google Scholar] [CrossRef] [Green Version]
- Berechet, M.D.; Calinescu, I.; Stelescu, M.D.; Manaila, E.; Craciun, G.; Purcareanu, B.; Mihaiescu, D.E.; Rosca, S.; Fudulu, A.; Niculescu-Aron, I.G. Composition of the essential oil of Rosa damascena Mill. cultivated in Romania. Rev. Chim 2015, 66, 1986–1991. [Google Scholar]
- Tabari, M.A.; Youssefi, M.R.; Nasiri, M.; Hamidi, M.; Kiani, K.; Samakkhah, S.A.; Maggi, F. Towards green drugs against cestodes: Effectiveness of Pelargonium roseum and Ferula gummosa essential oils and their main component on Echinococcus granulosus protoscoleces. Vet. Parasitol. 2019, 266, 84–87. [Google Scholar] [CrossRef]
- Carmen, G.; Carolroseum, C. Phytochemical study of the plant Pelargonium roseum (P. radula). Available online: https://www.umfst.ro/doctorat_arhiva/galea_carmen.pdf (accessed on 14 June 2023).
- Abouhosseini Tabari, M.; Hajizadeh Moghaddam, A.; Maggi, F.; Benelli, G. Anxiolytic and antidepressant activities of Pelargonium roseum essential oil on Swiss albino mice: Possible involvement of serotonergic transmission. Phytother. Res. 2018, 32, 1014–1022. [Google Scholar] [CrossRef]
- Ghannadi, A.; Bagherinejad, M.; Abedi, D.; Jalali, M.; Absalan, B.; Sadeghi, N. Antibacterial activity and composition of essential oils from Pelargonium graveolens L’Her and Vitex agnus-castus L. Iran. J. Microbiol. 2012, 4, 171. [Google Scholar] [PubMed]
- Verma, R.S.; Rahman, L.U.; Verma, R.K.; Chauhan, A.; Singh, A. Essential oil composition of Pelargonium graveolens L’Her ex Ait. cultivars harvested in different seasons. J. Essent. Oil Res. 2013, 25, 372–379. [Google Scholar] [CrossRef]
- Schmarr, H.-G.; Eisenreich, W.; Engel, K.-H. Synthesis and analysis of thio-, thiono-, and dithio-derivatives of whiskey lactone. J. Agric. Food Chem. 2001, 49, 5923–5928. [Google Scholar] [CrossRef]
- Wei, Y.; Du, J.; Lu, Y. Preparative separation of bioactive compounds from essential oil of Flaveria bidentis (L.) K untze using steam distillation extraction and one step high-speed counter-current chromatography. J. Sep. Sci. 2012, 35, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Fujimori, T.; Sato, H.; Ishikawa, G.; Kami, K.; Ohashi, Y. Statistical hypothesis testing of factor loading in principal component analysis and its application to metabolite set enrichment analysis. BMC Bioinform. 2014, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Feature No. | Compound | EC, % | k | ||||
|---|---|---|---|---|---|---|---|
| R. damascena cv. “Janina” | R. gallica | R. alba | R.centifolia | P. roseum | |||
| 1 | Citronellol I | 89.27 | 49.57 | 14.92 | 43.99 | 91.13 | 1.00 |
