The Foliar Anatomy and Micromorphology of Cyphostemma hypoleucum (Vitaceae)
Abstract
1. Introduction
2. Results
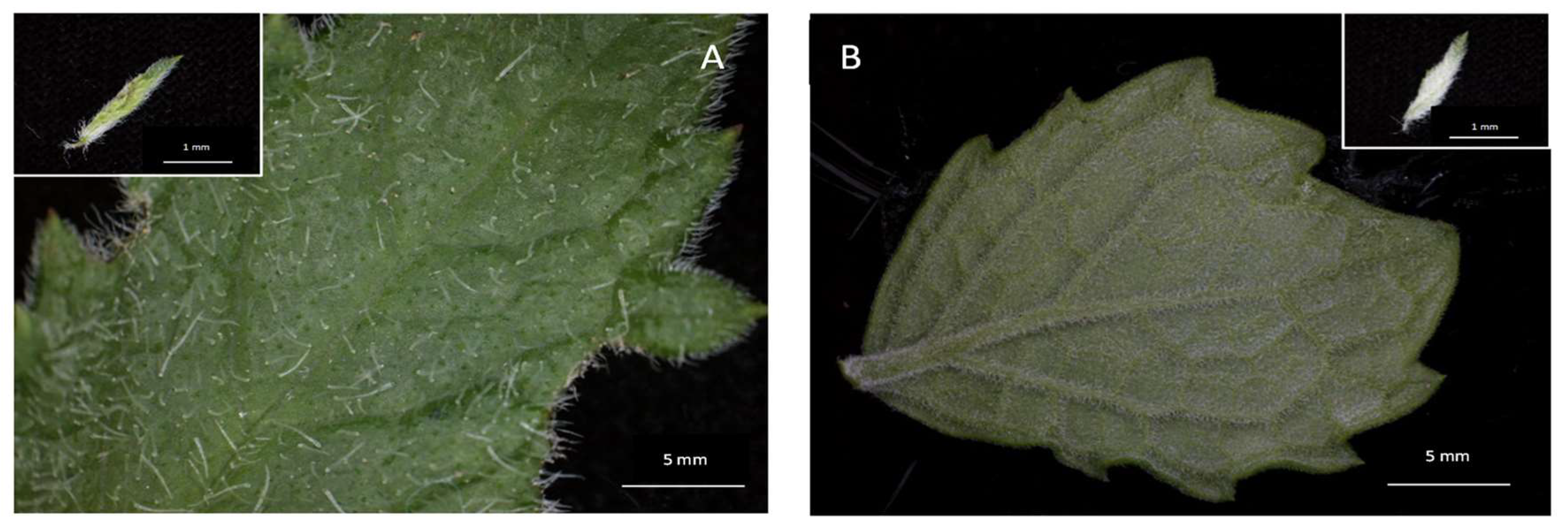
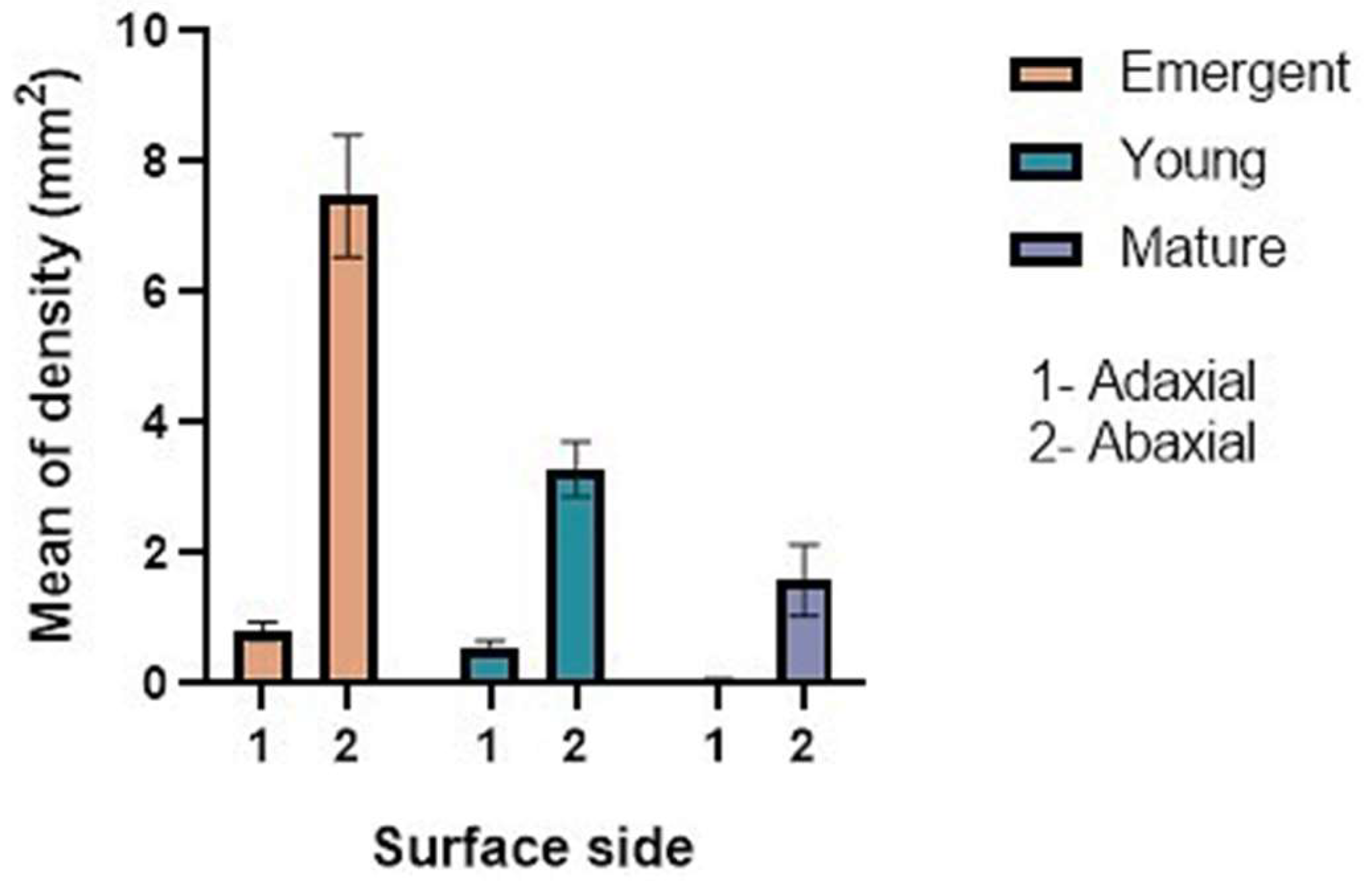
2.1. Trichome Density and Micromorphology
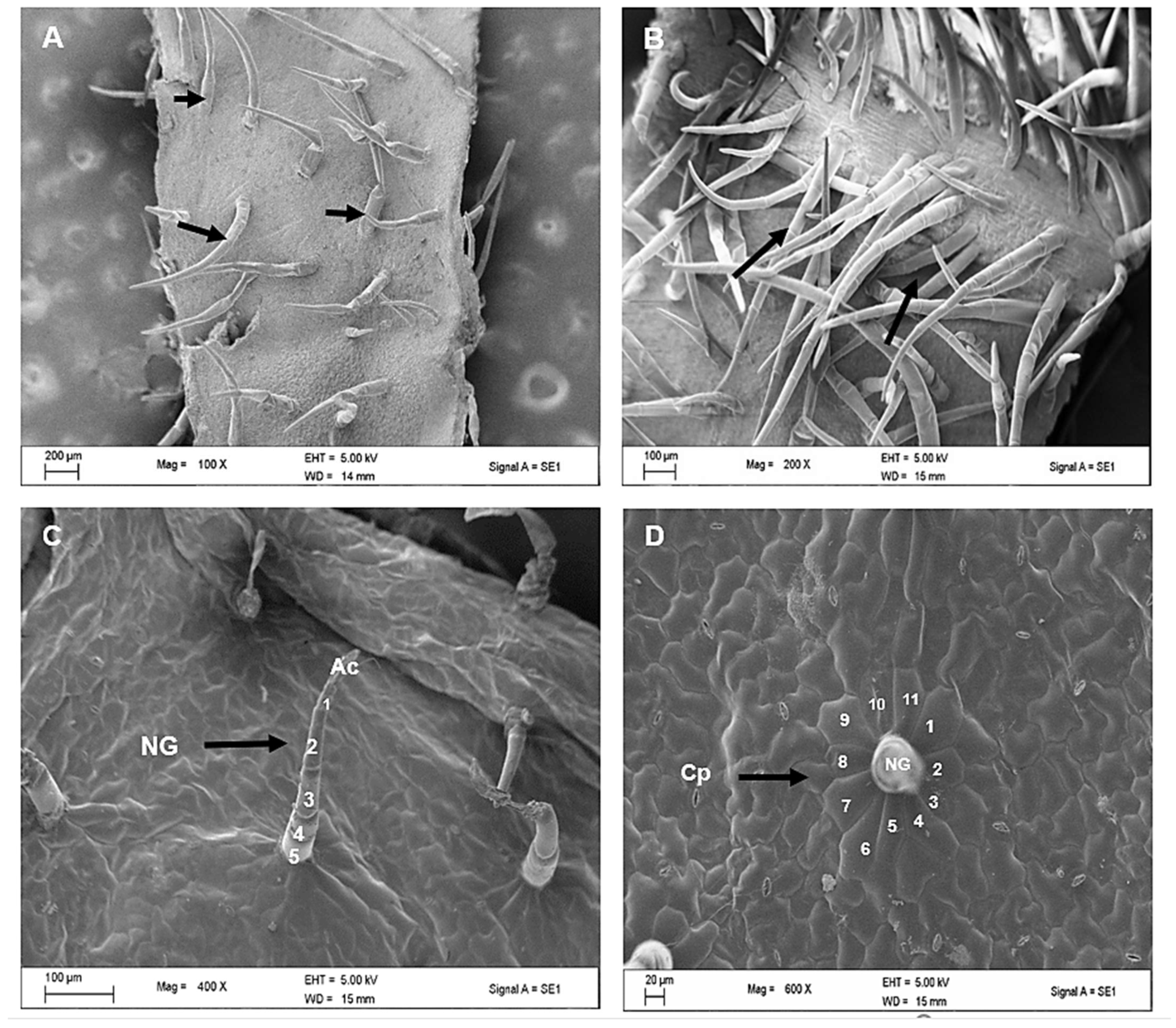
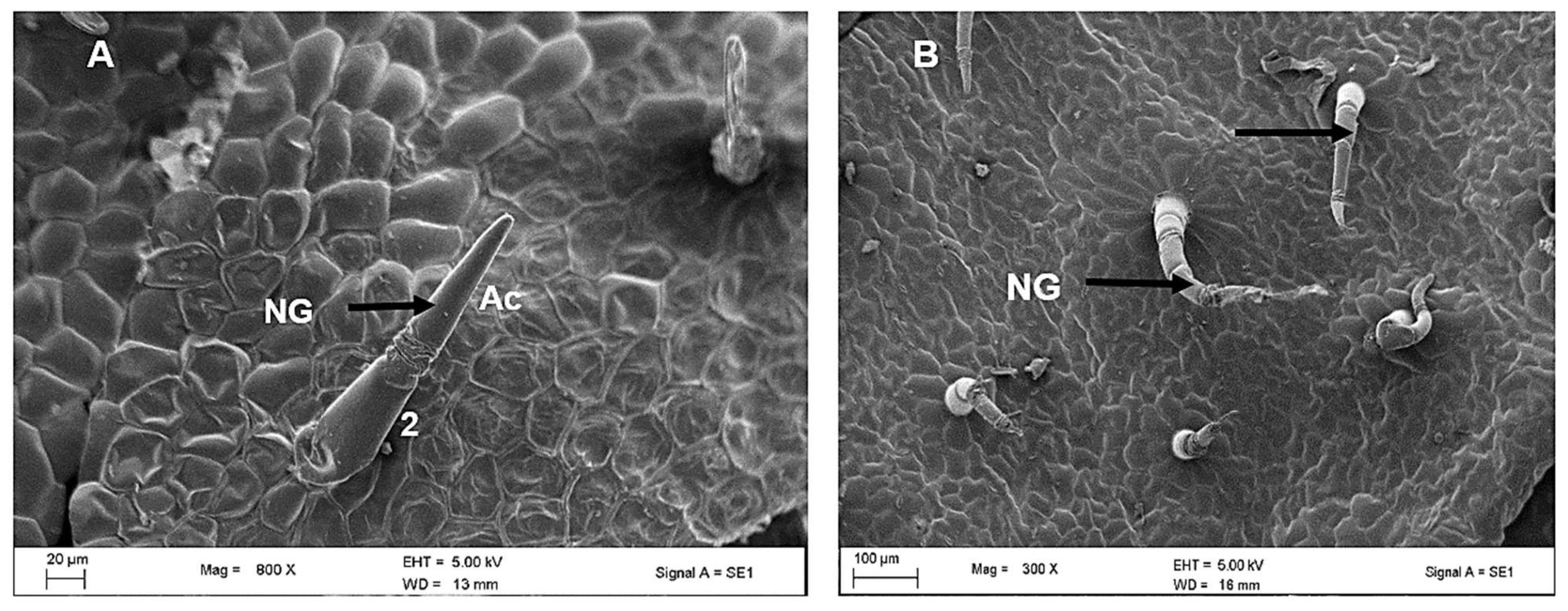
2.2. Scanning Electron Microscopy of Leaves of C. hypoleucum
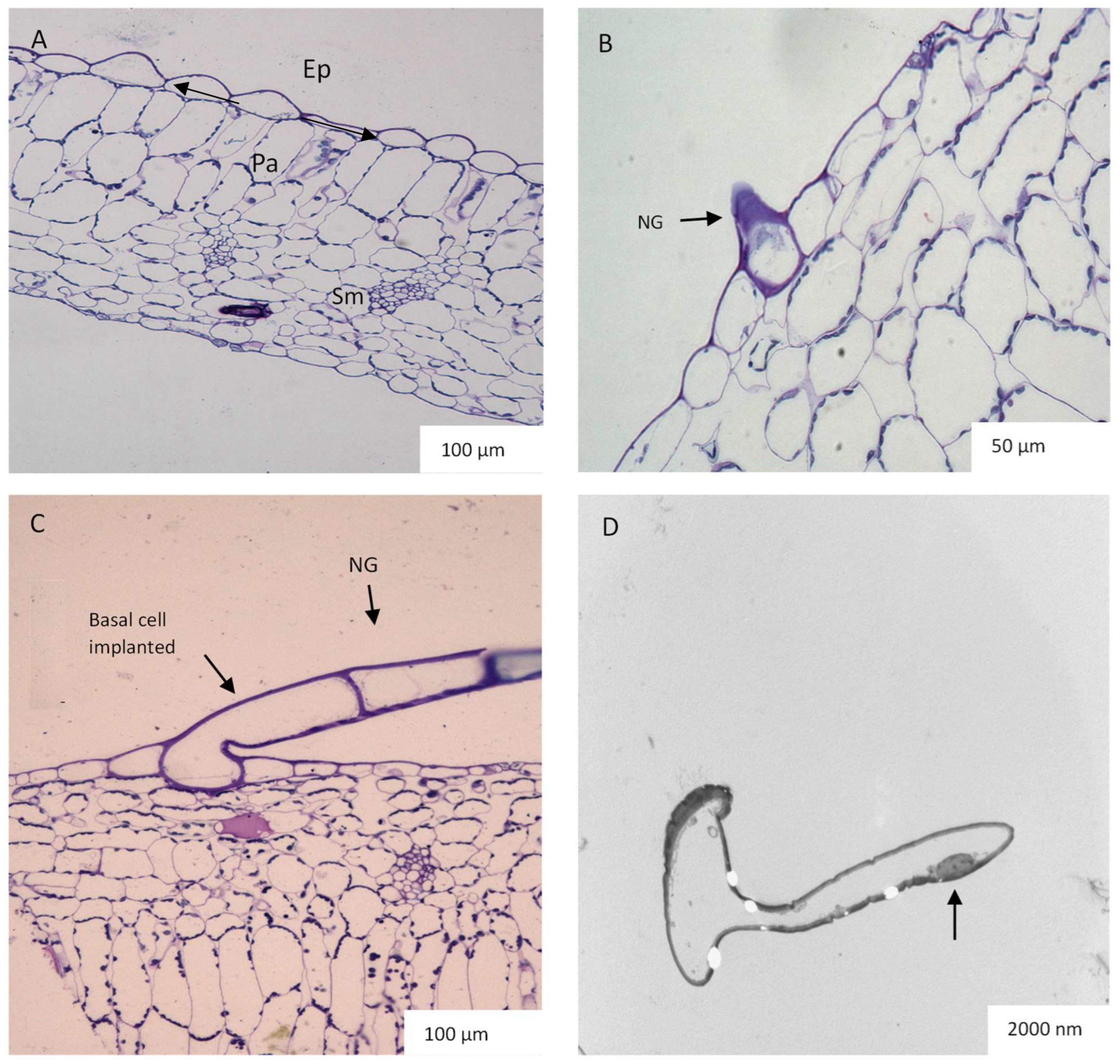
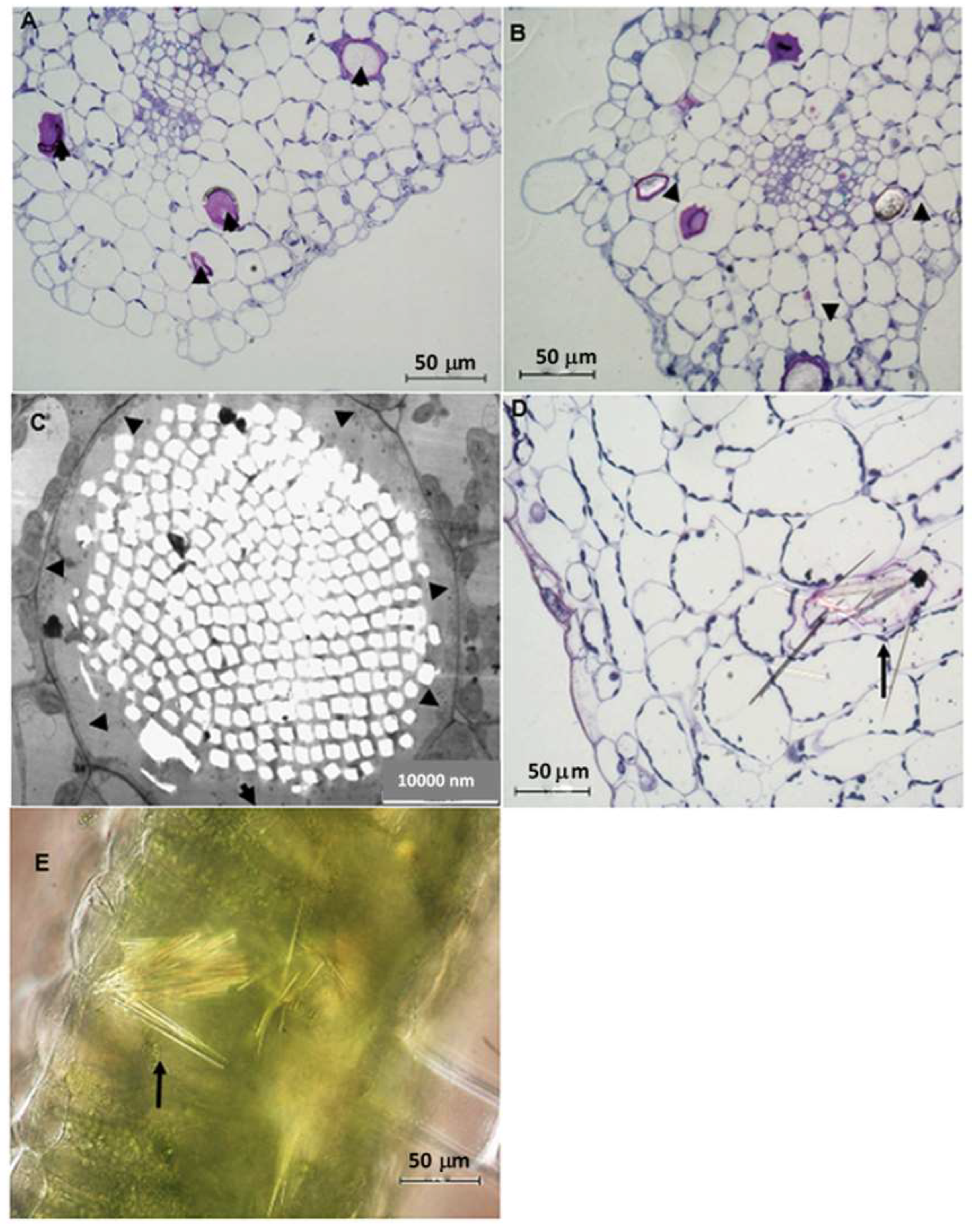
2.3. Leaf Anatomy Based on Light and Transmission Electron Microscopy (TEM)
3. Discussion
3.1. Trichome Density and Morphology
3.2. Idioblast Crystals
4. Materials and Methods
4.1. Plant Collection
4.2. Stereomicroscopy
4.3. Scanning Electron Microscopy (SEM)
4.4. Light Microscopy and Transmission Electron Microscopy (TEM)
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahn, A. Secretory tissues in vascular plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef]
- Roschina, V.V. Autofluorescence of plant secreting cells as a biosensor and bioindicator reaction. J. Fluoresc. 2003, 13, 403–420. [Google Scholar] [CrossRef]
- Retief, E.; van Wyk, A.E. A new species of Cyphostemma (Vitaceae) from South Africa. S. Afr. J. Bot. 1996, 62, 183–187. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, E.S. Structural features of glandular and non-glandular trichomes in three species of Mentha. Appl. Microsc. 2013, 43, 47–53. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Mayekiso, B. Morphological and chemical composition of the essential oil of the leaf of Schistostephium heptalobium. Afr. J. Biotechnol. 2009, 8, 1509–1519. [Google Scholar]
- Werker, E. Function of essential oil-secreting glandular hairs in aromatic plans of Lamiaceae—A review. Flavour. Fragr. J. 1993, 8, 249–255. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Wagner, G.J. Secreting glandular trichomes: More than just hairs. Plant Physiol. 1991, 96, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A. Glandular trichomes: What comes after expressed sequence tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Payne, W. A glossary of plant hair terminology. Brittonia 1978, 30, 239–255. [Google Scholar] [CrossRef]
