Plant Extract-Based Fabrication of Silver Nanoparticles and Their Effective Role in Antibacterial, Anticancer, and Water Treatment Applications
Abstract
1. Introduction
Rationales for the Activities Performed
- Cytotoxicity assay: The rationale for performing the cytotoxicity assay was to evaluate the potential of the green-synthesized silver nanoparticles (AgNPs) as an anticancer agent. The assay was performed on two different cell lines, MCF-7 and MDA-MB-231, to determine the toxicity of AgNPs towards breast cancer cells. The results of the assay were compared with the standard drug Idarubicin to assess the efficacy of AgNPs as a potential anticancer agent.
- Photocatalytic activity against Eosin Y: The rationale for performing the photocatalytic activity against Eosin Y was to evaluate the potential of AgNPs as a photocatalyst for the degradation of the Eosin Y dye. Eosin Y is a widely used water-soluble dye in the textile and paper industries, and its metabolites are carcinogenic to both human and aquatic ecosystems if disposed of untreated. The degradation of Eosin Y was performed in the presence of NaBH4 to better evaluate the role and the competence of green-synthesized AgNPs.
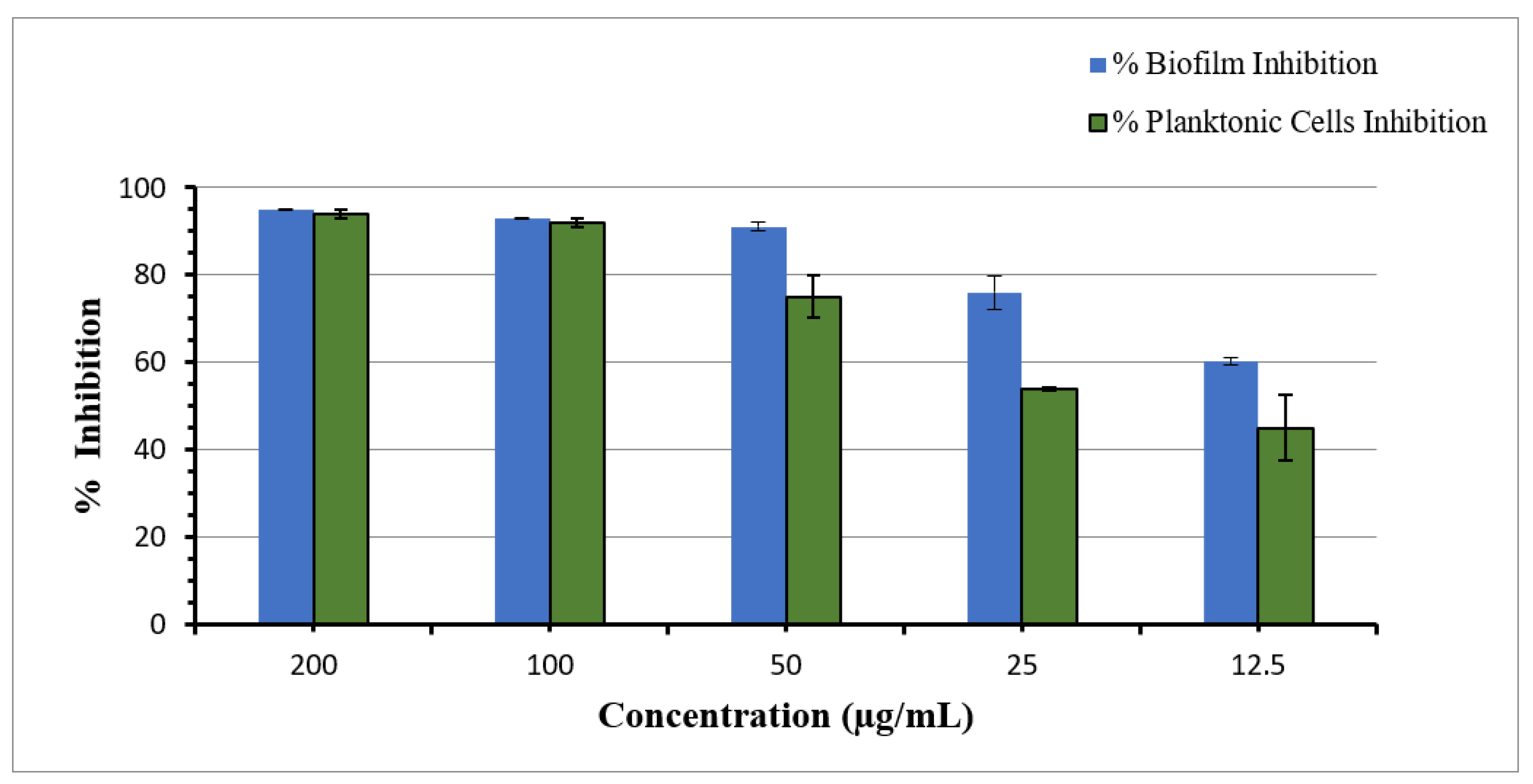
- Antibiofilm activity: The rationale for performing the antibiofilm activity was to evaluate the potential of AgNPs as an antibiofilm agent. Biofilm formation is responsible for about 65% of all microbial and 80% of acute infections. Microbial cells in biofilms possess 10–1000 times more antibiotic resistance in contrast with planktonic cells. Therefore, hindering the biofilms at the early stage of attachment may result in being a key factor in finding promising antibiofilm agents. The effect of AgNPs on the inhibition of initial biofilm formation by Staphylococcus aureus was determined by crystal violet assay.
2. Experimental Section
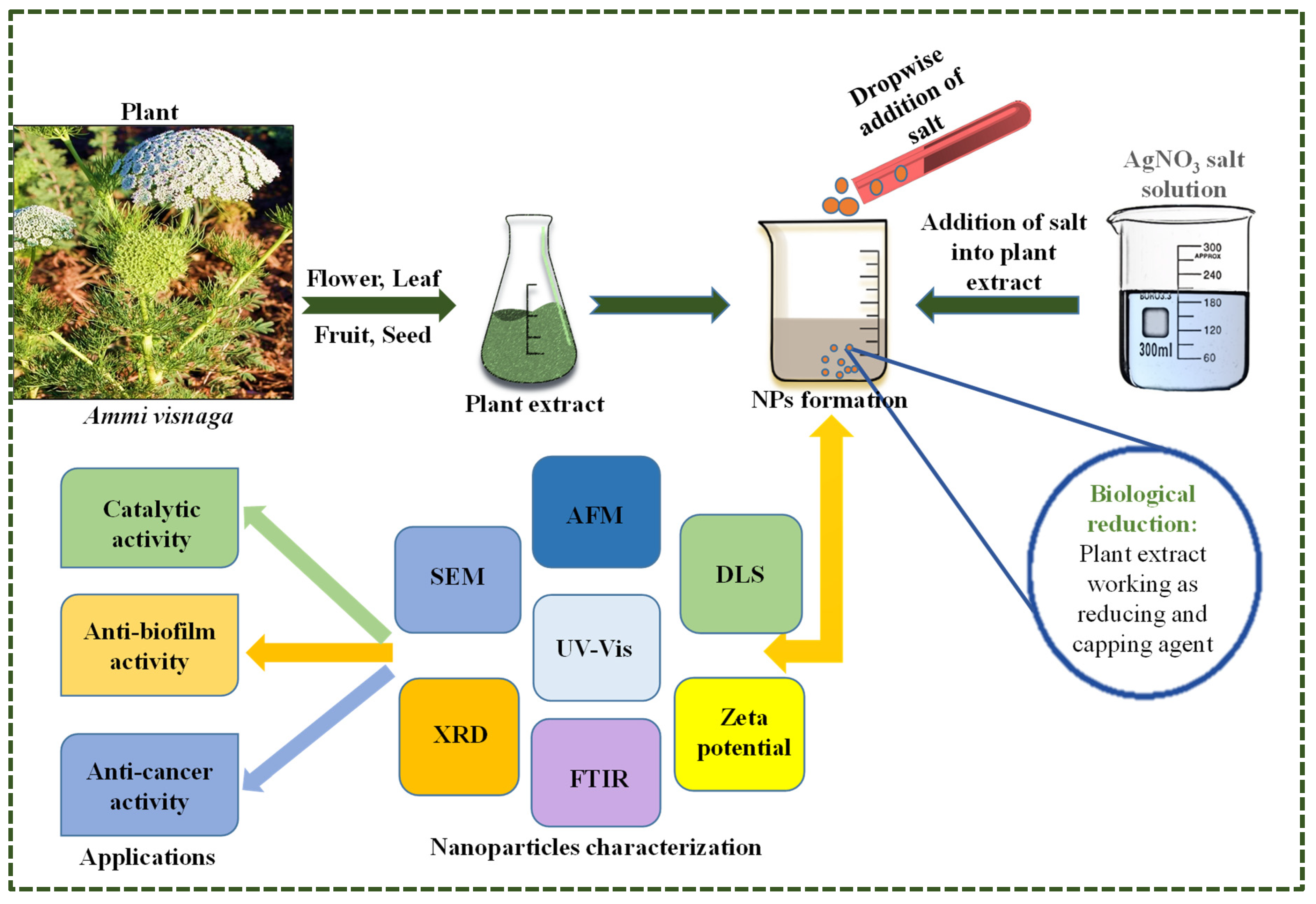
2.1. Ammi visnaga Plant
2.2. Important Chemical Constituents Reported from Ammi visnaga
2.3. Materials and Instrumentation
2.4. Synthesis of Silver Nanoparticles
