Interactive Effect of Cultivars, Crop Years and Rootstocks on the Biochemical Traits of Prunus persica (L.) Batsch Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Climatic Data Collection
2.2. Chemicals
2.3. Analytical Methods
2.3.1. Pomological Traits Determination
2.3.2. Phytochemical Content, Total Monomeric Anthocyanin and Antioxidant Activity Extraction
2.3.3. Total Phenolic Content Determination
2.3.4. Total Monomeric Anthocyanins Determination
2.3.5. Antioxidant Activity Evaluation
2.4. Statistical Analysis
3. Results
3.1. Climatic Condition
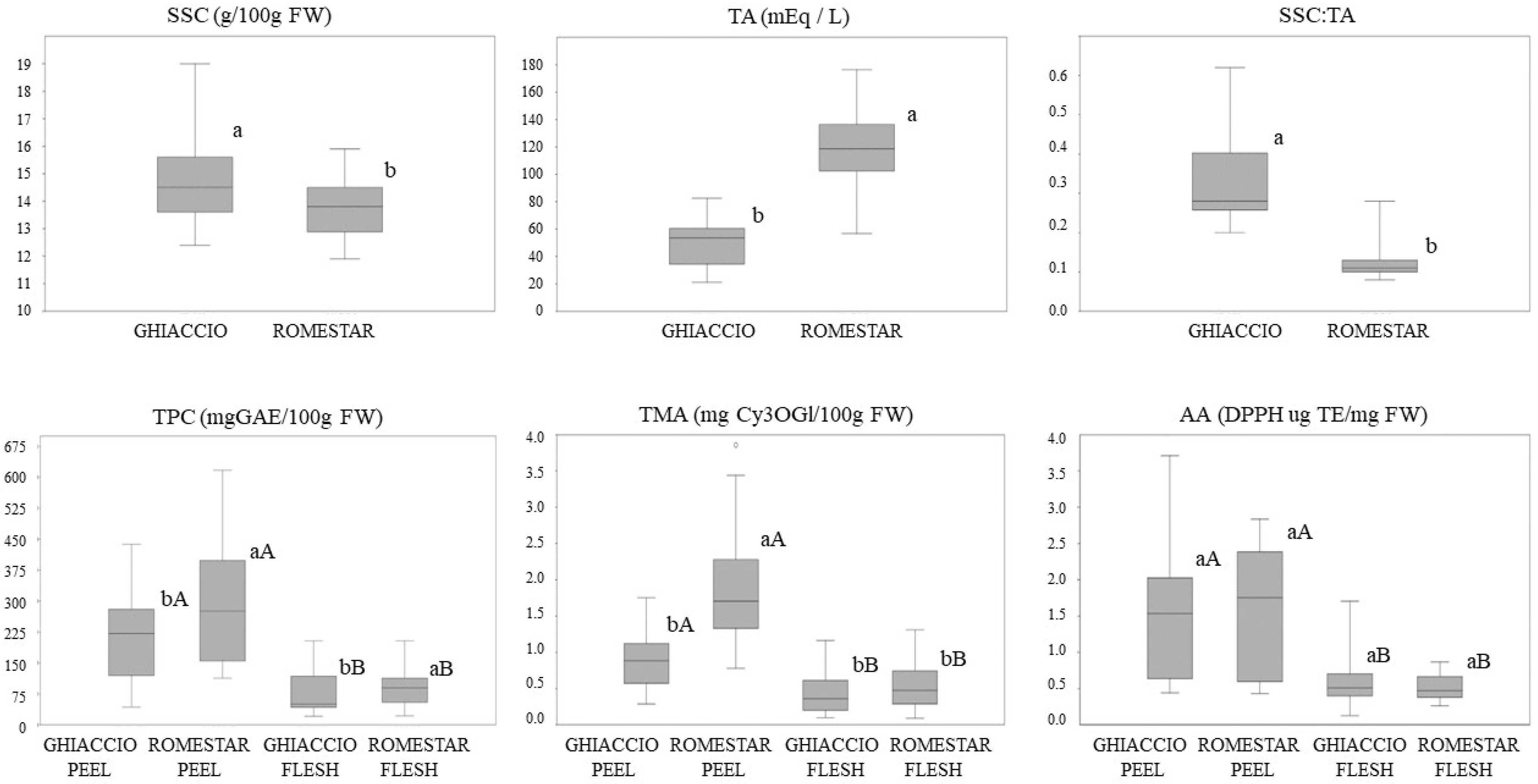
3.2. Peach Cultivars Biochemical Variability
3.3. Peach Years and Rootstock Biochemical Variability
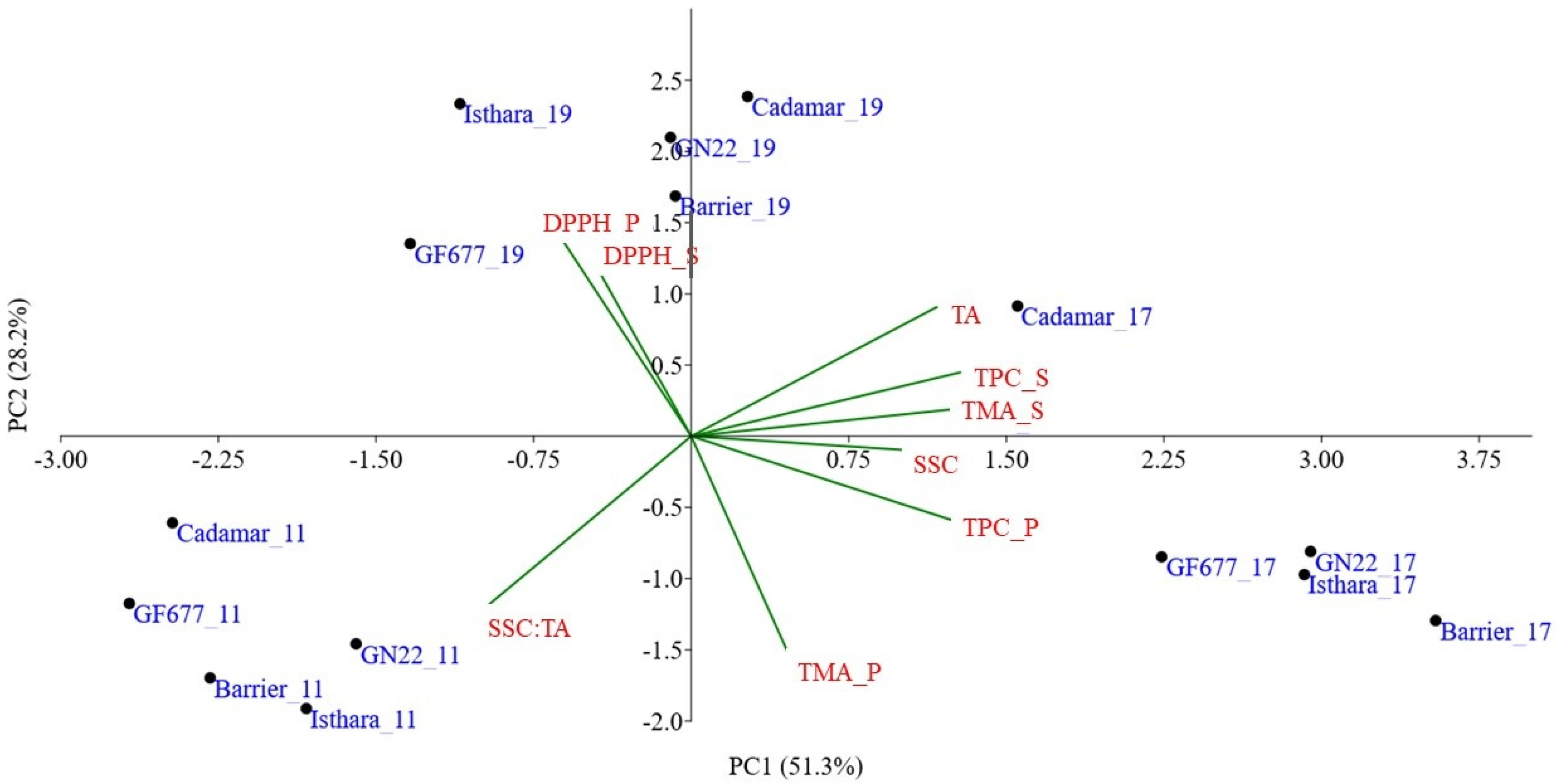
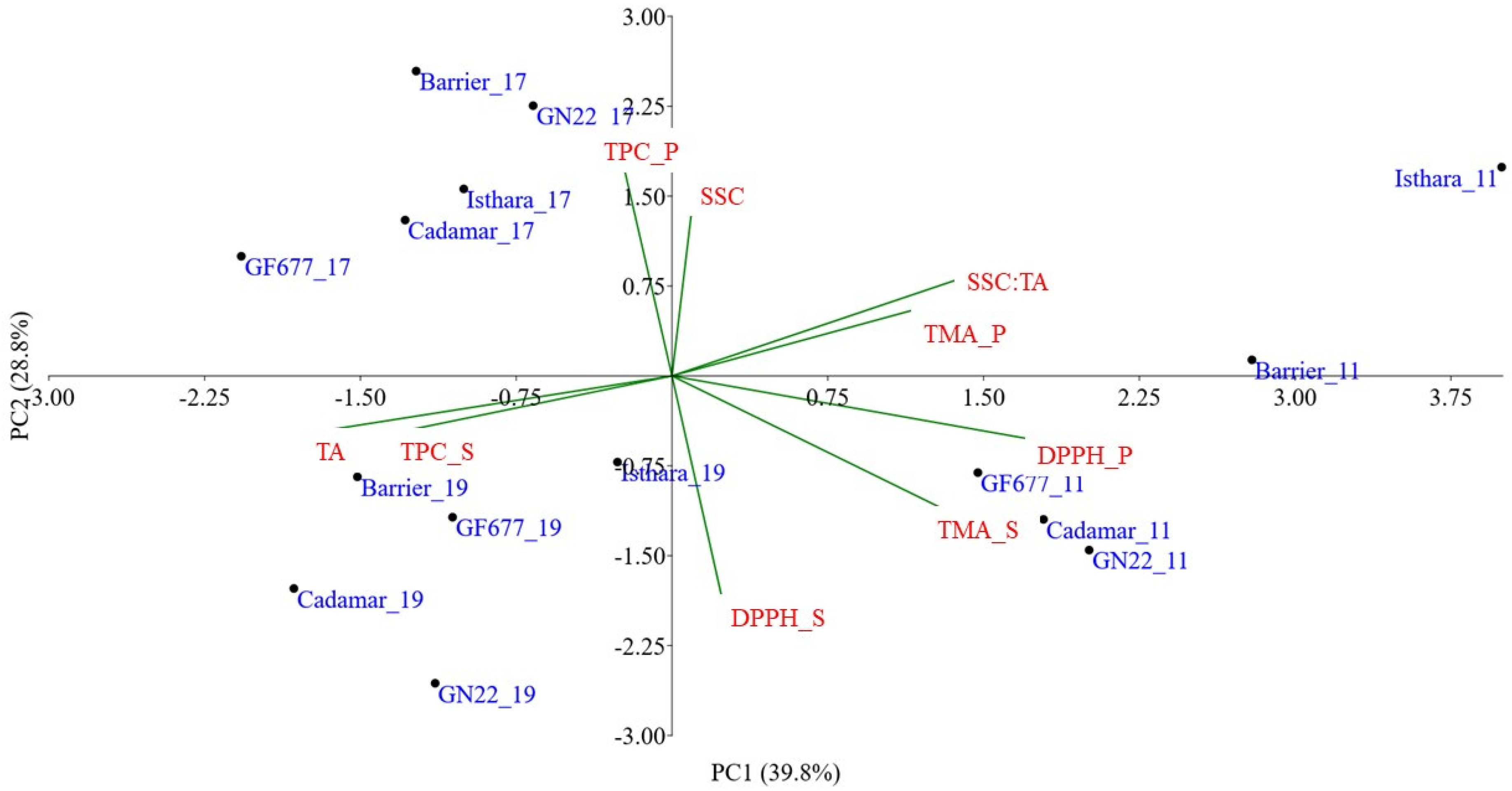
3.4. Chemometric Elaboration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the Fruit Quality and Phytochemical Compounds in Peach and Nectarine Cultivars. Plants 2023, 12, 1618. [Google Scholar] [CrossRef]
- Giorgi, M.; Capocasa, F.; Scalzo, J.; Murri, G.; Battino, M.; Mezzetti, B. The rootstock effects on plant adaptability, production, fruit quality, and nutrition in the peach (cv. “Suncrest”). Sci. Hortic. 2005, 107, 36–42. [Google Scholar] [CrossRef]
- Minas, I.S.; Anthony, B.M.; Pieper, J.R.; Sterle, D.G. Large-scale and accurate non-destructive visual to near infrared spectroscopy-based assessment of the effect of rootstock on peach fruit internal quality. Eur. J. Agron. 2023, 143, 126706. [Google Scholar] [CrossRef]
- Alfaro, J.M.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of rootstock on Citrus fruit quality: A review. Food Rev. Int. 2021, 1–19. [Google Scholar] [CrossRef]
- Reighard, G.L. Peach, plum and apricot rootstocks for the 21st century. Asp. Appl. Biol. 2013, 119, 59–66. [Google Scholar]
- Anthony, B.M.; Minas, I.S. Redefining the impact of preharvest factors on peach fruit quality development and metabolism: A review. Sci. Hortic. 2022, 297, 110919. [Google Scholar] [CrossRef]
