The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of EO of P. crispum Seeds
2.2. Phytotoxic Activity of the EO of P. crispum on Lactuca sativa Seeds
2.3. Oxidative Stress Markers in the Phytotoxic Activity of the EO of P. crispum on Lactuca sativa Seeds
2.4. Molecular Similarity
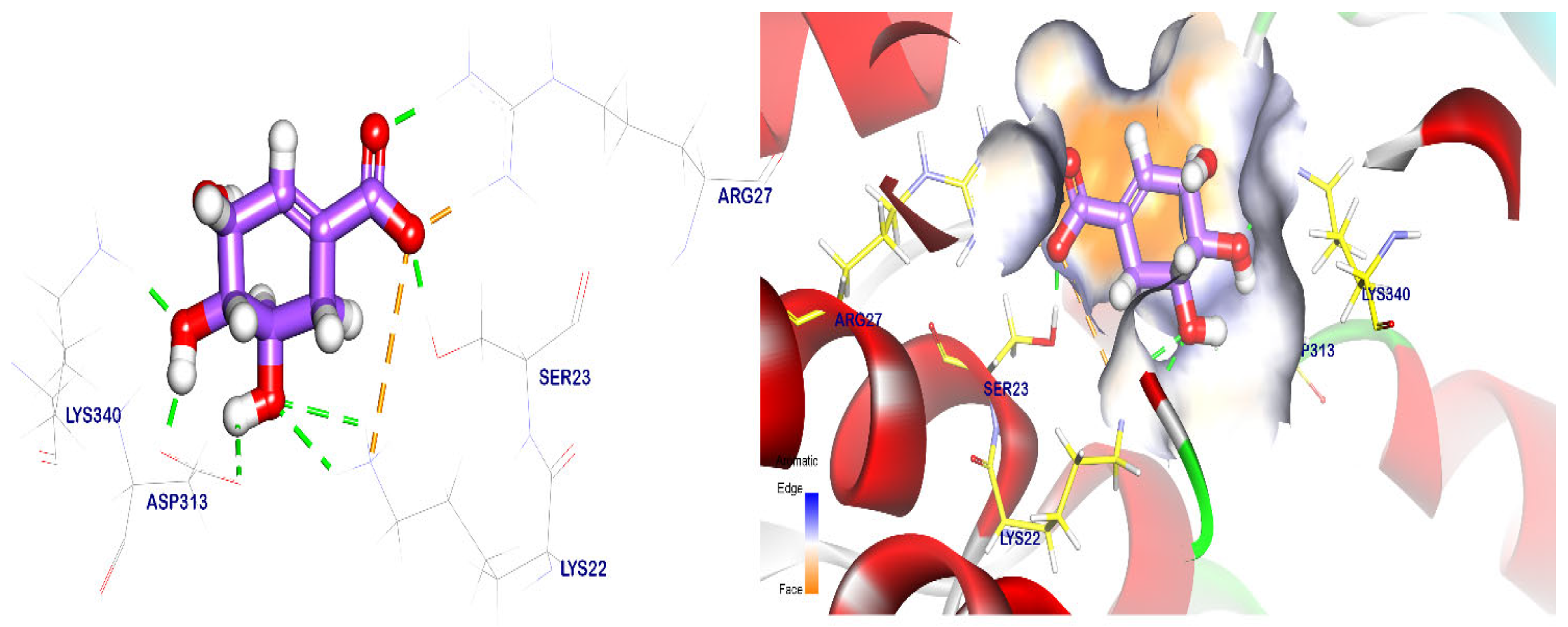
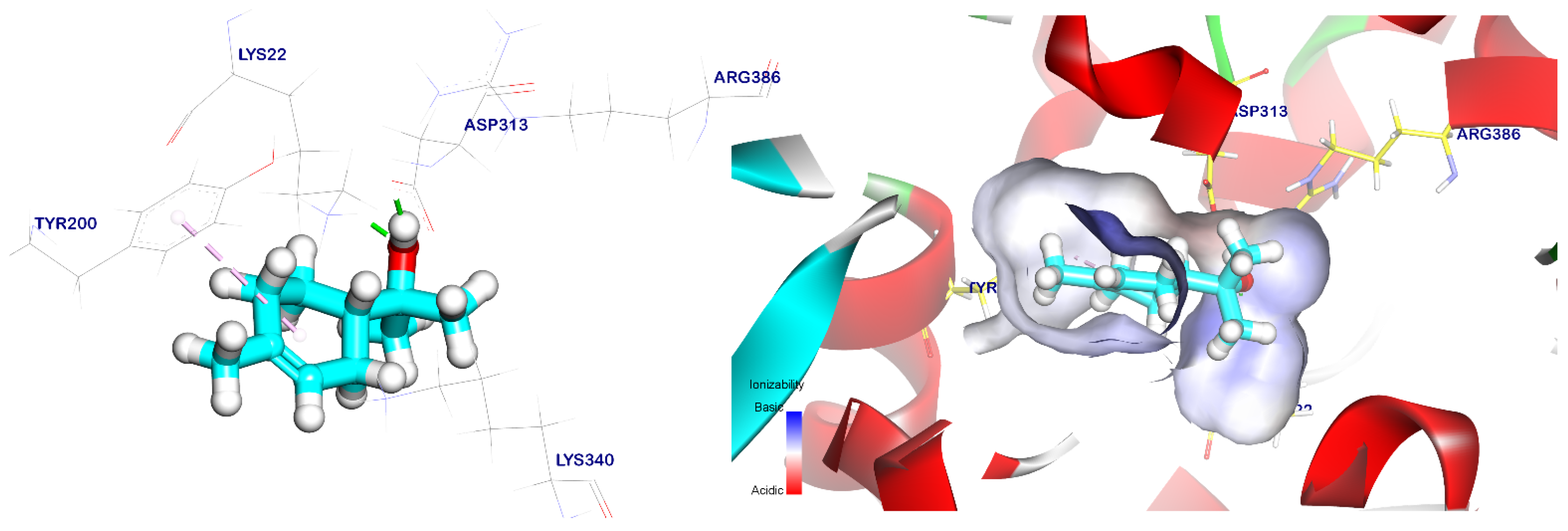
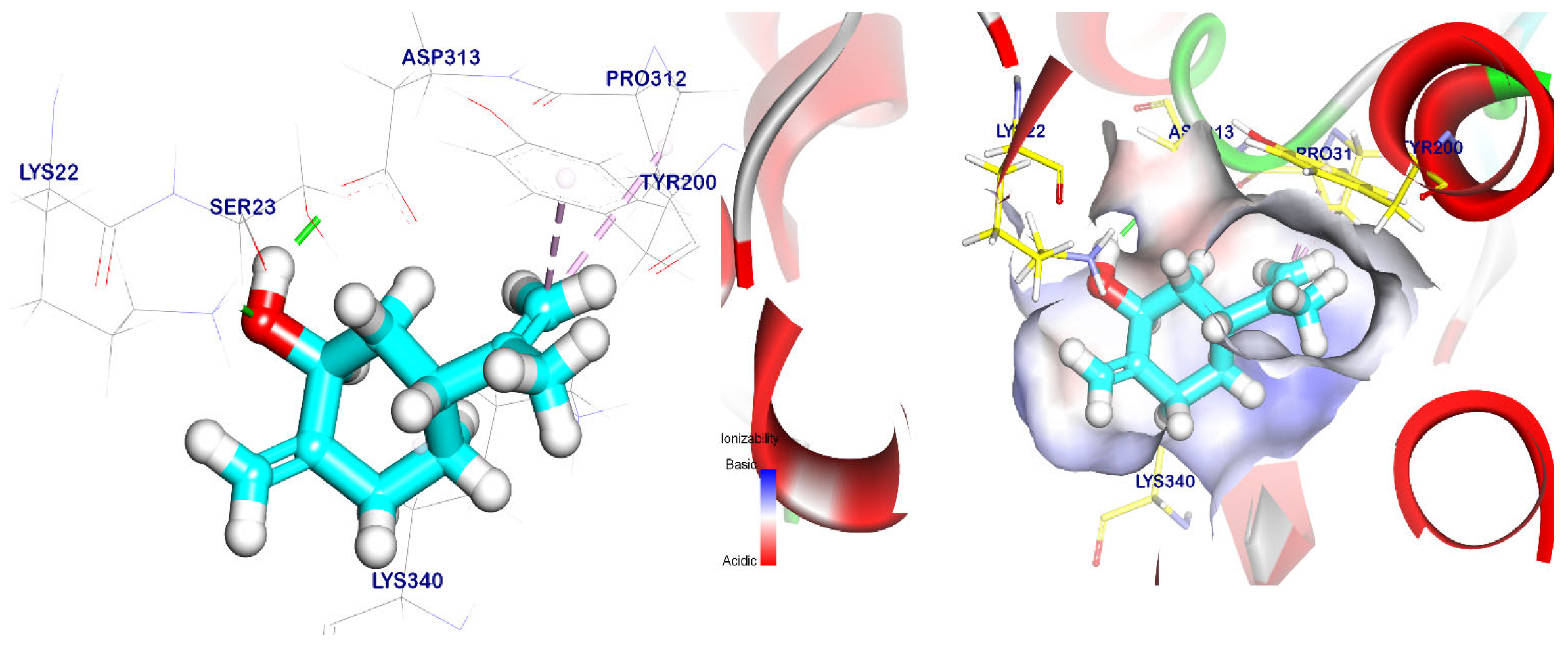
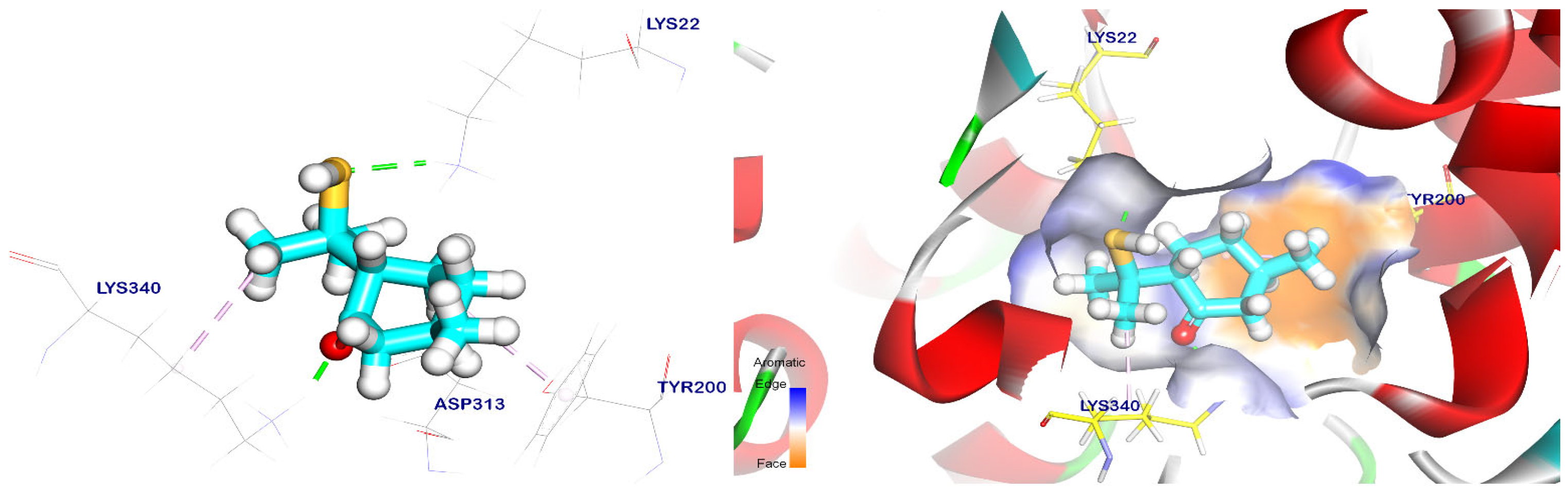
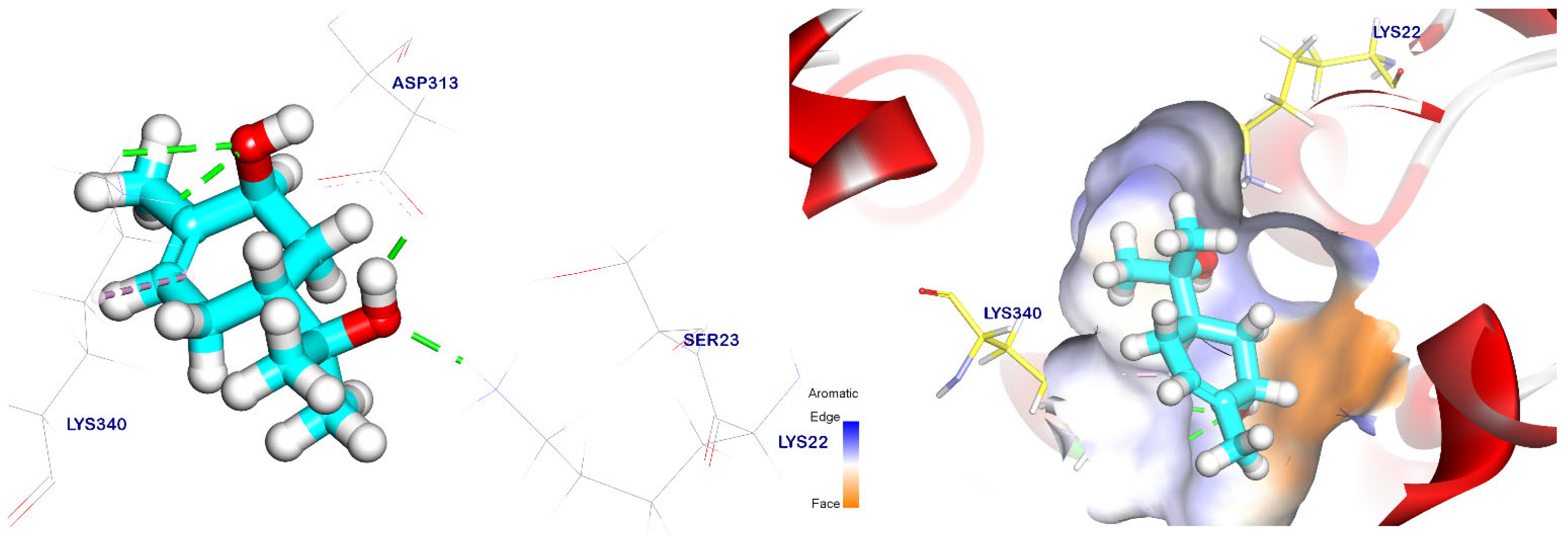
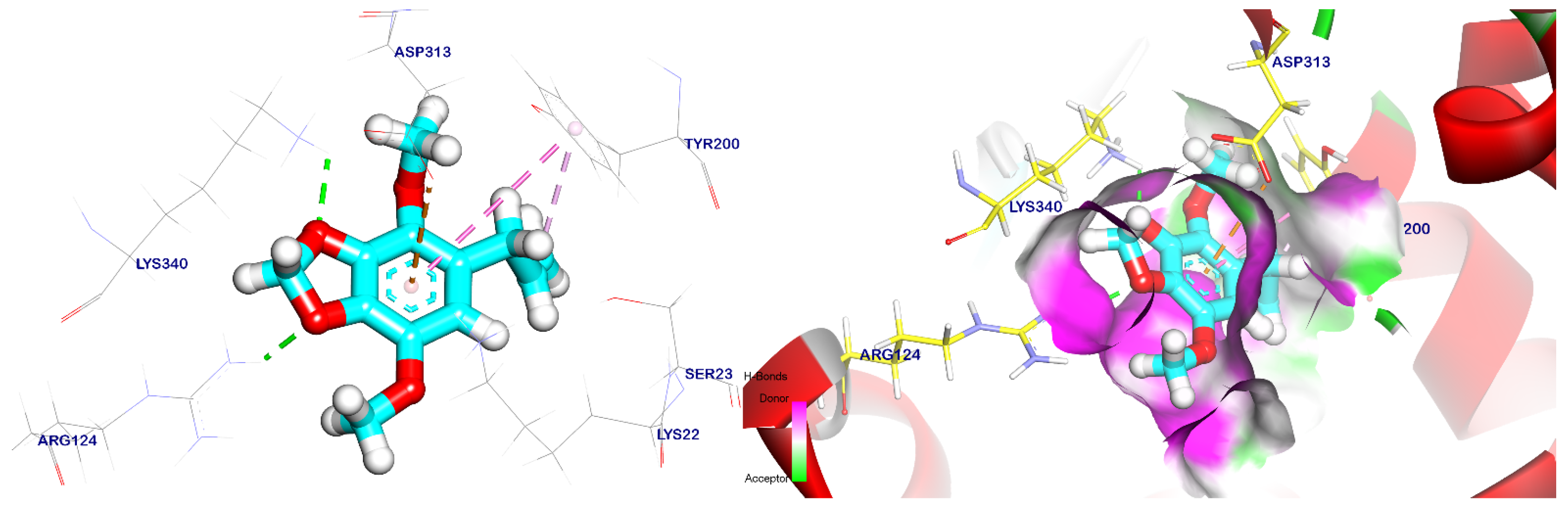
2.5. Docking Studies
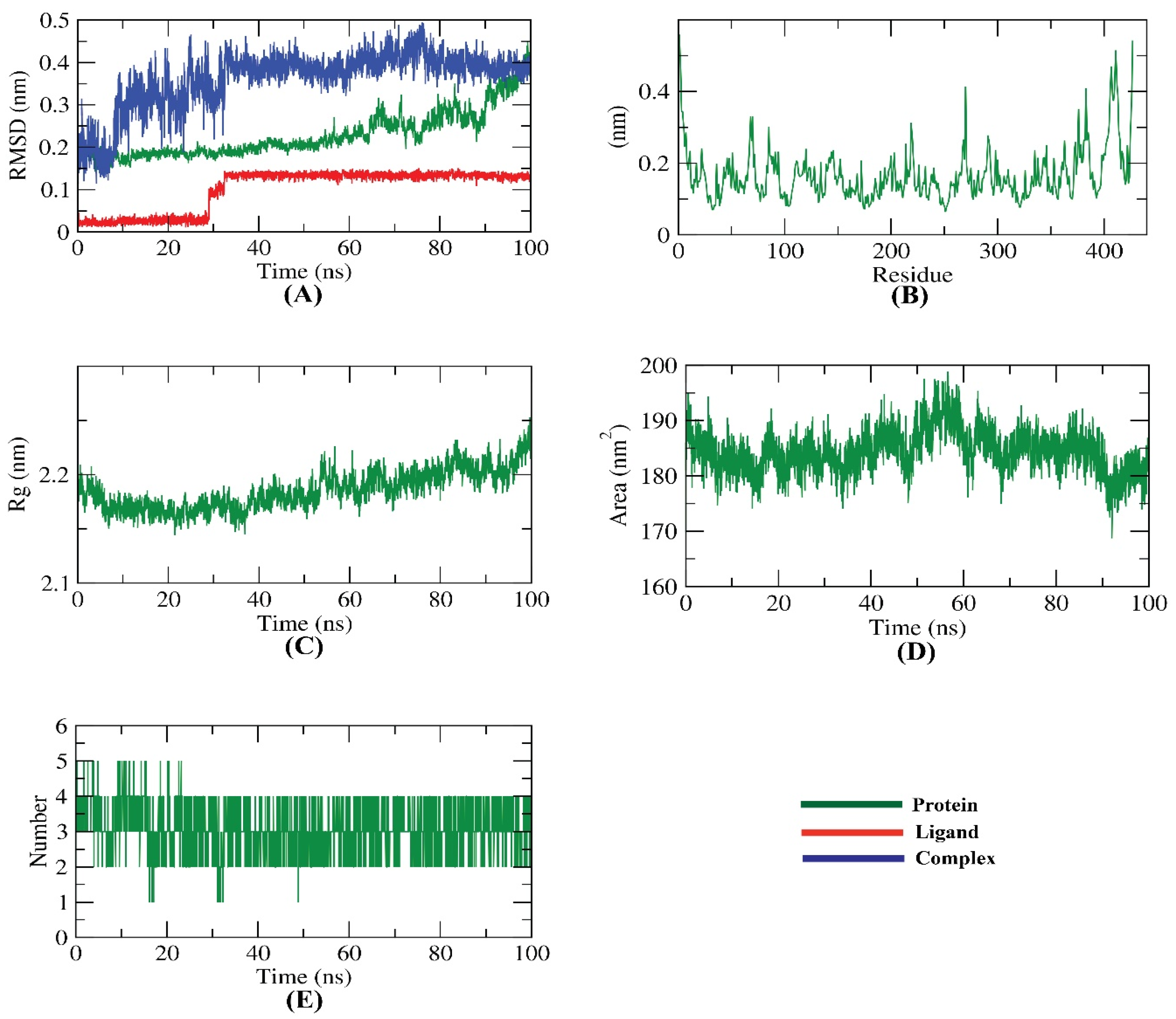
2.6. Molecular Dynamic Simulation
2.7. The Molecular Mechanics Poisson–Boltzmann Surface Area (MMPBSA)
3. Materials and Methods
3.1. Plant Material
3.2. Identification of the Volatile Compounds by Gas Chromatography–Mass Spectrometry (GC–MS)
3.3. Phytotoxicity Assay on Lactuca sativa Seeds
3.4. Determination of Oxidative Stress Parameters in L. sativa
3.5. Method of Molecular Similarity
3.6. Method of Docking Study
3.7. Method of Molecular Dynamic Simulation and MMPBSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, G.; Shrestha, S.; Kunwar, S.; Tseng, T.M. Crop Diversification for Improved Weed Management: A Review. Agriculture 2021, 11, 461. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 2833. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand Challenges in Weed Management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Zareen, S.; Fawad, M.; Haroon, M.; Ahmad, I.; Zaman, A. Allelopathic Potential of Summer Weeds on Germination and Growth Performance of Wheat and Chickpea. J. Nat. Pestic. Res. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Bailey, K.L. The Bioherbicide Approach to Weed Control Using Plant Pathogens. In Integrated Pest Management: Current Concepts and Ecological Perspective; Springer: Berlin/Heidelberg, Germany, 2014; pp. 245–266. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect Effects of the Herbicide Glyphosate on Plant, Animal and Human Health Through Its Effects on Microbial Communities. Front. Environ. Sci. 2021, 9, 464. [Google Scholar] [CrossRef]