2.5. Methodology for Antibiofilm Activity
2.5.1. Bacterial Strain and Medium
2.5.2. Crystal Violet Assay for Antibiofilm Activity
2.6. Anticancer Activity Protocol
2.7. Photocatalytic Activity against Eosin Y
3. Results
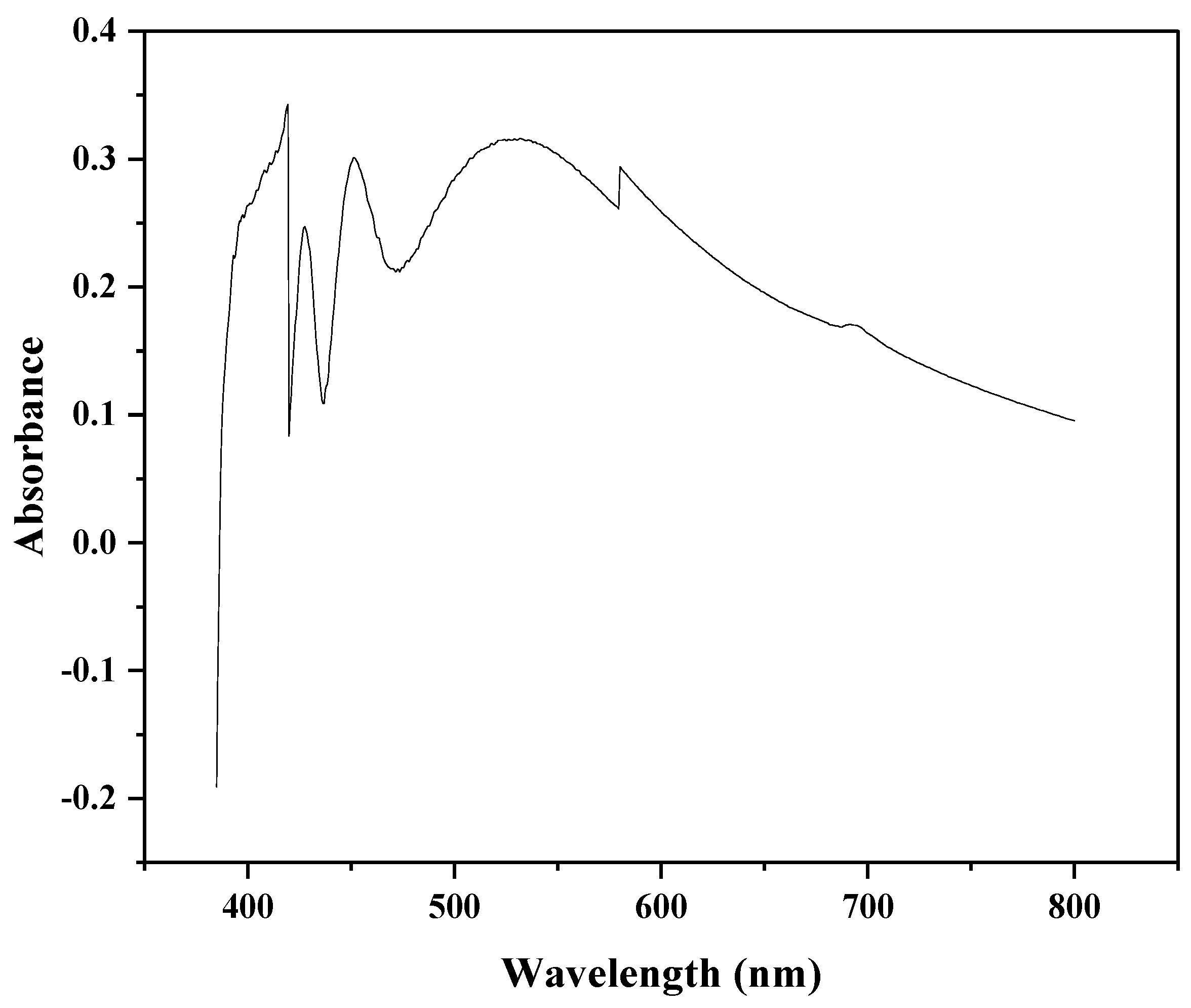
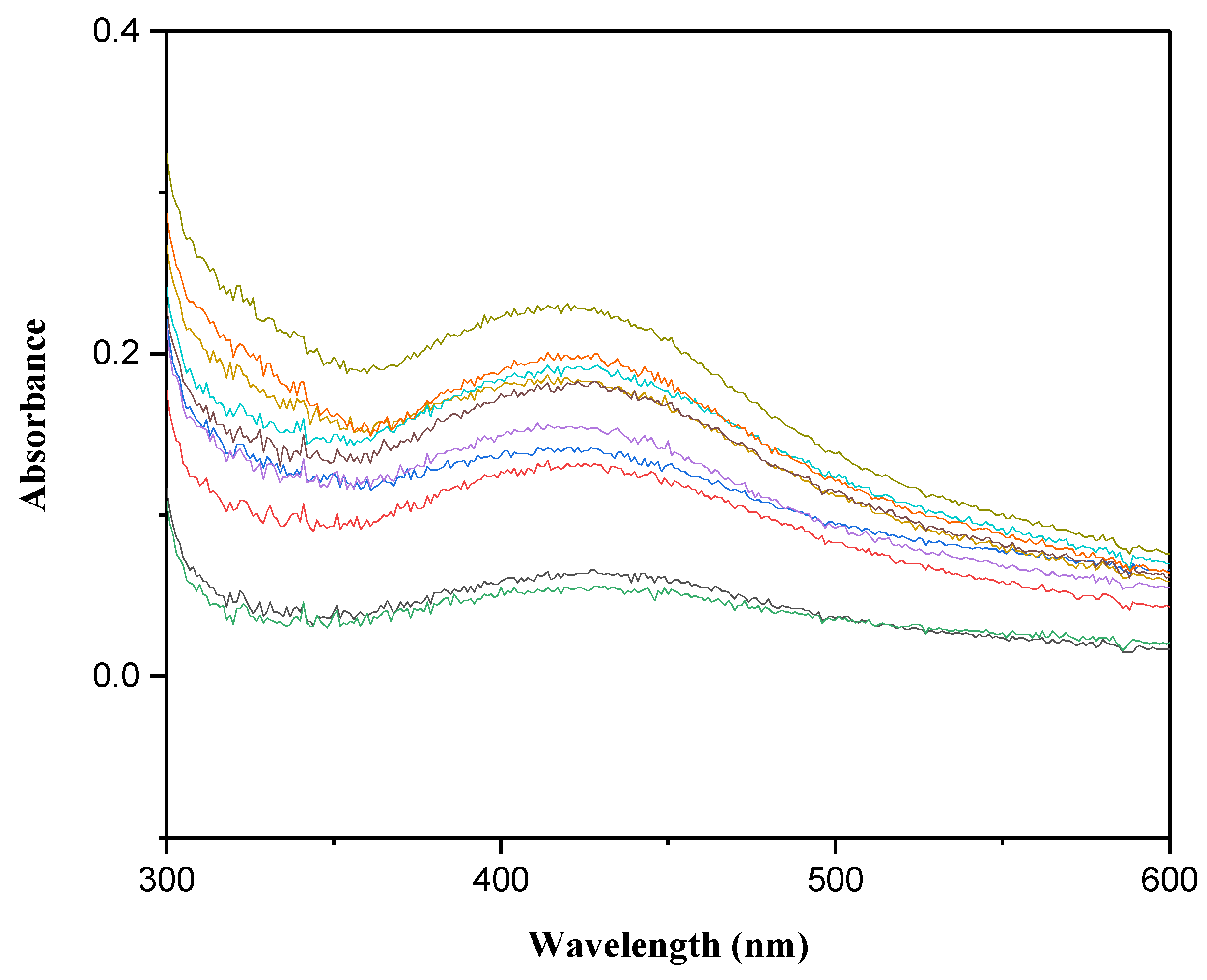
3.1. UV-Vis Spectroscopy
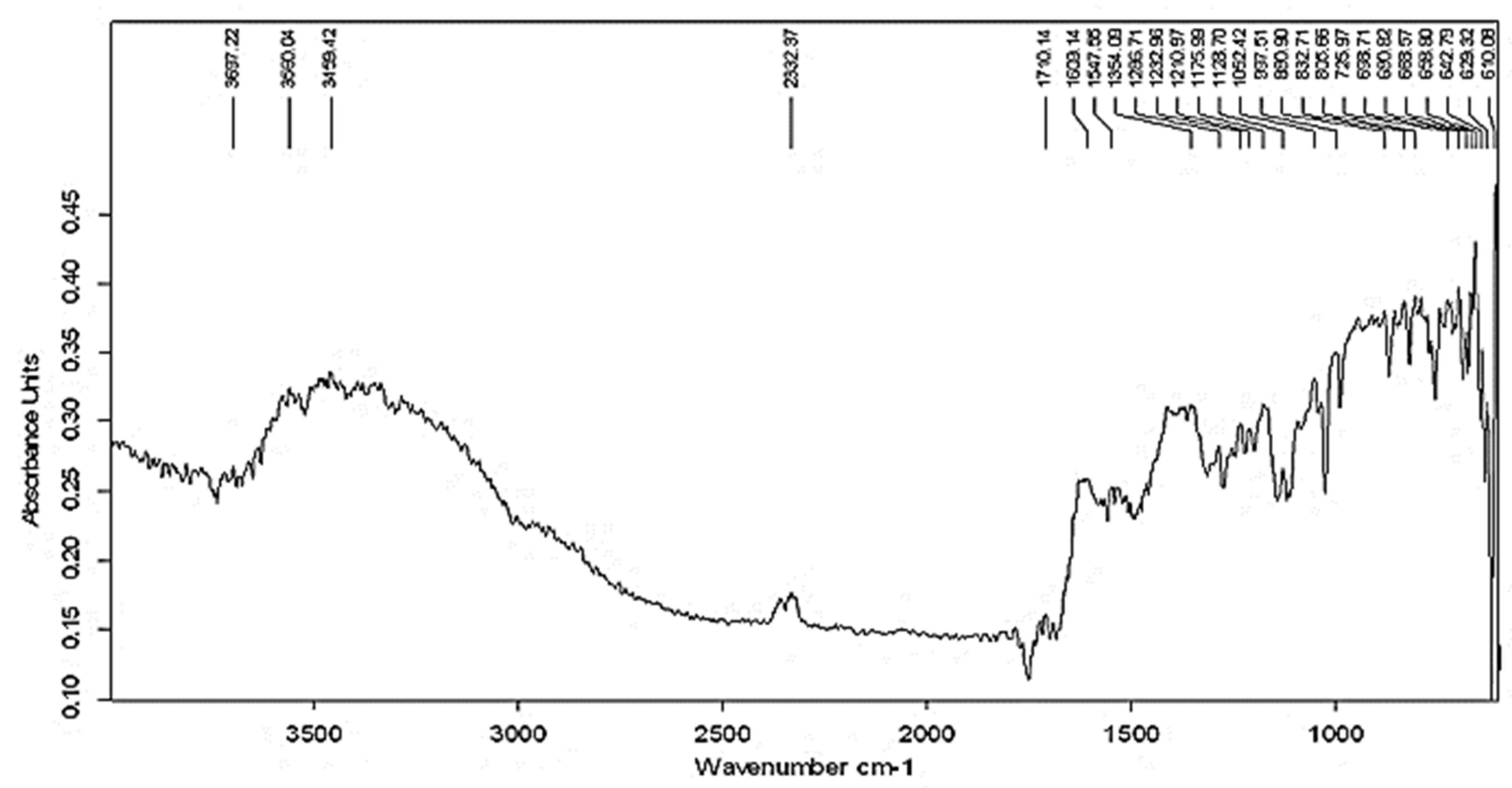
3.2. FTIR Analysis of Prepared AgNPs
3.3. Dynamic Light Scattering and Zeta Potential
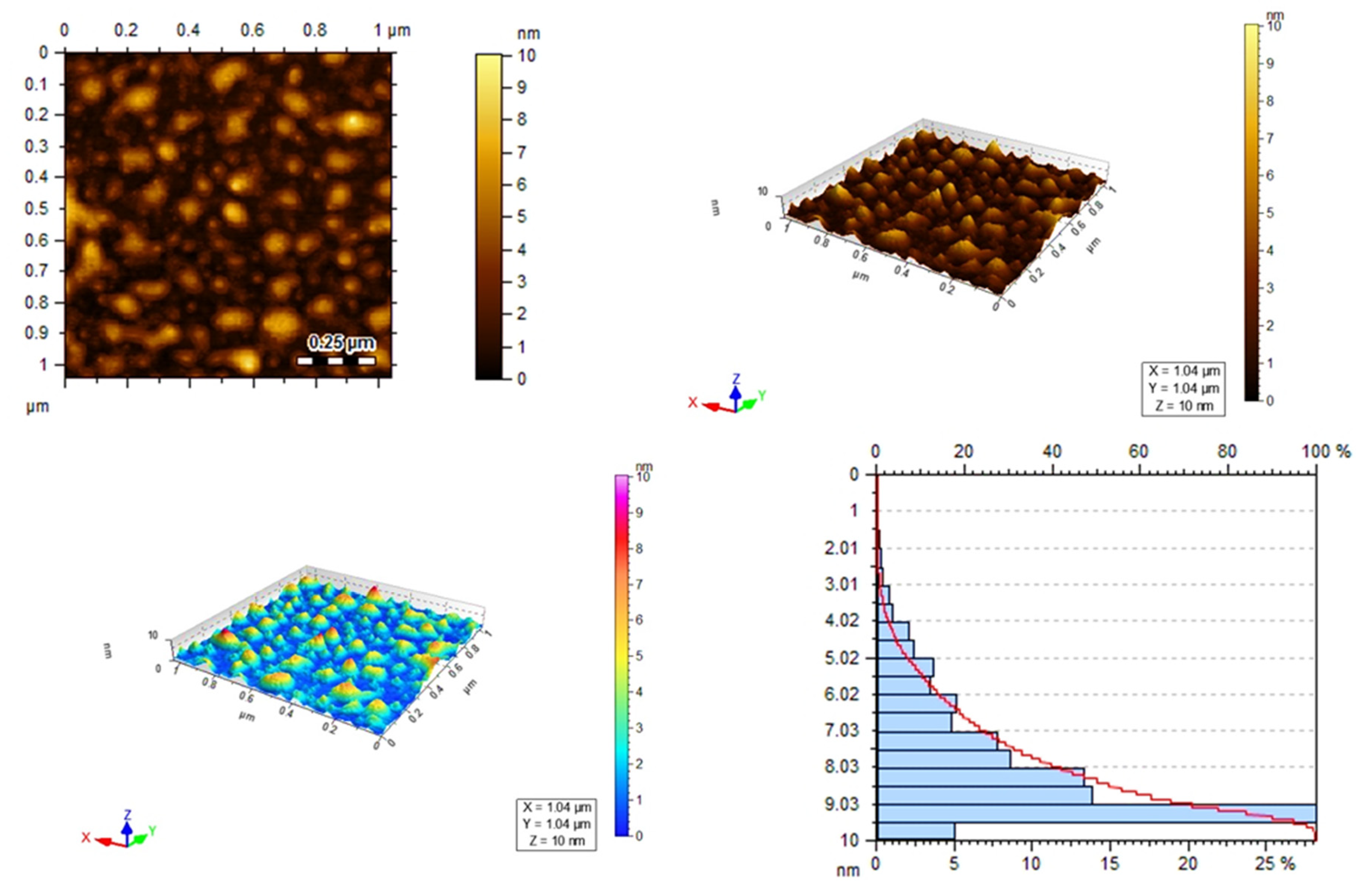
3.4. Atomic Force Microscopy
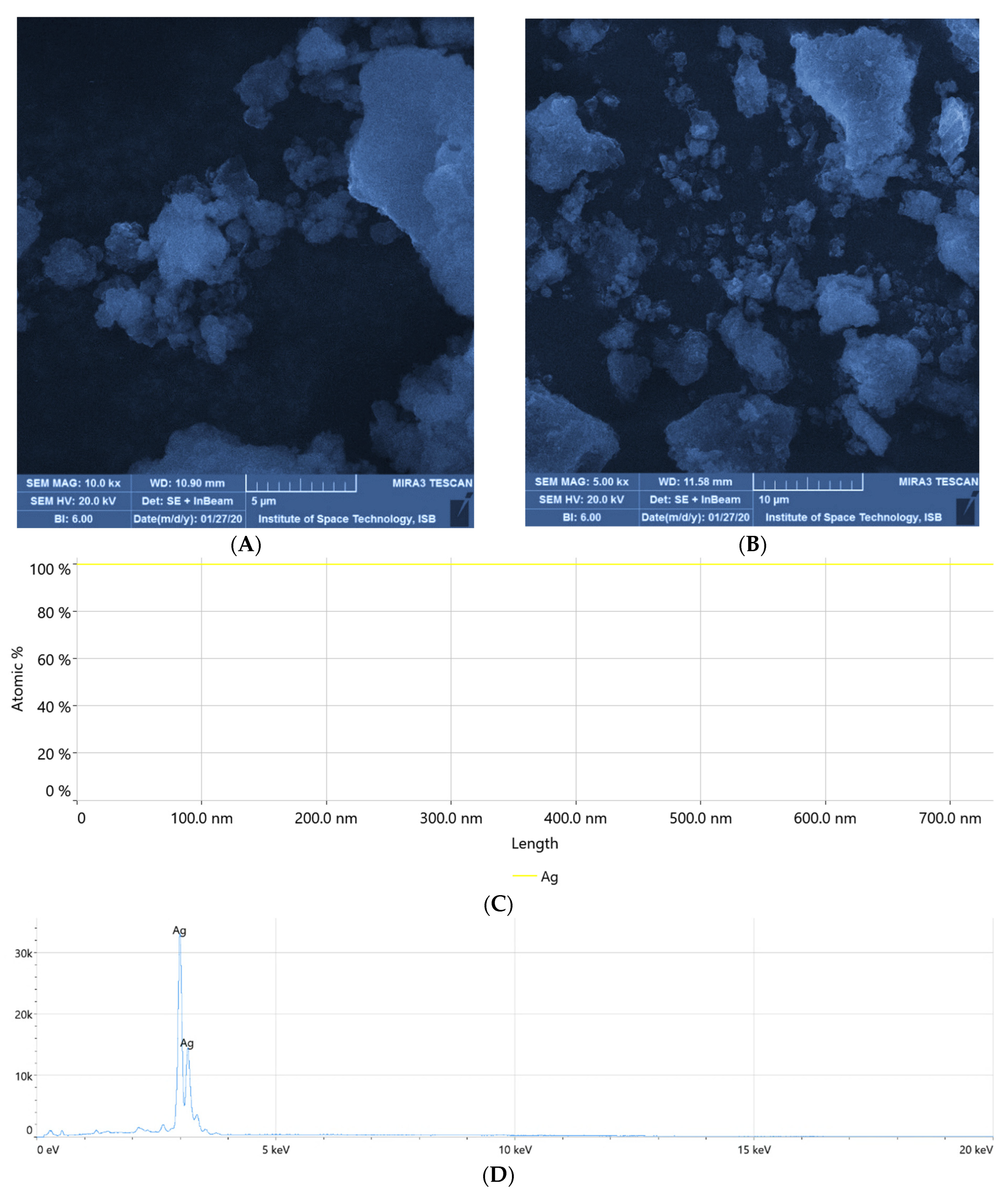
3.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
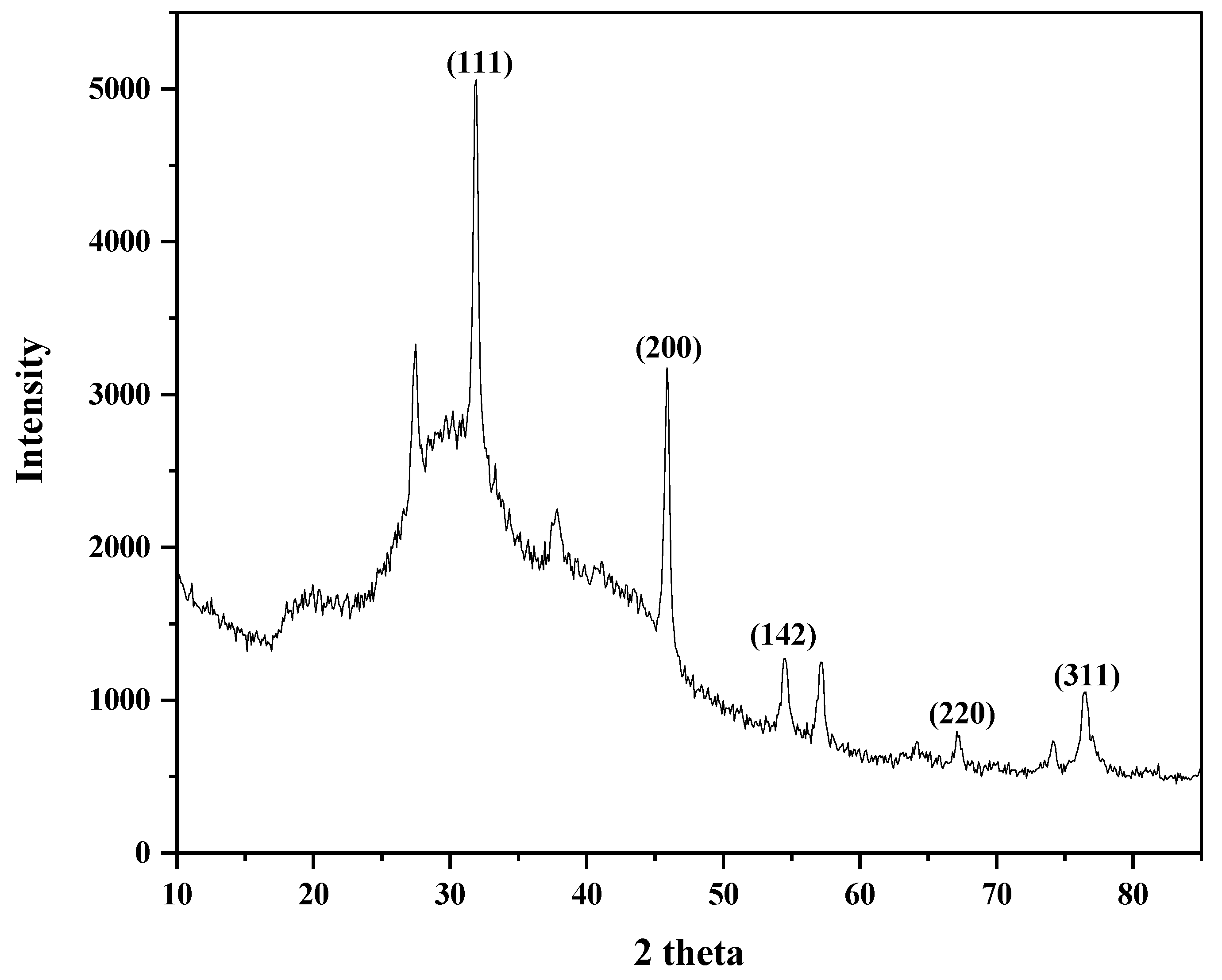
3.6. X-ray Diffraction
3.7. Antibiofilm Activity
3.7.1. Planktonic Cells and Biofilm Inhibition
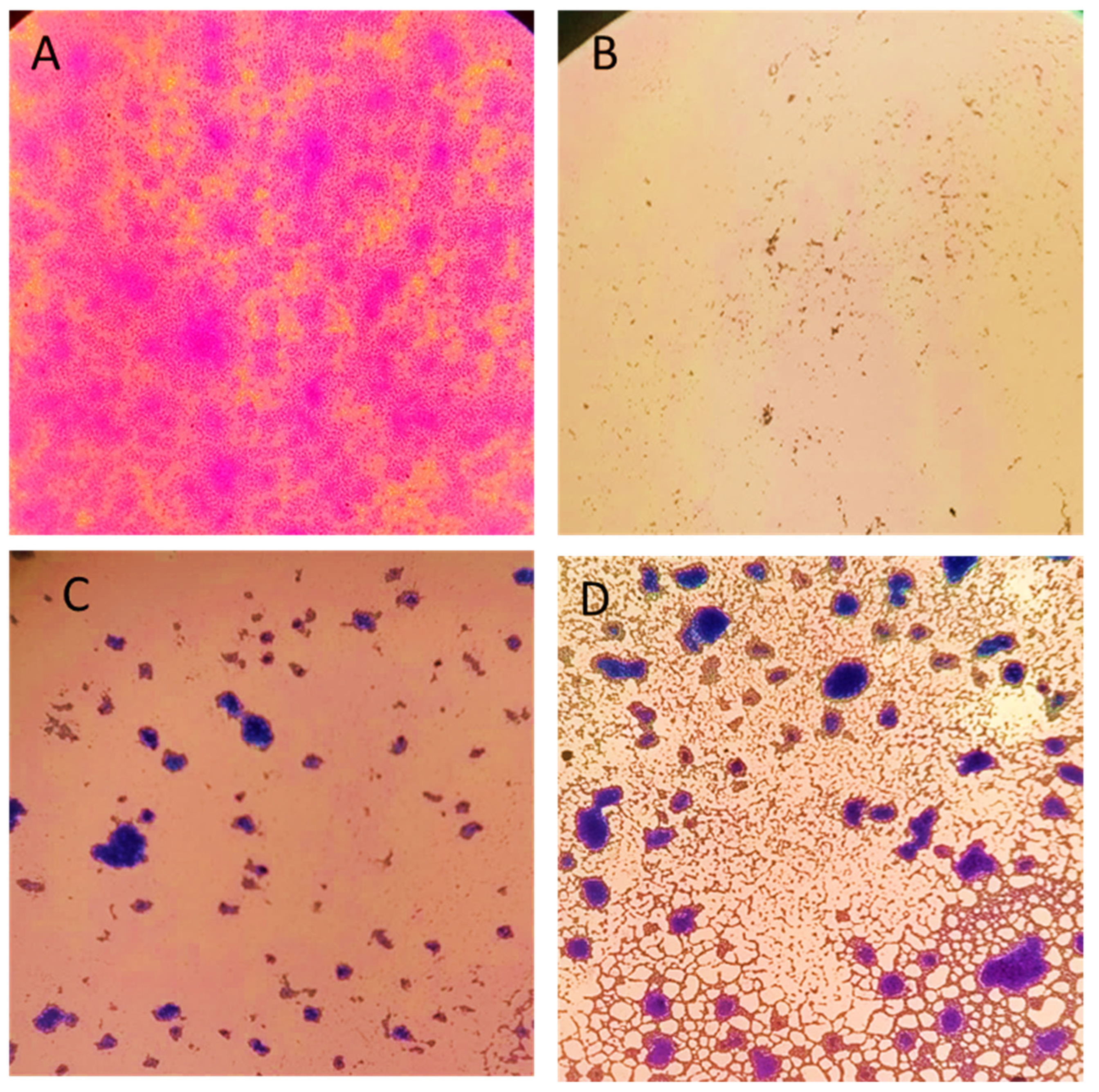
3.7.2. Light Microscopy
3.8. In Vitro Anticancer Activity
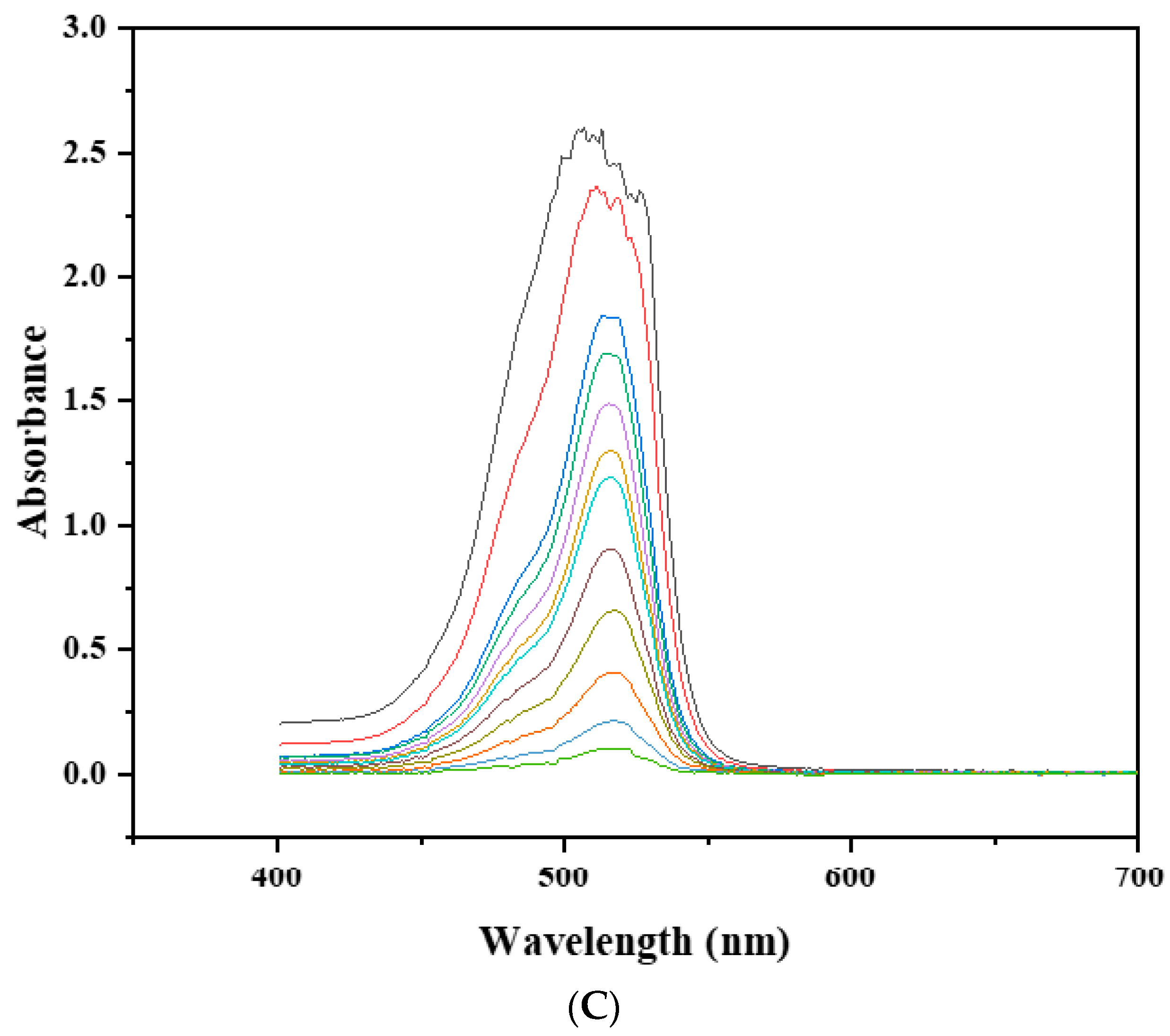
3.9. Catalytic Activity
3.9.1. Effect of pH
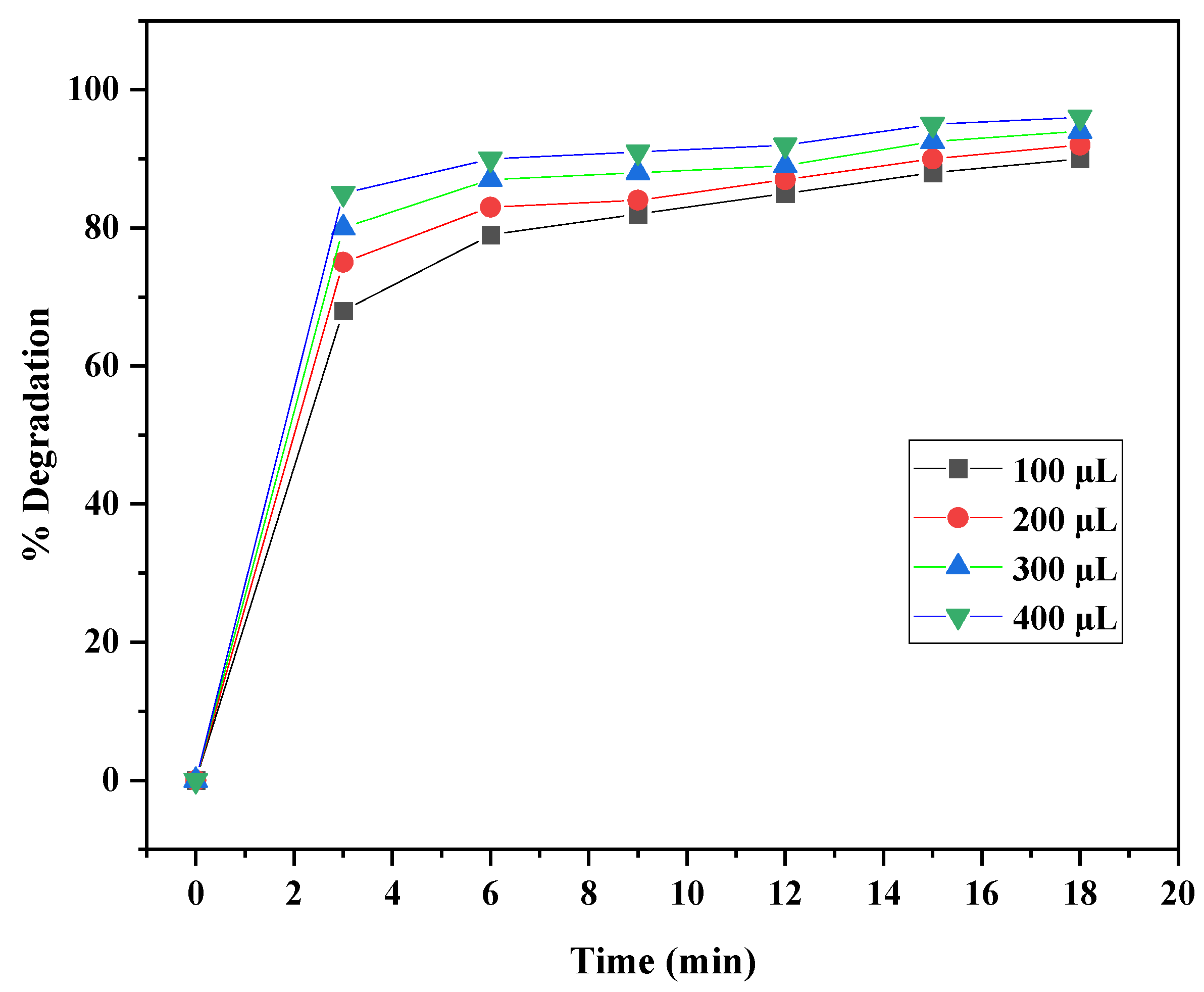
3.9.2. Effect of Photocatalyst Dosage
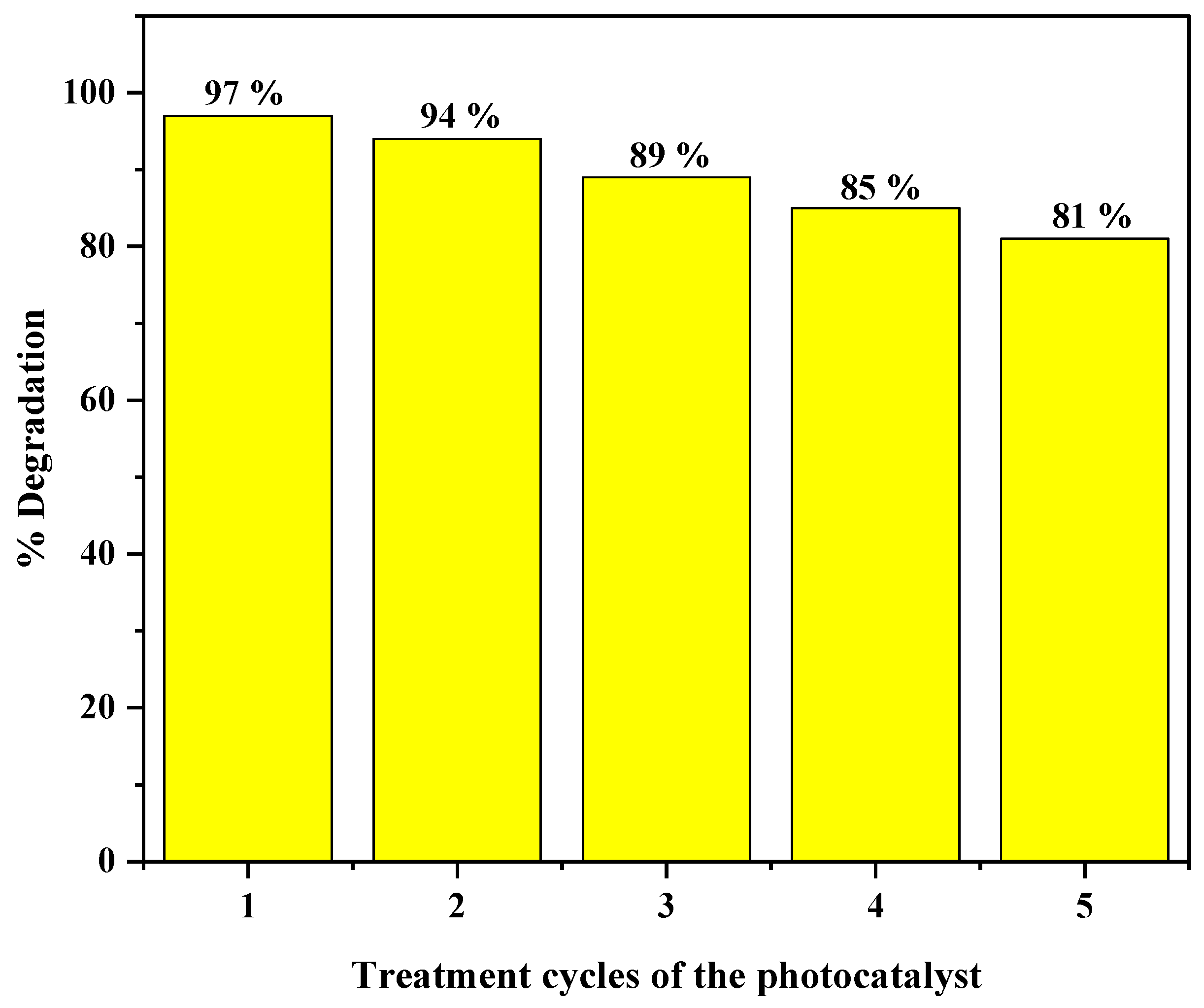
3.9.3. Reusability of the Photocatalyst
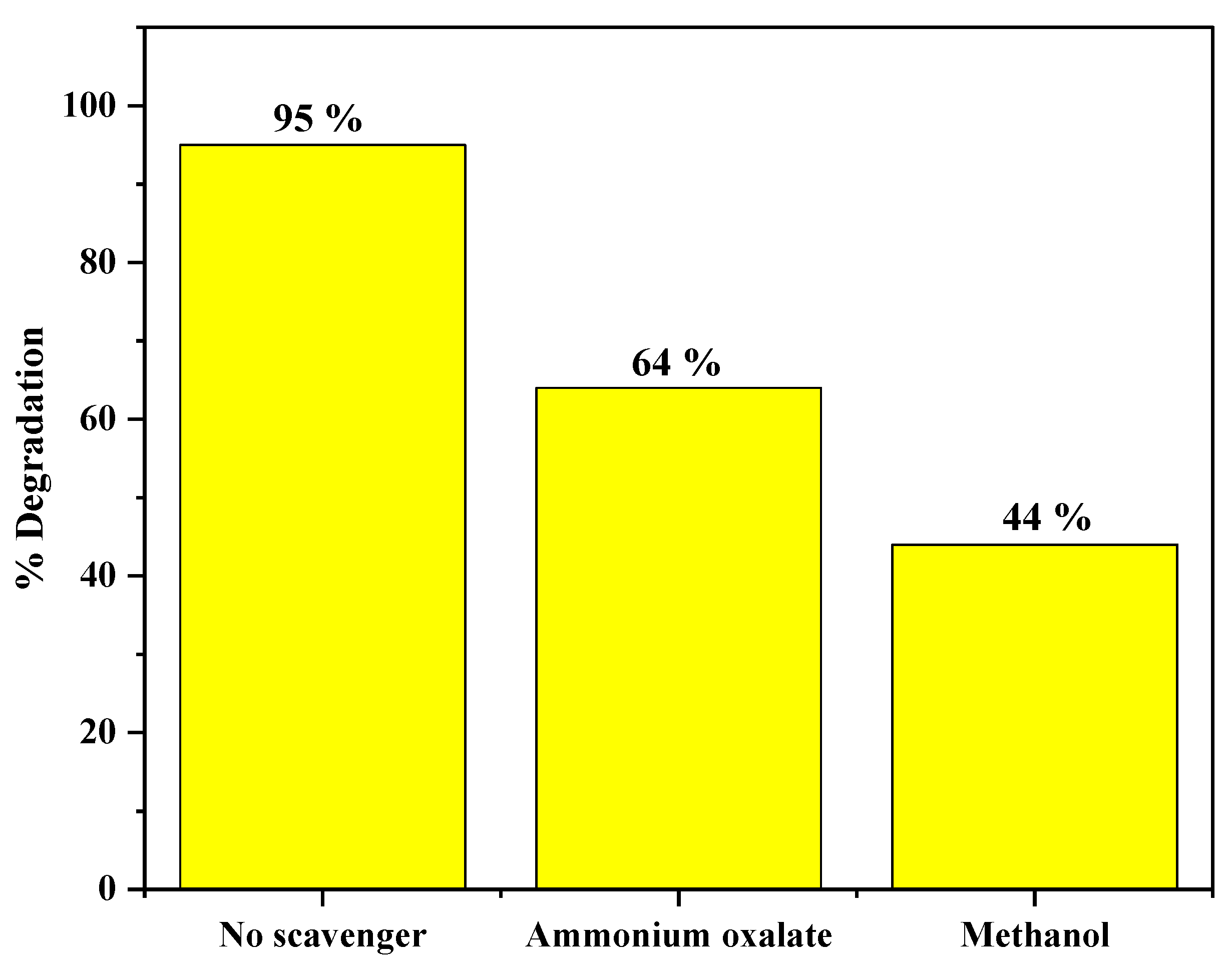
3.9.4. Effect of Different Types of Scavengers on Catalytic Performance
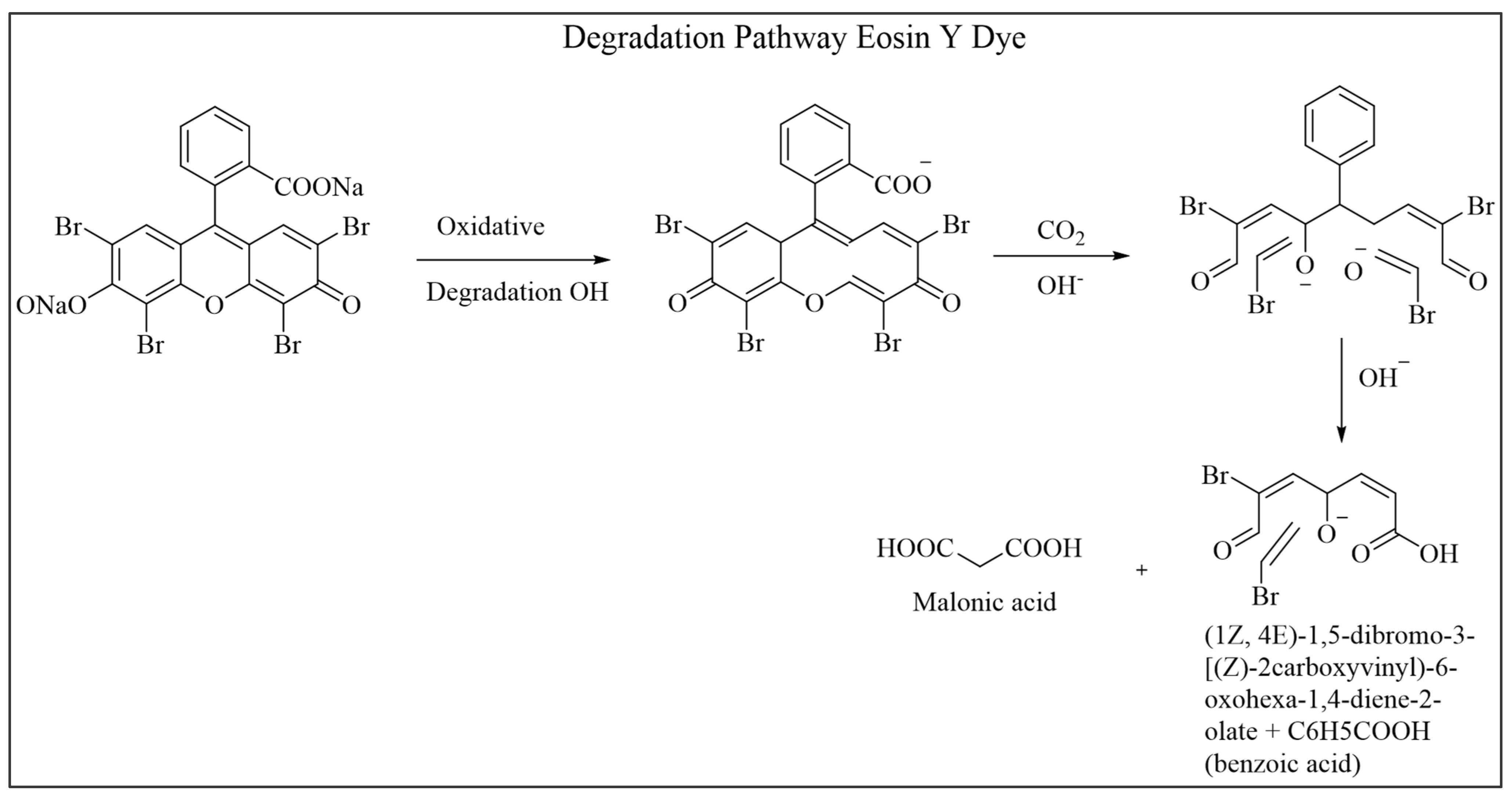
3.9.5. Mechanism Leading to the Degradation of Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- WHO. World Health Report 2008 (The) Chinese; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Elsherbini, A.M.; Shaldam, M.A. Glyceryl trinitrate blocks staphyloxanthin and biofilm formation in Staphylococcus aureus. Afr. Health Sci. 2019, 19, 1376–1384. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, M.K.; Sharma, A.K.; Jayaprakash, G.K.; Tonk, R.K.; Chellappan, D.K.; Singh, S.K.; Dua, K.; Ahmed, F.; Bhattacharyya, S. Metal-based nanomaterials, and nanocomposites as promising frontier in cancer chemotherapy. Med. Comm. 2023, 4, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, A.; Trojan, A.; Burnand, B.; Giannelli, M. Efficacy and side effects of cisplatin-and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: A systematic review of randomized controlled trials. Lung Cancer 2008, 59, 1–11. [Google Scholar] [PubMed]