| 2 | Citronellol II | 10.73 | 50.43 | 85.08 | 56.01 | 8.87 | |
| 3 | Citronellyl propionate I | 46.33 | 35.56 | 10.60 | 20.51 | 81.18 | 1.87 |
| 4 | Citronellyl propionate II | 53.67 | 64.44 | 89.40 | 79.49 | 18.82 | |
| 5 | Epicubenol I | 5.22 | 8.31 | 33.00 | 2.20 | 34.06 | 1.04 |
| 6 | Epicubenol II | 94.78 | 91.69 | 67.00 | 97.80 | 65.94 | |
| 7 | Farnesol I | 96.67 | 89.37 | 87.86 | 90.52 | 43.43 | 1.02 |
| 8 | Farnesol II | 3.33 | 10.63 | 12.14 | 9.48 | 56.57 | |
| 9 | Rose oxide I | 67.15 | 67.98 | 68.65 | 68.72 | 69.15 | 1.10 |
| 10 | Rose oxide II | 32.85 | 32.02 | 31.35 | 31.28 | 30.85 | |
| 11 | α-epi-Muurolol I | 56.79 | 86.29 | 48.92 | 64.98 | 55.00 | 1.03 |
| 12 | α-epi-Muurolol II | 43.21 | 13.71 | 51.08 | 35.02 | 45.00 | |
| 15 | α-Guaiene III | 38.34 | 42.92 | 33.42 | 36.95 | 38.02 | 1.00 1.16 |
| 13 | α-Guaiene I | 31.84 | 32.92 | 34.18 | 28.92 | 46.75 | |
| 14 | α-Guaiene II | 29.82 | 24.16 | 32.40 | 34.14 | 15.23 | |
| 16 | β-Bourbonene I | 56.95 | 1.87 | 3.28 | 8.73 | 9.47 | 1.01 1.45 |
| 17 | β-Bourbonene II | 42.84 | 94.86 | 75.76 | 86,45 | 74.00 | |
| 18 | β-Bourbonene III | 0.21 | 3.27 | 20.97 | 4.82 | 16.53 | |
| Feature No. | Name | Similarity | Probability | CAS | Quant Mass | F-Ratio | Mean 1tR (Sec) | Mean 2tR (Sec) | %RSD 1tR | %RSD 2tR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Geraniol | 889 | 5831 | 106-25-2 | 69 | 3.30 × 1015 | 1856 | 3.58 | 0 | 3.85 × 10−14 |
| 2 | Nerol | 922 | 8214 | 106-25-2 | 69 | 1.95 × 1015 | 1720 | 3.45 | 0 | 2.66 × 10−14 |
| 3 | Phenethyl alcohol | 943 | 9536 | 60-12-8 | 91 | 1.43 × 1015 | 1560 | 3.37 | 0 | 2.73 × 10−14 |
| 4 | Citronellol I | 949 | 5840 | 106-22-9 | 69 | 1.08 × 1015 | 1752 | 3.13 | 0 | 1.47 × 10−14 |
| 5 | Phenylethyl tigelate | 945 | 9688 | 55719-85-2 | 104 | 1.02 × 1015 | 2952 | 0.44 | 0 | 3.92 × 10−14 |
| 6 | Citronellyl formate | 954 | 6352 | 105-85-1 | 69 | 4.17 × 1014 | 1624 | 4.20 | 0 | 2.19 × 10−14 |
| 7 | Methyleugenol | 922 | 8332 | 93-15-2 | 178 | 3.30 × 1014 | 2200 | 0.38 | 0 | 1.51 × 10−14 |
| 8 | 2-Phenylethyl acetate | 961 | 8704 | 103-45-7 | 104 | 2.94 × 1014 | 1656 | 5.98 | 0 | 3.07 × 10−14 |
| 9 | Geranial | 892 | 7768 | 141-27-5 | 69 | 2.54 × 1014 | 1664 | 5.28 | 0 | 0 |
| 10 | Geraniol acetate | 878 | 2691 | 25905-14-0 | 69 | 2.29 × 1014 | 2032 | 5.61 | 0 | 0 |
| 11 | Eugenol | 937 | 6353 | 97-53-0 | 164 | 2.25 × 1014 | 2248 | 5.57 | 0 | 3.30 × 10−14 |
| 12 | γ-Eudesmol | 921 | 5617 | 1209-71-8 | 189 | 1.82 × 1014 | 3152 | 5.95 | 0 | 3.09 × 10−14 |
| 13 | Menthone | 944 | 5109 | 10458-14-7 | 112 | 1.21 × 1014 | 1216 | 4.33 | 0 | 2.12 × 10−14 |