- Jiménez, S.; Fattahi, M.; Bedis, K.; Nasrolahpour-Moghadam, S.; Irigoyen, J.J.; Gogorcena, Y. Interactional effects of climate change factors on the water status, photosynthetic rate, and metabolic regulation in peach. Front. Plant Sci. 2020, 11, 43. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Antonucci, F.; Costa, C.; Talento, C.; Ciccoritti, R. An artificial class modelling approach to identify the most largely diffused cultivars of sweet cherry (Prunus avium L.) in Italy. Food Chem. 2020, 333, 127515. [Google Scholar] [CrossRef]
- Conte, L.; Fantechi, P.; Insero, O.; Liverani, L.; Nicotra, A. Monografia di Cultivar di Pesco, Nettarine e Percoche; Ministero delle Politiche Agricole e Forestali: Rome, Italy; Istituto Sperimentale per la Frutticoltura: Rome, Italy, 2004; pp. 46, 75. [Google Scholar]
- Beckman, T.G. Register of New Fruit and Nut Cultivars (Almond Rootstock). Hortic. Sci. 2008, 43, 1321–1322. [Google Scholar]
- Fideghelli, C.; Loreti, F.; Ancarani, V.; Fei, C.; Godini, A.; Grandi, M.; Liverani, A.; Lugli, S.; Massai, R.; Palasciano, M.; et al. Monografia dei Portinnesti dei Fruttiferi; Progetto Finalizzato MIPAAF Regioni “Liste di orientamento varietale dei fruttiferi”; Ministero delle Risorse Agricole, Alimentari e Forestali: Rome, Italy, 2009; p. 239.
- Felipe, A.J. ‘Felinem’, ‘Garnem’, and ‘Monegro’ almond×peach hybrid rootstocks. Hortic. Sci. 2009, 44, 196–197. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. food Anal. Chem. 2001, 1, F1–F2. [Google Scholar] [CrossRef]
- Remorini, D.; Tavarini, S.; Degl’Innocenti, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of rootstocks and harvesting time on the nutritional quality of peel and flesh of peach fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef]