- Fonseca, E.C.M.; Da Costa, K.S.; Lameira, J.; Alves, C.N.; Lima, A.H. Investigation of the Target-Site Resistance of EPSP Synthase Mutants P106T and T102I/P106S against Glyphosate. RSC Adv. 2020, 10, 44352–44360. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the Herbicide Glyphosate with Its Target Enzyme 5-Enolpyruvylshikimate 3-Phosphate Synthase in Atomic Detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376. [Google Scholar] [CrossRef]
- Hertel, R.; Gibhardt, J.; Martienssen, M.; Kuhn, R.; Commichau, F.M. Molecular Mechanisms Underlying Glyphosate Resistance in Bacteria. Environ. Microbiol. 2021, 23, 2891–2905. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and Its Application as a Weed Management Tool: A Review. Front. Plant Sci. 2022, 13, 4766. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Leong, S.Y.; Khelfa, A.; Greiner, R.; Chemat, F. Extraction Methods of Essential Oils From Herbs and Spices. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 21–55. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.M.; Algefare, A.I.; Alfwuaires, M.A. Antioxidant Potential of Parsley Leaf (Petroselinum crispum) Essential Oil on Hypothyroidism and Testicular Injury in Mice Intoxicated by Carbon Tetrachloride. Biomed. Res. Int. 2021, 2021, 9989174. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, R.; Walasek-Janusz, M. Essential Oil Diversity of Turnip-Rooted Parsley Cultivars. Agronomy 2022, 12, 1949. [Google Scholar] [CrossRef]
- Tang, E.L.H.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum crispum Has Antioxidant Properties, Protects against DNA Damage and Inhibits Proliferation and Migration of Cancer Cells. J. Sci. Food Agric. 2015, 95, 2763. [Google Scholar] [CrossRef] [PubMed]
- Mara De Menezes Epifanio, N.; Rykiel Iglesias Cavalcanti, L.; Falcão Dos Santos, K.; Soares Coutinho Duarte, P.; Kachlicki, P.; Ozarowski, M.; Jorge Riger, C.; Siqueira De Almeida Chaves, D. Chemical Characterization and In Vivo Antioxidant Activity of Parsley (Petroselinum crispum) Aqueous Extract. Food Funct. 2020, 11, 5346–5356. [Google Scholar] [CrossRef]