- Maffei, M.; Codignola, A. Photorespiration and herbicide effect of terpene production in peppermint (Mentha piperita L.). J. Essent. Oil Res. 1990, 2, 275–286. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef]
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK; New York, NY, USA, 1979; p. 302. [Google Scholar]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New Phytol. 2020, 225, 2251–2266. [Google Scholar] [CrossRef]
- Valkama, E.; Salminen, J.; Koricheva, J.; Pihlaja, J. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann. Bot. 2003, 91, 643–655. [Google Scholar] [CrossRef]
- Ma, Z.; Wen, J.; Ickert-bond, S.M.; Chen, L.; Liu, X. Morphology, structure and ontogeny of trichomes of the grape genus (Vitis, Vitaceae). Front. Plant Sci. 2016, 7, 704. [Google Scholar] [CrossRef]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996; p. 464. [Google Scholar]
- Mlambo, N.P. The Screening of Medicinal Plants Traditionally Used to Treat Diarrhea, in Ongoye Area, Kwazulu Natal. Master’s Thesis, Faculty of Science, University of Zululand, Richards Bay, South Africa, 2008. [Google Scholar]
- Akwu, N.; Naidoo, Y.; Singh, M. The anatomy and histochemistry of Grewia lasiocarpa E. Mey. ex Harv. (Malvaceae). Suid-Afrik. Tydskr. Nat. Tegnol. 2020, 39, 91–107. [Google Scholar] [CrossRef]
- Gangaram, S.; Naidoo, Y.; Dewir, Y.H. Foliar micromorphology, ultrastructure, and histochemical analysis of Barleria albostellata CB Clarke. S. Afr. J. Bot. 2020, 1, 212–224. [Google Scholar] [CrossRef]
- Solereder, H. Systematic Anatomy of Dicotyledons: A Handbook for Laboratories of Pure and Applied Botany. Nature 1908, 79, 211–212. [Google Scholar]