| 14 | Rose oxide I | 881 | 9509 | 16409-43-1 | 139 | 1.10 × 1014 | 960 | 3.24 | 0 | 4.26 × 10−14 |
| 15 | Isomenthone | 943 | 5481 | 491-07-6 | 112 | 9.76 × 1013 | 1168 | 4.05 | 0 | 2.27 × 10−14 |
| 16 | β-Myrcene | 896 | 4554 | 123-35-3 | 69 | 8.61 × 1013 | 1720 | 4.89 | 0 | 0 |
| 17 | α-Humulene | 926 | 7531 | 6753-98-6 | 93 | 8.21 × 1013 | 2272 | 5.01 | 0 | 0 |
| 18 | Linalool | 941 | 8734 | 78-70-6 | 71 | 7.56 × 1013 | 1216 | 2.55 | 0 | 0 |
| 19 | Linalyl acetate | 899 | 3336 | 115-95-7 | 93 | 6.56 × 1013 | 1448 | 4.30 | 0 | 2.14 × 10−14 |
| 20 | Geranyl tiglate | 858 | 4553 | 7785-33-3 | 93 | 5.20 × 1013 | 3296 | 7.22 | 0 | 0 |
| 21 | β-Bourbonene II | 851 | 5710 | 119903-95-6 | 81 | 4.76 × 1013 | 1872 | 4.72 | 0 | 0 |
| 22 | Citronellol acetate | 939 | 3760 | 150-84-5 | 81 | 4.64 × 1013 | 1840 | 5.00 | 0 | 0 |
| 23 | trans Calamenene | 814 | 9135 | 73209-42-4 | 159 | 4.55 × 1013 | 2568 | 6.27 | 0 | 2.93 × 10−14 |
| 24 | Farnesol I | 909 | 2231 | 3790-71-4 | 69 | 4.08 × 1013 | 3688 | 5.16 | 0 | 3.56 × 10−14 |
| 25 | Citronellyl tiglate | 925 | 5599 | 24717-85-9 | 81 | 3.57 × 1013 | 3128 | 6.25 | 0 | 0 |
| 26 | α-Terpineol | 932 | 7680 | 98-55-5 | 59 | 2.75 × 1013 | 1672 | 3.19 | 0 | 1.44 × 10−14 |
| 27 | Lavanduol acetate | 935 | 5589 | 25905-14-0 | 69 | 2.45 × 1013 | 1584 | 4.74 | 0 | 0 |
| 28 | Rose oxide II | 901 | 9528 | 16409-43-1 | 139 | 2.39 × 1013 | 1048 | 3.34 | 0 | 2.75 × 10−14 |
| 29 | Valerianol | 947 | 4303 | 20489-45-6 | 161 | 2.26 × 1013 | 3336 | 5.82 | 0 | 3.16 × 10−14 |
| 30 | α-Pinene | 941 | 6777 | 80-56-8 | 93 | 2.16 × 1013 | 568 | 5.83 | 0 | 1.58 × 10−14 |
| 31 | Neryl propionate | 893 | 3355 | 105-91-9 | 69 | 1.97 × 1013 | 2392 | 5.86 | 0 | 0 |
| 32 | Geranyl isobutyrate | 855 | 1869 | 2345-26-8 | 69 | 1.95 × 1013 | 2744 | 5.90 | 0 | 1.56 × 10−14 |
| 33 | Germacrene D | 938 | 5363 | 23986-74-5 | 161 | 1.86 × 1013 | 2360 | 5.37 | 0 | 0.0 × 1000 |
| 34 | β-Phenylethyl butyrate | 947 | 6918 | 103-52-6 | 104 | 1.62 × 1013 | 2168 | 6.31 | 0 | 2.91 × 10−14 |
| 35 | Linalol acetate | 777 | 765 | 115-95-7 | 69 | 1.59 × 1013 | 1912 | 5.48 | 0 | 3.36 × 10−14 |
| 36 | α-Guaiene III | 940 | 5599 | 3691-12-1 | 105 | 1.56 × 1013 | 2192 | 4.51 | 0 | 2.04 × 10−14 |
| 37 | Citronellyl butyrate | 966 | 6244 | 141-16-2 | 81 | 1.44 × 1013 | 2584 | 5.30 | 0 | 1.73 × 10−14 |
| 38 | Caryophyllene | 966 | 5900 | 13877-93-5 | 93 | 1.36 × 1013 | 2128 | 4.68 | 0 | 1.96 × 10−14 |
| 39 | Dihydromyrcenol | 899 | 8797 | 18479-58-8 | 59 | 1.30 × 1013 | 2784 | 2.93 | 0 | 0 |
| 40 | Terpinen-4-ol | 902 | 6816 | 562-74-3 | 93 | 1.23 × 1013 | 1520 | 3.20 | 0 | 1.44 × 10−14 |
| 41 | Phenethyl propionate | 944 | 7758 | 122-70-3 | 104 | 1.17 × 1013 | 2008 | 6.54 | 0 | 4.22 × 10−14 |