- Wu, K.H.; Lee, W.J.; Cheng, T.C.; Chang, H.W.; Chen, L.C.; Chen, C.C.; Lien, H.M.; Lin, T.N.; Ho, Y.S. Study of the Antitumor Mechanisms of Apiole Derivatives (AP-02) from Petroselinum crispum through Induction of G0/G1 Phase Cell Cycle Arrest in Human COLO 205 Cancer Cells. BMC Complement. Altern. Med. 2019, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Cuadros-Siguas, C.F.; Herrera-Calderon, O.; Batiha, G.E.S.; Almohmadi, N.H.; Aljarba, N.H.; Apesteguia-Infantes, J.A.; Loyola-Gonzales, E.; Tataje-Napuri, F.E.; Kong-Chirinos, J.F.; Almeida-Galindo, J.S.; et al. Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru. Molecules 2023, 28, 3348. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical Extraction of Volatile and Fixed Oils from Petroselinum crispum L. Seeds: Chemical Composition and Biological Activity. Nat. Prod. Res. 2020, 36, 1883–1888. [Google Scholar] [CrossRef]
- Bouzekri, O.; Elgamouz, S.; El khatabi, K.; Amechrouq, A.; Aziz Ajana, M.; Bouachrine, M.; Lakhlifi, T.; El Idrissi, M.; Choukrad, M. Chemical Composition and In Silico Acetylcholinesterase Inhibitory Activity of Essential Oils of Six Apiaceae Species from South-East Morocco. Biointerface Res. Appl. Chem. 2023, 13, 36. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Fraternale, D.; Flamini, G. Photochemical Response of Parsley (Petroselinum crispum (Mill.) Fuss) Grown under Red Light: The Effect on the Essential Oil Composition and Yield. J. Photochem. Photobiol. B 2018, 185, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jiang, Z.T.; Jiang, S.; Li, R. Composition Comparison of Essential Oils Extracted by Hydrodistillation and Microwave-Assisted Hydrodistillation from Petroselinum crispum Grown in China. J. Essent. Oil Bear. Plants 2017, 20, 368–374. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and Essential Oil Phytotoxicity of Seven Annual Aromatic Plants. Weed Sci. 2010, 58, 457–465. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Çenet, M.; Öztürk, B.; Bozok, F.; Karabörklü, S.; Demirci, S.C. Chemical Characterization, Phytotoxic, Antimicrobial and Insecticidal Activities of Vitex Agnus-Castus’ Essential Oil from East Mediterranean Region. J. Essent. Oil Bear. Plants 2015, 18, 1500–1507. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Cenet, M.; Ince, H.; Yilmaztekin, M. Antimicrobial and Herbicidal Activities of the Essential Oil from the Mediterranean Thymus eigii. J. Essent. Oil Bear. Plants 2018, 21, 214–222. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Pavlovic, I.; Celis, R. Enantiomer-Selective Characterization of the Adsorption, Dissipation, and Phytotoxicity of the Plant Monoterpene Pulegone in Soils. Plants 2022, 11, 1296. [Google Scholar] [CrossRef]
- Silva, E.R.; Overbeck, G.E.; Soares, G.L.G. Phytotoxicity of Volatiles from Fresh and Dry Leaves of Two Asteraceae Shrubs: Evaluation of Seasonal Effects. S. Afr. J. Bot. 2014, 93, 14–18. [Google Scholar] [CrossRef]
- Abd-Elgawad, A.M.; El Gendy, A.E.N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Ates, B.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical Composition, Antimicrobial, Insecticidal, Phytotoxic and Antioxidant Activities of Mediterranean Pinus brutia and Pinus pinea Resin Essential Oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Liu, L.; Toshmatov, Z.; Huang, L.; Shi, K.; Zhang, C.; Shao, H. Evaluation of the Phytotoxic Effect of the Essential Oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 2021, 226, 112856. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Polito, F.; Kouki, H.; Khedhri, S.; Hamrouni, L.; Mabrouk, Y.; Amri, I.; Nazzaro, F.; Fratianni, F.; De Feo, V. Chemical Composition and Phytotoxic and Antibiofilm Activity of the Essential Oils of Eucalyptus bicostata, E. gigantea, E. intertexta, E. obliqua, E. pauciflora and E. tereticornis. Plants 2022, 11, 