- Gago, P.; Conéjéro, G.; Martínez, M.C.; Boso, S.; This, P.; Verdeil, J.-L. Microanatomy of leaf trichomes: Opportunities for improved ampelographic discrimination of grapevine (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 22, 494–503. [Google Scholar] [CrossRef]
- Rowson, M. Anatomy of the Dicotyledons, Volumes I and II, by C. R. Metcalfe and L. Chalk. J. Pharm. Pharmacol. 1950, 2, 862–863. [Google Scholar] [CrossRef]
- Levin, D.A. The role of trichomes in plant defence. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Lubna, Z.M.; Ahmad, M.; Shaheen, S.; Sultana, S.; Rehman, S.U.; Amina, H. Micromorphological investigation of leaf epidermis and seeds of Vitaceae from Pakistan using light microscopy and scanning electron microscopy. Microsc. Res. Tech. 2019, 82, 335–344. [Google Scholar] [CrossRef]
- Oksanen, E. Trichomes form an important first line of defence against adverse environment—New evidence for ozone stress mitigation. Plant Cell Environ. 2018, 41, 1497–1499. [Google Scholar] [CrossRef]
- Pshenichnikova, T.A.; Doroshkov, A.V.; Osipova, S.V.; Permyakov, A.V.; Permyakova, M.D.; Efimov, V.M.; Afonnikov, D.A. Quantitative characteristics of pubescence in wheat (Triticum aestivum L.) are associated with photosynthetic parameters under conditions of normal and limited water supply. Planta 2019, 249, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Smith, S.Y. Evaluating stasis in Metasequoia (Cupressaceae): Testing the relationship between leaf traits and climate. Int. J. Plant Sci. 2020, 181, 157–174. [Google Scholar] [CrossRef]
- Smith, S.Y.; Chitwood, D.H. Plant-Environment Interactions: A Sweeping Perspective. Int. J. Plant Sci. 2020, 181, 155–156. [Google Scholar] [CrossRef]
- Johnson, H.B. Plant pubescence: An ecological perspective. Bot. Rev. 1975, 41, 233–258. [Google Scholar] [CrossRef]