- Wang, Z.; Xie, C.; Huang, Y.; Lam, C.W.K.; Chow, M.S. Overcoming chemotherapy resistance with herbal medicines: Past, present, and future perspectives. Phytochem. Rev. 2014, 13, 323–337. [Google Scholar] [CrossRef]
- Castro-Aceituno, V.; Ahn, S.; Simu, S.Y.; Singh, P.; Mathiyalagan, R.; Lee, H.A.; Yang, D.C. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed. Pharmacother. 2016, 84, 158–165. [Google Scholar] [CrossRef]
- Ishida, H.; Ishida, Y.; Kurosaka, Y.; Otani, T.; Sato, K.; Kobayashi, H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1998, 42, 1641–1645. [Google Scholar] [CrossRef]
- Shigeta, M.; Tanaka, G.; Komatsuzawa, H.; Sugai, M.; Suginaka, H.; Usui, T. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: A simple method. Chemotherapy 1997, 43, 340–345. [Google Scholar] [CrossRef]
- Elkins, J.G.; Hassett, D.J.; Stewart, P.S.; Schweizer, H.P.; McDermott, T.R. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl. Environ. Microbiol. 1999, 65, 4594–4600. [Google Scholar] [CrossRef]
- Fernández, L.; Breidenstein, E.B.; Hancock, R.E. Creeping baselines and adaptive resistance to antibiotics. Drug Resist. Updat. 2011, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, A.; Haghighat Bayan, M.A.; Nakielski, P.; Petronella, F.; De Sio, L.; Pierini, F. Nanotechnology Transition Roadmap toward Multifunctional Stimuli-Responsive Face Masks. ACS Appl. Mater. Interfaces 2022, 14, 46123–46144. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle, A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally sustainable fabrication of Ag@g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Biogenic synthesis of a Ag–graphene nanocomposite with efficient photocatalytic degradation, electrical conductivity and photoelectrochemical performance. N. J. Chem. 2015, 39, 8121–8129. [Google Scholar] [CrossRef]