3017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus proximus Serg. Essential Oil and Its Major Constituents. Front. Plant. Sci. 2021, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic Activity of Essential Oil and Water Extract of Peppermint (Mentha × Piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Sturtz, G.; Wedge, D.E.; Schrader, K.K.; Duke, S.O. Phytotoxic and Antifungal Compounds from Two Apiaceae Species, Lomatium Californicum and Ligusticum Hultenii, Rich Sources of Z-Ligustilide and Apiol, Respectively. J. Chem. Ecol. 2005, 31, 1567–1578. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Đorđević, T.; Đurović-Pejčev, R.; Stevanović, M.; Sarić-Krsmanović, M.; Radivojević, L.; Šantrić, L.; Gajić-Umiljendić, J. Phytotoxicity and Allelopathic Potential of Juglans regia L. Leaf Extract. Front. Plant Sci. 2022, 13, 3083. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Sunohara, Y.; Baba, Y.; Matsuyama, S.; Fujimura, K.; Matsumoto, H. Screening and Identification of Phytotoxic Volatile Compounds in Medicinal Plants and Characterizations of a Selected Compound, Eucarvone. Protoplasma 2015, 252, 1047–1059. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.V.D.; Bittencourt Fernandes, G.M.; da Costa, K.S.; Vakal, S.; Lima, A.H. Virtual Screening of Natural Products against 5-Enolpyruvylshikimate-3-Phosphate Synthase Using the Anagreen Herbicide-like Natural Compound Library. RSC Adv. 2022, 12, 18834. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.F.; Franca, E.F.; Leite, F.L. Unbinding Pathway Energy of Glyphosate from the EPSPs Enzyme Binding Site Characterized by Steered Molecular Dynamics and Potential of Mean Force. J. Mol. Graph Model 2017, 72, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The Essential Oil of Cymbopogon Citratus Stapt and Carvacrol: An Approach of the Antitumor Effect on 7,12-Dimethylbenz-[α]-Anthracene (DMBA)-Induced Breast Cancer in Female Rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Marcon, C.; Droste, A. Phytotoxic and Cytogenotoxic Assessment of Glyphosate on Lactuca Sativa L. Braz. J. Biol. 2022, 84, e257039. [Google Scholar] [CrossRef] [PubMed]