- Valverde, P.L.; Fornoni, J.; Núñez-Farfán, J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. J. Evol. Biol. 2001, 14, 424–432. [Google Scholar] [CrossRef]
- Tozin, L.R.S.; Silva, S.C.M.; Rodrigues, T.M. Non-glandular trichomes in Lamiaceae and Verbenaceae species: Morphological and histochemical features indicate more than physical protection. N. Z. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- Cardoso, M.Z. Herbivore handling of a Plant’s Trichome: The case of Heliconius charithonia (L.) (Lepidoptera: Nymphalidae) and Passiflora lobata (Killip) Hutch. (Passifloraceae). Neotrop. Entomol. 2008, 37, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, G.J. Plants that lead: Do some surface features direct enemy traffic on leaves and stem? Biol. J. Linn. 2015, 116, 288–294. [Google Scholar] [CrossRef]
- Muganu, M.; Paolocci, M. Adaptation of local grapevine germplasm: Exploitation of natural defence mechanisms to biotic stresses. In The Mediterranean Genetic Code—Grapevine and Olive; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Bickford, C.P. Ecophysiology of leaf trichomes. Funct. Plant Biol. 2016, 43, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, Y.; Samuels, J.; Nicholas, A.; Rampersad, E. Leaf micromorphology of the African medicinal plant Xysmalobium undulatum. Microsc. Microanal. 2009, 15, 884–885. [Google Scholar] [CrossRef]
- Mayekiso, B.; Magwa, M.L.; Coopoosamy, R. The morphology and ultrastructure of glandular and non-glandular trichomes of Pteronia incana (Asteraceae). Afr. J. Plant Sci. 2008, 2, 52–60. [Google Scholar]
- Belmonte, E.; Arriaza, B.; Arismendi, M.; Sepúlveda, G. Foliar Anatomy of three native species of Tillandsia L. from the Atacama Desert, Cile. Plants 2022, 11, 870. [Google Scholar] [CrossRef]