- Rehman, I.; Gondal, H.Y.; Zamir, R.; Al-Hussain, S.A.; Batool, F.; Irfan, A.; Noreen, S.; Roheen, T.; Nisar, M.; Zaki, M.E. Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum. Nanomaterials 2023, 13, 1343. [Google Scholar] [CrossRef]
- Kong, H.; Jang, J. Antibacterial properties of novel poly (methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Petronella, F.; De Biase, D.; Zaccagnini, F.; Verrina, V.; Lim, S.-I.; Jeong, K.-U.; Miglietta, S.; Petrozza, V.; Scognamiglio, V.; Godman, N.P. Label-free and reusable antibody-functionalized gold nanorod arrays for the rapid detection of Escherichia coli cells in a water dispersion. Environ. Sci. Nano 2022, 9, 3343–3360. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N-and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Yu, L.; Du, A.; Yang, L.; Hu, Y.; Xie, W. Quantifying Hot Electron Energy Contributions in Plasmonic Photocatalysis Using Electrochemical Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 5495–5500. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga. A review. Int. J. Pharm. Ind. Res. 2013, 3, 257–265. [Google Scholar]
- Winderl, B.; Schwaiger, S.; Ganzera, M. Fast and improved separation of major coumarins in Ammi visnaga (L.) Lam. by supercritical fluid chromatography. J. Sep. Sci. 2016, 39, 4042–4048. [Google Scholar] [CrossRef]
- Feirouz, B.; Salima, K.-G. Antibacterial activity and chemical composition of Ammi visnaga L. essential oil collected from Boumerdes (Algeria) during three periods of the plant growth. J. Essent. Oil Bear. Plants 2014, 17, 1317–1328. [Google Scholar] [CrossRef]
- Khalfallah, A.; Labed, A.; Semra, Z.; Kaki, B.; Kabouche, A.; Touzani, R.; Kabouche, Z. Antibacterial activity and chemical composition of the essential oil of Ammi visnaga L.(Apiaceae) from Constantine, Algeria. Int. J. Med. Aromat. Plant 2011, 1, 302–305. [Google Scholar]
- Khadhri, A.; El Mokni, R.; Mguis, K.; Ouerfelli, I.; Araujo, M. Variability of two essential oils of Ammi visnaga (L.) Lam. a traditional Tunisian medicinal plant. J. Med. Plants Res. 2011, 5, 5079–5082. [Google Scholar]