- Vujčić Bok, V.; Gerić, M.; Gajski, G.; Gagić, S.; Domijan, A.M. Phytotoxicity of Bisphenol A to Allium cepa Root Cells Is Mediated through Growth Hormone Gibberellic Acid and Reactive Oxygen Species. Molecules 2023, 28, 2046. [Google Scholar] [CrossRef]
- Priestman, M.A.; Healy, M.L.; Funke, T.; Becker, A.; Schönbrunn, E. Molecular Basis for the Glyphosate-Insensitivity of the Reaction of 5-Enolpyruvylshikimate 3-Phosphate Synthase with Shikimate. FEBS Lett. 2005, 579, 5773–5780. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, Z.; Zhan, Y.; Ke, X.; Yan, Y.; Lin, M.; Li, P.; Jiang, S.; Wang, J.; Lu, W. A Highly Glyphosate-Resistant EPSPS Mutant from Laboratory Evolution. Appl. Sci. 2022, 12, 5723. [Google Scholar] [CrossRef]
- Elkaeed, E.B.; Metwaly, A.M.; Alesawy, M.S.; Saleh, A.M.; Alsfouk, A.A.; Eissa, I.H. Discovery of Potential SARS-CoV-2 Papain-like Protease Natural Inhibitors Employing a Multi-Phase In Silico Approach. Life 2022, 12, 1407. [Google Scholar] [CrossRef]






| # | Compounds | IR cal | IR ref | % | S.D. |
|---|---|---|---|---|---|
| 1 | α-Pinene | 932 | 932 | 2.24 | 0.75 |
| 2 | Sabinene | 973 | 969 | 0.20 | 0.10 |
| 3 | β-Pinene | 979 | 974 | 1.43 | 0.02 |
| 4 | Myrcene | 991 | 988 | 5.93 | 0.89 |
| 5 | δ-2-Carene | 1016 | 1001 | 0.09 | 0.01 |
| 6 | α-Phellandrene | 1018 | 1002 | 1.15 | 0.02 |
| 7 | ο-Cymene | 1037 | 1022 | 0.27 | 0.22 |
| 8 | Limonene | 1040 | 1024 | 1.16 | 0.61 |
| 9 | β-Phellandrene | 1042 | 1025 | 15.02 | 2.01 |
| 10 | ϒ-Terpinene | 1069 | 1054 | 0.14 | 0.02 |
| 11 | ρ-Mentha-2,4(8)-diene | 1097 | 1085 | 2.73 | 0.91 |
| 12 | ρ-Cymenene | 1106 | 1089 | 3.65 | 0.69 |
| 13 | 1,3,8-ρ-Menthatriene | 1119 | 1108 | 22.59 | 2.31 |
| 14 | cis-ρ-Mentha-2,8-dien-1-ol | 1138 | 1133 | 0.13 | 0.01 |
| 15 | ρ-methyl-Acetophenone | 1197 | 1179 | 0.29 | 0.12 |
| 16 | Cryptone | 1198 | 1183 | 0.21 | 0.09 |
| 17 | Terpineol | 1215 | 1199 | 0.20 | 0.04 |