- Gil, M.; Tomas-Barberan, A.T.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids and vitamin C content of nectarine and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.Á. Agronomical parameters, sugar profile and antioxidant compounds of “Catherine” peach cultivar influenced by different plum rootstocks. Int. J. Mol. Sci. 2014, 15, 2237–2254. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Tsipouridis, C.G. Effects of Cultivar and Rootstock on the Antioxidant Content and Physical Characters of Clingstone Peaches. Sci. Hortic. 2007, 115, 34–39. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Moreno, M.Á. Influence of plum rootstocks on agronomic performance, leaf mineral nutrition and fruit quality of ‘Catherina’ peach cultivar in heavy-calcareous soil conditions. Span. J. Agric. Res. 2017, 15, e0901. [Google Scholar] [CrossRef]
- Gullo, G.; Motisi, A.; Zappia, R.; Dattola, A.; Diamanti, J.; Mezzetti, B. Rootstock and fruit canopy position affect peach [Prunus persica (L.) Batsch] (cv. Rich May) plant productivity and fruit sensorial and nutritional quality. Food Chem. 2014, 153, 234–242. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Talento, C.; Sartori, A.; Terlizzi, M.; Caboni, E.; Carbone, K. Comparative characterization of fruit quality, phenols and antioxidant activity of de-pigmented “Ghiaccio-1*” and white flesh peaches. Adv. Hortic. Sci. 2016, 30, 175–182. [Google Scholar]
- De Salvador, F.R.; Proietti, G.; Tomasone, R.; Cedrola, C. Field performance of several hybrid rootstocks with six peach cultivars. In Proceedings of the X International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Stellenbosch, South Africa, 3–6 December 2012; pp. 577–583. [Google Scholar]
- Cipriani, G.; Terlizzi, M.; Bevilacqua, D.; Di Cintio, A.; Rosato, T.; Sartori, A. Peach breeding programme for new cultivars and different traits-pomological and phenological data analysis with a ranking method. In Proceedings of the II International Symposium on Horticulture in Europe, Angers, France, 1–5 July 2012; pp. 695–702. [Google Scholar]