| 42 | α-Copaene | 932 | 5272 | 138874-68-7 | 105 | 1.05 × 1013 | 1824 | 4.50 | 0 | 0 |
| 43 | α-Guaiene II | 883 | 1031 | 3691-12-1 | 107 | 9.42 × 1012 | 2544 | 4.94 | 0 | 1.86 × 10−14 |
| 44 | γ-Muurolene | 942 | 5736 | 39029-41-9 | 161 | 8.49 × 1012 | 2552 | 5.33 | 0 | 1.72 × 10−14 |
| 45 | β-Citral | 910 | 6118 | 106-26-3 | 41 | 8.36 × 1012 | 1544 | 5.01 | 0 | 0 |
| 46 | β-Famesene | 857 | 1624 | 18794-84-8 | 69 | 7.95 × 1012 | 3592 | 4.90 | 0 | 0 |
| 47 | epi-γ-Eudesmol | 919 | 5350 | 1209-71-8 | 91 | 7.56 × 1012 | 3136 | 5.84 | 0 | 3.15 × 10−14 |
| 48 | Ledene | 910 | 2766 | 21747-46-6 | 105 | 7.49 × 1012 | 2488 | 4.91 | 0 | 3.74 × 10−14 |
| 49 | α-Eudesmol | 906 | 5903 | 473-16-5 | 59 | 6.94 × 1012 | 3320 | 5.91 | 0 | 3.11 × 10−14 |
| 50 | α-epi-Muurolol II | 937 | 3695 | 19912-62-0 | 95 | 6.19 × 1012 | 3448 | 5.28 | 0 | 0 |
| 51 | α-epi-Cadinol | 916 | 4366 | 5937-11-1 | 161 | 6.14 × 1012 | 3432 | 4.86 | 0 | 0 |
| 52 | Epicubenol I | 923 | 5221 | 19912-67-5 | 119 | 4.73 × 1012 | 3064 | 5.79 | 0 | 3.18 × 10−14 |
| 53 | Citronellyl propionate I | 939 | 5397 | 141-14-0 | 69 | 4.61 × 1012 | 2232 | 5.06 | 0 | 1.82 × 10−14 |
| 54 | Lavandulyl isobutyrate | 879 | 1592 | 51117-20-5 | 93 | 4.47 × 1012 | 2536 | 5.54 | 0 | 3.32 × 10−14 |
| 55 | Citronellol II | 913 | 4838 | 1117-61-9 | 67 | 4.37 × 1012 | 1760 | 4.36 | 0 | 0 |
| 56 | n-Hexanol | 951 | 8404 | 111-27-3 | 56 | 4.36 × 1012 | 672 | 1.36 | 0 | 3.38 × 10−14 |
| 57 | β-Bourbonene I | 923 | 9138 | 119903-95-6 | 80 | 4.32 × 1012 | 1848 | 4.76 | 0 | 0 |
| 58 | Citronellyl isohexanoate | 909 | 3155 | 71662-18-5 | 81 | 4.27 × 1012 | 3216 | 5.29 | 0 | 1.74 × 10−14 |
| 59 | Methyl geraniate | 890 | 8608 | 2349-14-6 | 69 | 4.06 × 1012 | 1704 | 5.73 | 0 | 3.21 × 10−14 |
| 60 | δ-Cadinene | 953 | 6110 | 16729-01-4 | 81 | 3.97 × 1012 | 2600 | 5.28 | 0 | 0 |
| 61 | β-Pinene | 943 | 6657 | 127-91-3 | 93 | 3.59 × 1012 | 672 | 6.14 | 0 | 0 |
| 62 | Farnesal | 860 | 6270 | 502-67-0 | 69 | 3.32 × 1012 | 3552 | 6.84 | 0 | 4.03 × 10−14 |
| 63 | Geranyl propionate | 834 | 2474 | 105-90-8 | 69 | 3.25 × 1012 | 2896 | 5.72 | 0 | 0 |
| 64 | para-Cymene | 884 | 4466 | 99-87-6 | 119 | 3.20 × 1012 | 712 | 2.53 | 0 | 1.82 × 10−14 |
| 65 | Phytol acetate | 865 | 1716 | 10236-16-5 | 95 | 3.10 × 1012 | 5152 | 6.03 | 0 | 0 |
| 66 | Neryl hexanoate | 880 | 1283 | 68310-59-8 | 69 | 2.98 × 1012 | 3376 | 5.98 | 0 | 3.07 × 10−14 |
| 67 | α-Calacorene | 940 | 8993 | 21391-99-1 | 157 | 2.85 × 1012 | 2576 | 7.38 | 0 | 2.49 × 10−14 |
| 68 | Spathulenol | 944 | 7254 | 72203-24-8 | 91 | 2.68 × 1012 | 3112 | 5.17 | 0 | 1.78 × 10−14 |