- Rasooli, I.; Taghizadeh, M.; Astaneh, S.; Rezaei, M.; Jaimand, K. Phytobiological properties of Ammi visnaga L. and Lavandula angustifolia Mill. essential oils. Int. J. Essent. Oil 2007, 1, 72–78. [Google Scholar]
- Talaat, I.M.; Khattab, H.I.; Ahmed, A.M. Changes in growth, hormones levels and essential oil content of Ammi visnaga L. plants treated with some bioregulators. Saudi J. Biol. Sci. 2014, 21, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jalil, T.Z.; Saour, K.; Nasser, A. Phytochemical study of some flavonoids present in the fruits of two Ammi L. species wildly grown in Iraq. Iraqi J. Pharm. Sci. 2010, 19, 48–57. [Google Scholar] [CrossRef]
- Bencheraiet, R.; Kherrab, H.; Kabouche, A.; Kabouche, Z.; Maurice, J. Flavonols and antioxidant activity of Ammi visnaga L.(Apiaceae). Rec. Nat. Prod. 2011, 5, 52–67. [Google Scholar]
- Asli, A.; Brouillette, E.; Ster, C.; Ghinet, M.G.; Brzezinski, R.; Lacasse, P.; Jacques, M.; Malouin, F. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS ONE 2017, 12, 176988–177011. [Google Scholar] [CrossRef]
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not. J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning Electron Microscopy (SEM): A Review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; Volume 2018, pp. 7–9. [Google Scholar]
- Rajawat, S.; Qureshi, M. Electrolytic deposition of silver nanoparticles under “Principles of Green Chemistry”. Arab. J. Sci. Eng. 2014, 39, 563–568. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Sowmyya, T. Spectroscopic investigation on catalytic and bactericidal properties of biogenic silver nanoparticles synthesized using Soymida febrifuga aqueous stem bark extract. J. Environ. Chem. Eng. 2018, 6, 3590–3601. [Google Scholar]
- Vignesh, K.; Priyanka, R.; Hariharan, R.; Rajarajan, M.; Suganthi, A. Fabrication of CdS and CuWO4 modified TiO2 nanoparticles and its photocatalytic activity under visible light irradiation. J. Ind. Eng. Chem. 2014, 20, 435–443. [Google Scholar] [CrossRef]
- Mittal, A.; Jhare, D.; Mittal, J. Adsorption of hazardous dye Eosin Yellow from aqueous solution onto waste material De-oiled Soya: Isotherm, kinetics, and bulk removal. J. Mol. Liq. 2013, 179, 133–140. [Google Scholar] [CrossRef]