- Fernandez, V.; Sancho-Knapik, D.; Guzman, P.; Peguero-Pina, J.J.; Gil, L.; Karabourniotis, G.; Khayet, M.; Fasseas, C.; Heredia-Guerrero, J.A.; Heredia, A.; et al. Wettability, polarity, and water absorption of holm oak leaves: Effect of leaf size and age. Plant Physiol. 2014, 166, 168–180. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Chaney, R.L.; Angle, J.S.; Maugel, T.K.; Erbe, E.F.; Murphy, C.A. Simultaneous hyperaccumulation of nickel, manganese, and calcium in Alyssum leaf trichomes. Environ. Sci. Technol. 2004, 38, 5797–5802. [Google Scholar] [CrossRef]
- Li, W.; Chen, T.; Chen, Y.; Lei, M. Role of trichome of Pteris vittata L. in arsenic hyperaccumulation. Sci. China Ser. C Life Sci. 2005, 48, 148–154. [Google Scholar]
- Qiu, R.; Zhao, X.; Tang, Y.T.; Yu, F.M.; Hu, P.J. Antioxidative response to Cd in a newly discovered cadmium hyperaccumulator, Arabis paniculata F. Chemosphere 2008, 74, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Barzanti, R.; Colzi, I.; Arnetoli, M.; Gallo, A.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Cadmium phytoextraction potential of different Alyssum species. J. Hazard. Mater. 2011, 196, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Höreth, S.; Pongrac, P.; van Elteren, J.T.; Debeljak, M.; Vogel-Mikuš, K.; Weber, M.; Braun, M.; Pietzenuk, B.; Pečovnik, M.; Vavpetič, P. Arabidopsis halleri shows hyperbioindicator behaviour for Pb and leaf Pb accumulation spatially separated from Zn. New Phytol. 2020, 226, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hu, J.; Gao, W.; Gao, P.; Cao, Z.; Liu, N.; Wang, X.; Liu, W.; Zhao, J.; Dong, J.; et al. Mechanosensation triggers enhanced heavy metal ion uptake by non-glandular trichomes. J. Hazard. Mater. 2022, 426, 127983. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Buono, R.A.; Lombardi, J.A. Food bodies in Cissus verticillata (Vitaceae): Ontogenesis, structure and functional aspects. Ann. Bot. 2009, 103, 517–524. [Google Scholar] [CrossRef]
- Jones, I.M.; Koptur, S.; von Wettberg, E. The use of extrafloral nectar in pest management: Overcoming context dependence. J. Appl. Ecol. 2016, 54, 489–499. [Google Scholar] [CrossRef]