- Crisosto, C.H.; Day, K.R.; Crisosto, G.M.; Garner, D. Quality attributes of white flesh peaches and nectarines grown under California conditions. J. Am. Pomol. Soc. 2001, 55, 45–51. [Google Scholar]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Response of plum trees to deficit irrigation under two crop levels: Tree growth, yield and fruit quality. Irrig. Sci. 2010, 28, 525–534. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff-Hoenninger, S. Quality assessment of 178 varieties of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef]
- Tavarini, S.; Gil, M.I.; Tomas-Barberan, F.A.; Buendia, B.; Remorini, D.; Massai, R.; Degl’Innocenti, E.; Guidi, L. Effects of water stress and rootstocks on fruit phenolic composition and physical/chemical quality in Suncrest peach. Ann. Appl. Biol. 2011, 158, 226–233. [Google Scholar] [CrossRef]

| Rootstocks | Origin | Characteristics |
|---|---|---|
| GF677 | Prunus persica (L.) Batsch x Prunus amygdalus Batsch | Induces high tree vigor, rapid entry into production, high production yields (both dry and irrigated). Superior adaptability even to difficult soils, tolerating active lime levels up to 12%. Tolerates water deficiency and stumpiness fairly well. Poor resistance to Agrobacterium, nematodes and Armillaria. |
| Cadaman® Avimag | Prunus davidiana (Carrière) Franch. x Prunus persica (L.) Batsch | Medium-to-high vigor (similar to GF677) with good growth rate. Suitable for fresh, poor and even tendentially asphyctic soils. Induces slight earliness of maturity and increase in size. Resistant to some types of nematodes. Presents some polloniferous aptitude. |
| Barrier® | Prunus davidiana (Carrière) Franch. x Prunus persica (L.) Batsch | Slightly lower vigor than GF677. Good anchorage, good-to-high productivity, induces better fruit size and coloration, slightly delays both flowering and ripening. Suitable for replanting. Resistant to some nematodes, asphyctic soils and chlorosants. |