- Vidhu, V.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Cheng, Y.-H.; Muthukrishnan, P.; Padmavathy, S.; Elangovan, A. Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. J. Photochem. Photobiol. B Biol. 2017, 170, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Truppi, A.; Sibillano, T.; Giannini, C.; Striccoli, M.; Comparelli, R.; Curri, M.L. Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant. Catal. Today 2017, 284, 100–106. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, Z. Photocatalytic hot-carrier chemistry. MRS Bull. 2020, 45, 20–25. [Google Scholar] [CrossRef]
- Abdellah, M.; Nosier, S.; El-Shazly, A.; Mubarak, A. Photocatalytic decolorization of methylene blue using TiO2/UV system enhanced by air sparging. Alex. Eng. J. 2018, 57, 3727–3735. [Google Scholar] [CrossRef]
- Khan, M.E.; Mohammad, A.; Ali, W.; Khan, A.U.; Hazmi, W.; Zakri, W.; Yoon, T. Excellent visible-light photocatalytic activity towards the degradation of tetracycline antibiotic and electrochemical sensing of hydrazine by SnO2–CdS nanostructures. J. Clean. Prod. 2022, 349, 131249–131265. [Google Scholar] [CrossRef]
- Awais, M.; Khursheed, S.; Tehreem, R.; Mok, Y.S.; Siddiqui, G.U. pH regulated rapid photocatalytic degradation of methylene blue dye via niobium-nitrogen co-doped titanium dioxide nanostructures under sunlight. Appl. Catal. A Gen. 2022, 643, 118764–118778. [Google Scholar] [CrossRef]
- Khan, M.E. State-of-the-art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, Z.; Dojcinovic, M.; Vujancevic, J.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.; Stojadinovic, S.; Brankovic, G.; Nikolic, M. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020, 7, 200708–200725. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, 3663–3669. [Google Scholar] [CrossRef]