- Ozawa, M.; Yano, S. Pearl bodies of Cayratia japonica (Thunb.) Gagnep. (Vitaceae) as alternative food for a predatory mite Euseius sojaensis (Ehara) (Acari: Phytoseiidae). Ecol. Res. 2009, 24, 257–262. [Google Scholar] [CrossRef]
- Gerrath, J.; Posluszny, U.; Melville, L. Vegetative Features of the Vitaceae. In Taming the Wild Grape; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- O’Dowd, D.J. Pearl bodies as ant food: An ecological role for some leaf emergences of tropical plants. Biotropica 1982, 14, 40–49. [Google Scholar] [CrossRef]
- Periyanayagam, K.; Kasirajan, B.; Karthikeyan, V.; Gracelet, R.J.; Kumuda, T. Quality assessment profile of the leaves of Vitis vinifera L. (Vitaceae)—An important phytotherapy component of tropical diseases control. Innovare J. Health Sci. 2013, 1, 26–31. [Google Scholar]
- de Oliveira, A.B.; de Mendonça, M.S.; Azevedo, A.A.; Meira, R.M.S.A. Anatomy and histochemistry of the vegetative organs of Cissus verticillata—A native medicinal plant of the Brazilian Amazon. Bras. J. Pharmacogn. 2012, 22, 1201–1211. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. 601. [Google Scholar]
- Cody, A.M.; Horner, H.T. Twin raphides in the Vitaceae and Araceae and a model for their growth. Bot. Gaz. 1983, 144, 318–330. [Google Scholar] [CrossRef]
- Şeker, S.S.; Akbulut, M.K.; Şenel, G. Calcium oxalate crystal (CaOx) composition at different growth stages of petiole in Vitis vinifera (Vitaceae). Adv. Stud. Biol. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Webb, M.A. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell. 1999, 11, 751–761. [Google Scholar] [CrossRef]
- Horner, H.T.; Wagner, L.B. The association of druse crystals with the developing stomium of Capsicum annuum (Solanaceae) anthers. Am. J. Bot. 1980, 67, 1347–1360. [Google Scholar] [CrossRef]