| Isthara® | (Prunus cerasifera Ehrh. x Prunus salicina Lindl.) x (Prunus cerasifera Ehrh. x Prunus persica (L.) Batsch) | Medium vigor, with lower development than “franco” seedling rootstocks, but with good vegetative renewal and decent adaptability. Moderate suckering activity. Induces early fruiting, high yields and good-sized fruit. It is susceptible to Armillaria, but resistant to some nematodes. |
| GxN22 (Felinem) | Prunus amygdalus Batsch x Prunus persica (L.) Batsch | In grafted cultivars induces vigor and productivity similar to GF-677 or Hansen 536. High resistance to the main root nematode species that attack Prunus. Adapts well to calciferous soil. Resistant toward chlorosis. |
| Ghiaccio-1* | |||||||||||
| (A) | Pitted Fruit | Peel | Flesh | ||||||||
| Source | D.F. 1 | TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | |
| Crop Years (Y) | 2 | 15,254.1 ** | 79.32 ** | 0.68 ** | 776,527 ** | 7.7 ** | 25.5 ** | 201,693 ** | 2.7 ** | 0.96 ** | |
| Rootstock (R) | 4 | 661.6 ** | 28.67 ** | 0.02 ns | 70,126.8 ** | 1.5 ** | 22.5 ** | 11,088 ** | 0.7 ** | 1.66 ** | |
| Y × R | 5 | 3210.3 * | 64.22 ** | 0.03 ns | 130,864 ** | 2.1 ** | 15.2 ** | 11,878 ** | 0.8 ** | 6.91 ** | |
| Within | 75 | 42.8 ns | 44.56 ns | 0.19 ns | 62,892 ns | 0.9 ns | 4.5 ns | 13,910 ns | 1.6 ns | 0.52 ns | |
| Total | 89 | 19,877.4 ns | 219.79 ns | 0.92 ns | 1.1 × 106 ns | 12.2 ns | 67.6 ns | 238,570 ns | 5.8 ns | 10.05 ns | |
| (B) | TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | ||
| ROOTSTOCKS | Barrier® | 48 ± 8 ab | 15.0 ± 1.1 ab | 0.4 ± 0.1 a | 235 ± 27 a | 1.0 ± 0.1 a | 1.4 ± 0.1 bc | 96 ± 17 a | 0.5 ± 0.1 a | 0.5 ± 0.2 a | |
| Cadaman® | 51 ± 7 a | 14.2 ± 0.9 b | 0.3 ± 0.1 a | 223 ± 20 a | 0.9 ± 0.1 a | 2.5 ± 0.2 a | 76 ± 16 ab | 0.3 ± 0.1 b | 0.6 ± 0.1 a | ||
| GF677 | 48.0 ± 6 ab | 14.8 ± 1.0 ab | 0.3 ± 0.1 a | 155 ± 17 b | 0.7 ± 0.1 b | 1.2 ± 0.1 c | 67 ± 10 b | 0.3 ± 0.1 b | 0.5 ± 0.2 a | ||