| 18 | cis-ρ-Mentha-1(7),8-dien-2-ol | 1218 | 1227 | t | 0.01 |
| 19 | Pulegone | 1232 | 1233 | 1.50 | 0.12 |
| 20 | Carvotanacetone | 1242 | 1244 | 0.15 | 0.03 |
| 21 | Not identified | 1248 | - | t | 0.01 |
| 22 | E-Ocimenone | 1250 | 1235 | 0.19 | 0.06 |
| 23 | Methyl octine carbonate | 1300 | 1295 | 0.41 | 0.21 |
| 24 | cis-Piperitrol acetate | 1322 | 1332 | 0.39 | 0.11 |
| 25 | trans-Carvyl acetate | 1333 | 1339 | 1.32 | 0.98 |
| 26 | Not identified | 1353 | - | 0.10 | 0.03 |
| 27 | trans-p-Mentha-8-thiol-3-one | 1372 | 1371 | 0.12 | 0.09 |
| 28 | trans-p-Menth-6-en-2,8-diol | 1377 | 1371 | 0.10 | 0.01 |
| 29 | Isobornyl propanoate | 1399 | 1383 | 0.95 | 0.72 |
| 30 | Not identified | 1407 | - | 0.37 | 0.12 |
| 31 | β-Isocomene | 1410 | 1407 | 0.92 | 0.21 |
| 32 | α-Santalene | 1417 | 1416 | 0.50 | 0.42 |
| 33 | E-Caryophyllene | 1419 | 1417 | 0.62 | 0.31 |
| 34 | γ-Muurolene | 1483 | 1478 | 0.66 | 0.02 |
| 35 | Not identified | 1486 | - | 0.06 | 0.01 |
| 36 | γ-Himachalene | 1491 | 1481 | 0.36 | 0.22 |
| 37 | Bicyclogermacrene | 1510 | 1500 | 0.10 | 0.06 |
| 38 | Cubebol | 1524 | 1514 | 0.09 | 0.01 |
| 39 | δ-Cadinene | 1528 | 1522 | 0.38 | 0.21 |
| 40 | Kessane | 1533 | 1529 | 0.12 | 0.07 |
| 41 | Myristicin | 1535 | 1517 | 3.01 | 1.01 |
| 42 | Elemicin | 1562 | 1555 | 0.14 | 0.12 |
| 43 | cis-Muurol-5-en-4-α-ol | 1567 | 1559 | 0.44 | 0.12 |
| 44 | 6-methoxy-Elemicin | 1597 | 1595 | 6.96 | 1.23 |
| 45 | 1,10-di-epi-Cubenol | 1610 | 1618 | 0.12 | 0.06 |
| 46 | γ-Eudesmol | 1615 | 1630 | t | 0.01 |
| 47 | Apiole | 1692 | 1677 | 22.41 | 1.54 |
| Monoterpene hydrocarbons (%) | 56.61 | ||||
| Oxygenated monoterpenes (%) | 2.07 | ||||
| Sesquiterpene hydrocarbons (%) | 2.62 | ||||
| Oxygenated sesquiterpenes (%) | 0.79 | ||||
| Phenylpropenes (%) | 32.52 | ||||
| Others (%) | 4.84 | ||||
| TOTAL IDENTIFIED (%) | 99.45 | ||||
| Groups | Seed Germination (%) | Root Length (cm) | Hypocotyl Length (cm) | Root Length/ Stem Length |
|---|---|---|---|---|
| Negative control: DMSO 0.1% | 96.00 ± 5.29 * | 2.13 ± 0.15 * | 5.50 ± 0.50 * | 0.28 |