- Zheng, S.; Rupa, E.J.; Chokkalingam, M.; Piao, X.; Han, Y.; Ahn, J.C.; Nahar, J.; Kong, B.M.; Kwak, G.Y.; Kim, J.H. Photocatalytic activity of orchid-flower-shaped ZnO nanoparticles, toward cationic and anionic dye degradation under visible light, and its anti-cancer potential. Coatings 2022, 12, 946. [Google Scholar] [CrossRef]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-light triggered photocatalytic performance of ZnO. SiO2 nanocomposite for water pollutant compound methyl orange dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Synthesis, characterization, and photocatalytic property of novel ZnO/bone char composite. Mater. Res. Bull. 2018, 102, 45–50. [Google Scholar] [CrossRef]
- Hossain, A.; Rayhan, A.S.; Raihan, M.J.; Nargis, A.; Ismail, I.M.; Habib, A.; Mahmood, A.J. Kinetics of degradation of eosin Y by one of the advanced oxidation processes (AOPs)—Fenton’s process. Am. J. Anal. Chem. 2016, 7, 863–879. [Google Scholar] [CrossRef]





| S. No. | Compound Name | Structures |
|---|---|---|
| 1 | Khellin | 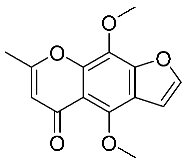 |
| 2 | Visnagin | 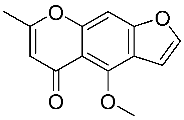 |
| 3 | Khellinol | 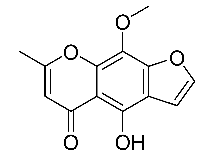 |
| 4 | Ammiol | 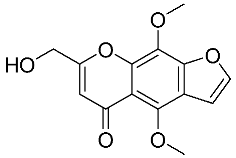 |
| 5 | Khellol | 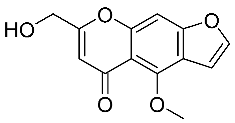 |
| 6 | Visnadine | 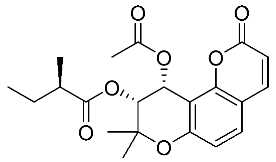 |
| 7 | Samidin |  |
| 8 | Dihydro Samidin | 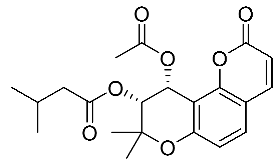 |
| 9 | Bornyl acetate | 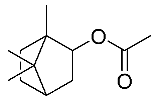 |
| 10 | Croweacin | 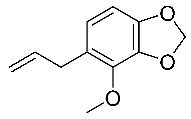 |
| 11 | 2,2 dimethyl butanoic acid | 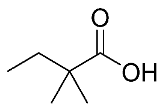 |
| 12 | Isobutyl isobutyrate | 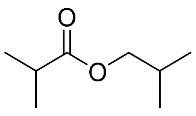 |
| 13 | Thymol | 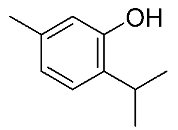 |
| 14 | Linalool | 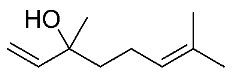 |
| 15 | (E)-β-Ocimene | 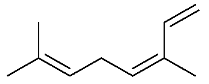 |
| 16 | α-Terpinene | 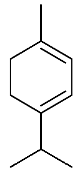 |
| 17 | Trans thujene |  |
| 18 | Cis-pinene hydrate |  |
| 19 | Linalool | 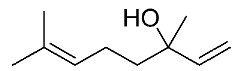 |
| 20 | Methyl octadecanoate | 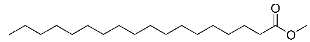 |
| 21 | Isoamyl-2-methyl butyrate |  |
| 22 | Isopentyl isovalerate | 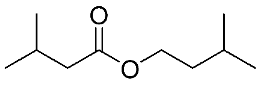 |