| GXN22 | 54 ± 6 a | 15.9 ± 1.3 a | 0.3 ± 0.1 a | 204 ± 26 ab | 0.9 ± 0.2 ab | 1.2 ± 0.1 c | 68 ± 8 b | 0.4 ± 0.1 ab | 0.5 ± 0.2 a | ||
| Isthara® | 46 ± 5 b | 14.2 ± 0.8 b | 0.3 ± 0.1 a | 219 ± 12 a | 1.0 ± 0.2 a | 1.6 ± 0.2 b | 88 ± 12 a | 0.6 ± 0.2 a | 0.6 ± 0.1 a | ||
| TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | |||
| YEARS | 2011 | 31.5 ± 5.5 b | 13.8 ± 0.8 bc | 0.45 ± 0.08 a | 90 ± 27 b | 0.6 ± 0.1 b | 1.5 ± 0.4 a | 43.8 ± 12.0 b | 0.5 ± 0.3 b | 0.5 ± 0.1 a | |
| 2017 | 60.8 ± 11.9 a | 16.0 ± 1.9 a | 0.27 ± 0.05 b | 307 ± 80 a | 1.2 ± 0.3 a | 1.0 ± 0.1 b | 142 ± 36 a | 0.6 ± 0.1 b | 0.6 ± 0.2 a | ||
| 2019 | 55.4 ± 5.1 a | 14.6 ± 0.9 b | 0.26 ± 0.03 b | 223 ± 66 ab | 0.9 ± 0.3 ab | 0.22 ± 0.08 c | 49 ± 20 b | 2.2 ± 0.8 a | 0.7 ± 0.1 a | ||
| Romestar* | |||||||||||
| (A) | Pitted Fruits | Peel | Flesh | ||||||||
| Source | D.F. 1 | TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | |
| Crop Years (Y) | 2 | 20,291.8 * | 6.9 * | 0.04 * | 1.1 × 106 * | 29.3 * | 56.4 * | 132,714 * | 5.74 * | 1.77 * | |
| Rootstock (R) | 4 | 12,499.2 * | 22.6 * | 0.05 * | 72,425.7 * | 2.6 * | 1.3 * | 16,196.2 * | 0.56 * | 0.14 * | |
| Y × R | 5 | 10,201.6 * | 36.3 * | 0.07 * | 383,575 * | 10.5 * | 1.1 * | 20,998.6 * | 1.94 * | 0.24 * | |
| Within | 75 | 8779.4 ns | 35.0 ns | 0.01 ns | 81,072.5 ns | 5.3 ns | 2.7 ns | 2.1 × 105 ns | 0.56 ns | 0.32 ns | |
| Total | 89 | 51,772.1 ns | 100.9 ns | 0.16 ns | 1.6 × 106 ns | 47.7 ns | 61.5 ns | 1.9 × 105 ns | 8.81 ns | 2.45 | |
| (B) | TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | ||
| ROOTSTOCKS | Barrier® | 119 ± 9 a | 14.3 ± 0.8 a | 0.12 ± 0.02 ab | 263 ± 18 b | 1.7 ± 0.4 a | 1.4 ± 0.2 a | 101 ± 17 a | 0.6 ± 0.2 a | 0.5 ± 0.1 a | |
| Cadaman® | 123 ± 11 a | 13.2 ± 0.9 b | 0.11 ± 0.02 ab | 276 ± 11 b | 1.9 ± 0.3 a | 1.5 ± 0.3 a | 81 ± 7 b | 0.4 ± 0.1 a | 0.5 ± 0.1 a | ||
| GF677 | 131 ± 10 a | 13.5 ± 0.8 b | 0.10 ± 0.01 ab | 282 ± 13 b | 1.9 ± 0.3 a | 1.5 ± 0.3 a | 80 ± 6 b | 0.5 ± 0.1 a | 0.5 ± 0.1 a | ||