- Franceschi, V.R. Calcium oxalate formation is a rapid and reversible process in Lemna minor L. Protoplasma 1989, 148, 130–137. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Ann. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Zindler-Frank, E. On the formation of the pattern of crystal idioblasts in Canavalia ensiformis DC. VII. Calcium and oxalate content of the leaves in dependence of calcium nutrition. Z. Pflanzenphysiol. 1975, 77, 80–85. [Google Scholar] [CrossRef]
- Kinzel, H. Calcium in the vacuoles and cell walls of plant tissue. Flora 1989, 182, 99–125. [Google Scholar] [CrossRef]
- Ifrim, C.; Jităreanu, C.D.; Slabu, C.; Marta, A.E. Aspects regarding the calcium oxalate crystals at the grapevines cultivated in Iaşi and Cotnari vineyards. Lucr. Ştiinţifice 2012, 55, 73–78. [Google Scholar]
- Thurston, E.L. Morphology, fine structure and ontogeny of the stinging emergence of Tragia ramosa and Tragia saxicola (Euphorbiaceae). Am. J. Bot. 1976, 63, 710–718. [Google Scholar] [CrossRef]
- Arnott, H.J.; Pautard, F.G.E.; Steinfink, H. Structure of calcium oxalate monohydrate. Nature 1965, 208, 1197–1198. [Google Scholar] [CrossRef]
- Wattendorff, J. A third type of raphide crystal in the plant kingdom: Six-sided raphides with laminated sheaths in Agave americana L. Planta 1976, 130, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A. The probable function of calcium oxalate crystals in plants. Bot. Gaz. 1901, 32, 142–144. [Google Scholar] [CrossRef]
- Coté, G.G. Diversity and distribution of idioblasts producing calcium oxalate crystals in Dieffenbachia seguine (Araceae). Am. J. Bot. 2009, 96, 1245–1254. [Google Scholar] [CrossRef]
- Arnott, H.J.; Webb, M.A. Twinned raphides of calcium oxalate in grape (Vitis): Implications for crystal stability and function. Int. J. Plant Sci. 2000, 161, 133–142. [Google Scholar] [CrossRef]
- Patil, U.H.; Gaikwad, D.K. Effect of varying environmental conditions on mineral status of stem bark of Anogeissus latifolia. J. Pharm. Res. 2012, 5, 1140–1143. [Google Scholar]