| 69 | β-Bourbonene III | 873 | 7103 | 5208-59-3 | 81 | 2.61 × 1012 | 2672 | 5.69 | 0 | 3.23 × 10−14 |
| 70 | Farnesol II | 786 | 1101 | 3790-71-4 | 69 | 2.11 × 1012 | 3760 | 4.65 | 0 | 0 |
| 71 | β-Dihydroagarofuran | 957 | 5868 | 5956-09-2 | 137 | 2.01 × 1012 | 2440 | 5.89 | 0 | 0 |
| 72 | β-Farnesene | 912 | 3136 | 18794-84-8 | 69 | 1.97 × 1012 | 2240 | 4.70 | 0 | 1.96 × 10−14 |
| 73 | Nerolidol | 945 | 4521 | 40716-66-3 | 69 | 1.86 × 1012 | 3000 | 4.33 | 0 | 2.12 × 10−14 |
| 74 | α-Farnesene | 943 | 6646 | 502-61-4 | 93 | 1.72 × 1012 | 2464 | 5.08 | 0 | 1.81 × 10−14 |
| 75 | Geranic acid | 956 | 7882 | 4698-08-2 | 69 | 1.64 × 1012 | 2928 | 2.52 | 0 | 1.82 × 10−14 |
| 76 | Aromandendrene | 920 | 2321 | 489-39-4 | 91 | 1.60 × 1012 | 2312 | 4.91 | 0 | 3.74 × 10−14 |
| 77 | Myrcene | 925 | 7292 | 123-35-3 | 41 | 1.53 × 1012 | 616 | 1.48 | 0 | 1.55 × 10−14 |
| 78 | 10-epi-α-Eudesmol | 874 | 4859 | 473-16-5 | 59 | 1.39 × 1012 | 3200 | 5.09 | 0 | 3.61 × 10−14 |
| 79 | 2-Phenylethyl dodecanoate | 930 | 6162 | 6309-54-2 | 104 | 1.38 × 1012 | 5312 | 0.24 | 0 | 4.79 × 10−14 |
| 80 | Elemol | 916 | 5631 | 639-99-6 | 93 | 1.32 × 1012 | 2968 | 4.76 | 0 | 0 |
| 81 | α-Copaen-11-ol | 868 | 8105 | 41370-56-3 | 59 | 1.26 × 1012 | 3448 | 5.98 | 0 | 3.07 × 10−14 |
| 82 | Linalyl butyrate | 865 | 1853 | 78-36-4 | 69 | 1.26 × 1012 | 3520 | 6.10 | 0 | 1.51 × 10−14 |
| 83 | Aciphyllene | 941 | 2394 | 87745-31-1 | 105 | 1.11 × 1012 | 2424 | 5.26 | 0 | 0 |
| 84 | Epicubenol II | 909 | 5249 | 19912-67-5 | 161 | 1.10 × 1012 | 3184 | 5.75 | 0 | 0 |
| 85 | n-Hexadecanoic acid | 908 | 7998 | 57-10-3 | 73 | 1.04 × 1012 | 5048 | 3.25 | 0 | 0 |
| 86 | Phenylethyl octanoate | 880 | 6941 | 5457-70-5 | 104 | 9.90 × 1011 | 3944 | 7.35 | 0 | 2.50 × 10−14 |
| 87 | β-Copaene | 941 | 4437 | 147515-11-5 | 161 | 8.78 × 1011 | 2080 | 4.95 | 0 | 1.86 × 10−14 |
| 88 | α-epi-Muurolol I | 950 | 6183 | 19912-62-0 | 105 | 8.12 × 1011 | 3352 | 5.36 | 0 | 0 |
| 89 | β-Elemene | 927 | 2299 | 33880-83-0 | 93 | 8.12 × 1011 | 2480 | 5.49 | 0 | 1.67 × 10−14 |
| 90 | Benzaldehyde | 942 | 9578 | 100-52-7 | 105 | 8.12 × 1011 | 712 | 2.99 | 0 | 3.07 × 10−14 |
| 91 | Ledol | 901 | 2809 | 577-27-5 | 105 | 7.89 × 1011 | 3080 | 5.51 | 0 | 1.67 × 10−14 |
| 92 | Norbourbonane | 904 | 8790 | 13844-03-6 | 81 | 7.62 × 1011 | 2768 | 7.56 | 0 | 2.43 × 10−14 |
| 93 | Citronellyl propionate II | 886 | 3313 | 141-14-0 | 81 | 7.56 × 1011 | 4080 | 5.65 | 0 | 1.63 × 10−14 |
| 94 | β-Selinene | 931 | 1656 | 17066-67-0 | 93 | 7.24 × 1011 | 2384 | 5.37 | 0 | 0 |
| 95 | α-Guaiene I | 783 | 1722 | 3691-12-1 | 105 | 7.05 × 1011 | 2200 | 4.86 | 0 | 0 |