| 23 | ɑ-isophorone | 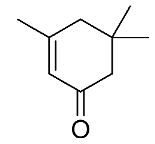 |
| 24 | Quercetin |  |
| 25 | Kaempferol | 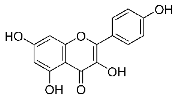 |
| 26 | Rhamnetin |  |
| 27 | Isorhamnetin | 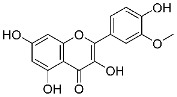 |
| 28 | Rhamnazin | 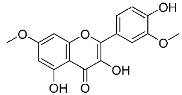 |
| 29 | 3-O-glucoside of rhamnetin | 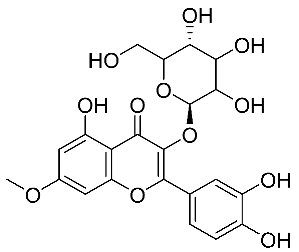 |
| 30 | 3-O-glucoside of isorhamnetin |  |
| 31 | 3-O-glucoside of rhamnazin |  |
| 32 | 7-O-glucoside of isorhamnetin |  |
| 33 | 3-O-rutin of quercetin | 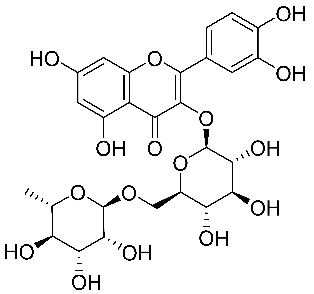 |
| 34 | 3-O-rutin of isorhamnetin |  |
| 35 | Quercetin 7, 3, 4′ –O-triglucoside | 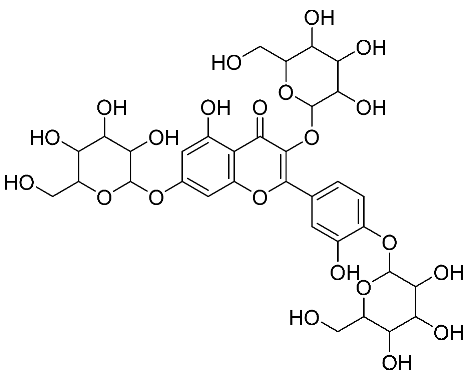 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, U.; Qureshi, A.K.; Noor, H.; Farhan, M.; Khan, M.E.; Hamed, O.A.; Bashiri, A.H.; Zakri, W. Plant Extract-Based Fabrication of Silver Nanoparticles and Their Effective Role in Antibacterial, Anticancer, and Water Treatment Applications. Plants 2023, 12, 2337. https://doi.org/10.3390/plants12122337
Farooq U, Qureshi AK, Noor H, Farhan M, Khan ME, Hamed OA, Bashiri AH, Zakri W. Plant Extract-Based Fabrication of Silver Nanoparticles and Their Effective Role in Antibacterial, Anticancer, and Water Treatment Applications. Plants. 2023; 12(12):2337. https://doi.org/10.3390/plants12122337
Chicago/Turabian StyleFarooq, Umar, Ahmad Kaleem Qureshi, Hadia Noor, Muhammad Farhan, Mohammad Ehtisham Khan, Osama A. Hamed, Abdullateef H. Bashiri, and Waleed Zakri. 2023. "Plant Extract-Based Fabrication of Silver Nanoparticles and Their Effective Role in Antibacterial, Anticancer, and Water Treatment Applications" Plants 12, no. 12: 2337. https://doi.org/10.3390/plants12122337
APA StyleFarooq, U., Qureshi, A. K., Noor, H., Farhan, M., Khan, M. E., Hamed, O. A., Bashiri, A. H., & Zakri, W. (2023). Plant Extract-Based Fabrication of Silver Nanoparticles and Their Effective Role in Antibacterial, Anticancer, and Water Treatment Applications. Plants, 12(12), 2337. https://doi.org/10.3390/plants12122337