| GxN22 | 115 ± 5 ab | 13.2 ± 0.9 b | 0.12 ± 0.02 ab | 315 ± 18 a | 2.0 ± 0.4 a | 1.6 ± 0.2 a | 81 ± 5 b | 0.7 ± 0.2 a | 0.5 ± 0.1 a | ||
| Isthara® | 96 ± 15 b | 14.5 ± 0.9 a | 0.17 ± 0.07 a | 314 ± 19 a | 1.8 ± 0.2 a | 1.7 ± 0.3 a | 113 ± 12 a | 0.5 ± 0.1 a | 0.6 ± 0.1 a | ||
| TA | SSC | SSC:TA | TPC | TMA | AA | TPC | TMA | AA | |||
| YEARS | 2011 | 95.9 ± 20.8 b | 13.5 ± 1.2 b | 0.15 ± 0.06 a | 145 ± 20 b | 1.3 ± 0.3 b | 0.6 ± 0.3 b | 76 ± 15 b | 0.8 ± 0.3 a | 0.7 ± 0.1 a | |
| 2017 | 125.4 ± 24.1 a | 14.2 ± 1.0 a | 0.12 ± 0.04 a | 311 ± 73 ab | 2.7 ± 0.6 a | 1.7 ± 0.4 a | 144 ± 37 a | 0.6 ± 0.1 a | 0.37 ± 0.04 b | ||
| 2019 | 127.9 ± 15.5 a | 13.6 ± 0.9 b | 0.11 ± 0.02 a | 412 ± 116 a | 2.4 ± 0.4 a | 1.7 ± 0.5 a | 57 ± 13 b | 0.5 ± 0.1 ab | 0.23 ± 0.03 c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccoritti, R.; Manganiello, R.; Antonucci, F.; Ceccarelli, D. Interactive Effect of Cultivars, Crop Years and Rootstocks on the Biochemical Traits of Prunus persica (L.) Batsch Fruits. Plants 2023, 12, 2325. https://doi.org/10.3390/plants12122325
Ciccoritti R, Manganiello R, Antonucci F, Ceccarelli D. Interactive Effect of Cultivars, Crop Years and Rootstocks on the Biochemical Traits of Prunus persica (L.) Batsch Fruits. Plants. 2023; 12(12):2325. https://doi.org/10.3390/plants12122325
Chicago/Turabian StyleCiccoritti, Roberto, Rossella Manganiello, Francesca Antonucci, and Danilo Ceccarelli. 2023. "Interactive Effect of Cultivars, Crop Years and Rootstocks on the Biochemical Traits of Prunus persica (L.) Batsch Fruits" Plants 12, no. 12: 2325. https://doi.org/10.3390/plants12122325
APA StyleCiccoritti, R., Manganiello, R., Antonucci, F., & Ceccarelli, D. (2023). Interactive Effect of Cultivars, Crop Years and Rootstocks on the Biochemical Traits of Prunus persica (L.) Batsch Fruits. Plants, 12(12), 2325. https://doi.org/10.3390/plants12122325