| 96 | Citronellyl caprate | 875 | 999 | 72934-06-6 | 95 | 6.58 × 1011 | 4752 | 5.95 | 0 | 3.09 × 10−14 |
| 97 | Benzyl tiglate | 905 | 9511 | 37526-88-8 | 91 | 6.58 × 1011 | 2584 | 0.36 | 0 | 1.60 × 10−14 |
| 98 | Nonanal | 913 | 6842 | 124-19-6 | 57 | 6.26 × 1011 | 1000 | 3.12 | 0 | 1.47 × 10−14 |
| 99 | α-Muurolene | 906 | 2115 | 10208-80-7 | 105 | 6.17 × 1011 | 2312 | 5.46 | 0 | 3.37 × 10−14 |
| 100 | Linalool oxide | 925 | 7468 | 5989-33-3 | 59 | 5.94 × 1011 | 1096 | 2.37 | 0 | 0 |
| Sample | Column Set-Up 1 | Column Set-Up 2 | Column Set-Up 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area Total (Pixel) | Area Used (Pixel) | Separation Space (%) | Area Total (Pixel) | Area Used (Pixel) | Separation Space (%) | Area Total (Pixel) | Area Used (Pixel) | Separation Space (%) | |
| R. damascena cv. “Janina” | 63.42 | 35.76 | 56.38 | 63.70 | 31.70 | 49.77 | 63.34 | 14.90 | 23.52 |
| R. gallica | 63.22 | 29.01 | 45.88 | 63.34 | 30.62 | 48.35 | 63.35 | 16.50 | 26.04 |
| R. alba | 63.95 | 30.28 | 47.35 | 63.50 | 25.39 | 39.98 | 63.34 | 14.80 | 23.36 |
| R.centifolia | 63.54 | 31.20 | 49.11 | 63.58 | 28.99 | 45.60 | 63.34 | 16.60 | 26.21 |
| P. roseum | 63.34 | 32.50 | 51.31 | 63.34 | 24.33 | 38.41 | 63.34 | 15.87 | 25.06 |
| Essential Oil | Sample Code |
|---|---|
| Rosa damascena cv. “Janina” | AK3 |
| Rosa gallica | IREMK1 |
| Rosa alba | IREMK2 |
| Rosa centifolia | IREMK4 |
| Prasophyllum roseum | IREMK5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koljančić, N.; Vyviurska, O.; Špánik, I. Aroma Compounds in Essential Oils: Analyzing Chemical Composition Using Two-Dimensional Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry Combined with Chemometrics. Plants 2023, 12, 2362. https://doi.org/10.3390/plants12122362
Koljančić N, Vyviurska O, Špánik I. Aroma Compounds in Essential Oils: Analyzing Chemical Composition Using Two-Dimensional Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry Combined with Chemometrics. Plants. 2023; 12(12):2362. https://doi.org/10.3390/plants12122362
Chicago/Turabian StyleKoljančić, Nemanja, Olga Vyviurska, and Ivan Špánik. 2023. "Aroma Compounds in Essential Oils: Analyzing Chemical Composition Using Two-Dimensional Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry Combined with Chemometrics" Plants 12, no. 12: 2362. https://doi.org/10.3390/plants12122362
APA StyleKoljančić, N., Vyviurska, O., & Špánik, I. (2023). Aroma Compounds in Essential Oils: Analyzing Chemical Composition Using Two-Dimensional Gas Chromatography–High Resolution Time-of-Flight Mass Spectrometry Combined with Chemometrics. Plants, 12(12), 2362. https://doi.org/10.3390/plants12122362