- Osuji, J.O. Probable functions of calcium oxalate crystals in different tissues of the edible aroids (Xanthosoma and Colocasia spp.) in Nigeria. Afr. J. Biotechnol. 2013, 12, 3952–3956. [Google Scholar]
- Prychid, C.J.; Jabaily, R.S.; Rudall, P.J. Cellular ultrastructure and crystal development in Amorphophallus (Araceae). Ann. Bot. 2008, 101, 983–995. [Google Scholar] [CrossRef]
- Masram, H.G.; Harisha, C.R. Importance of calcium oxalate crystals in family Vitaceae. Pharm. Glob. 2012, 3, 1–3. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Boix, Y.F.; Victório, C.P.; Defaveri, A.C.A.; Arruda, R.D.C.D.O.; Sato, A.; Lage, C.L.S. Glandular trichomes of Rosmarinus officinalis L.: Anatomical and phytochemical analysis of leaf volatiles. Plant Biosyst. 2011, 145, 848–856. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rambau, U.; Naidoo, Y.; Sadashiva, C.T.; Baijnath, H.; Dewir, Y.H.; Magyar-Tábori, K. The Foliar Anatomy and Micromorphology of Cyphostemma hypoleucum (Vitaceae). Plants 2023, 12, 2312. https://doi.org/10.3390/plants12122312
Rambau U, Naidoo Y, Sadashiva CT, Baijnath H, Dewir YH, Magyar-Tábori K. The Foliar Anatomy and Micromorphology of Cyphostemma hypoleucum (Vitaceae). Plants. 2023; 12(12):2312. https://doi.org/10.3390/plants12122312
Chicago/Turabian StyleRambau, Unarine, Yougasphree Naidoo, Channangihalli Thimmegowda Sadashiva, Himansu Baijnath, Yaser Hassan Dewir, and Katalin Magyar-Tábori. 2023. "The Foliar Anatomy and Micromorphology of Cyphostemma hypoleucum (Vitaceae)" Plants 12, no. 12: 2312. https://doi.org/10.3390/plants12122312
APA StyleRambau, U., Naidoo, Y., Sadashiva, C. T., Baijnath, H., Dewir, Y. H., & Magyar-Tábori, K. (2023). The Foliar Anatomy and Micromorphology of Cyphostemma hypoleucum (Vitaceae). Plants, 12(12), 2312. https://doi.org/10.3390/plants12122312