| P. crispum 0.1% | 27.67 ± 2.52 * | 1.83 ± 0.29 * | 4.17 ± 0.29 * | 0.31 |
| P. crispum 0.5% | 15.00 ± 3.00 | 0.47 ± 0.06 | 4.67 ± 0.58 * | 0.09 |
| P. crispum 1% | 7.67 ± 2.52 | 0.40 ± 0.10 | 3.00 ± 0.50 * | 0.12 |
| Positive control: glyphosate 1% | 5.67 ± 1.15 | 0.09 ± 0.01 | 1.77 ± 0.25 | 0.05 |
| # | A-LogP | M.wt. | HBA | HBD | Rotatable Bonds | Rings | Aromatic Rings | MFPSA | Minimum Distance | Is Similar |
|---|---|---|---|---|---|---|---|---|---|---|
| 38 | 3.202 | 222.366 | 1 | 1 | 1 | 3 | 0 | 0.08 | 3.11745 | True |
| 28 | 1.313 | 170.249 | 2 | 2 | 1 | 1 | 0 | 0.195 | 2.07929 | True |
| 27 | 2.491 | 186.314 | 2 | 1 | 1 | 1 | 0 | 0.248 | 2.40701 | True |
| 14 | 2.159 | 152.233 | 1 | 1 | 1 | 1 | 0 | 0.107 | 2.74091 | True |
| 18 | 2.454 | 152.233 | 1 | 1 | 1 | 1 | 0 | 0.11 | 2.78232 | True |
| 17 | 2.415 | 154.249 | 1 | 1 | 1 | 1 | 0 | 0.102 | 2.79147 | True |
| 25 | 2.779 | 194.27 | 2 | 0 | 3 | 1 | 0 | 0.113 | 3.03988 | True |
| 16 | 2.25 | 138.207 | 1 | 0 | 1 | 1 | 0 | 0.104 | 3.05145 | True |
| 47 | 2.573 | 222.237 | 4 | 0 | 4 | 2 | 1 | 0.154 | 3.0781 | True |
| 41 | 2.589 | 192.211 | 3 | 0 | 3 | 2 | 1 | 0.136 | 3.07947 | True |
| 24 | 3.043 | 196.286 | 2 | 0 | 3 | 1 | 0 | 0.111 | 3.08852 | True |
| Glyphosate | −2.628 | 173.143 | 5 | 3 | 1 | 1 | 0 | 0.581 | - | Reference |
| 9 | 3.308 | 136.234 | 0 | 0 | 1 | 1 | 0 | 0 | 3.53118 | False |
| 10 | 3.448 | 136.234 | 0 | 0 | 1 | 1 | 0 | 0 | 3.55272 | False |
| 11 | 3.448 | 136.234 | 0 | 0 | 0 | 1 | 0 | 0 | 3.55933 | False |
| 8 | 3.502 | 136.234 | 0 | 0 | 1 | 1 | 0 | 0 | 3.56113 | False |
| 1 | 2.872 | 136.234 | 0 | 0 | 0 | 3 | 0 | 0 | 3.56803 | False |
| 3 | 2.926 | 136.234 | 0 | 0 | 0 | 3 | 0 | 0 | 3.57562 | False |
| 12 | 3.314 | 132.202 | 0 | 0 | 1 | 1 | 1 | 0 | 3.66638 | False |
| 4 | 3.687 | 136.234 | 0 | 0 | 4 | 0 | 0 | 0 | 3.67158 | False |
| 7 | 3.51 | 134.218 | 0 | 0 | 1 | 1 | 1 | 0 | 3.69333 | False |
| 31 | 4.131 | 204.351 | 0 | 0 | 0 | 3 | 0 | 0 | 3.75387 | False |
| 37 | 4.699 | 204.351 | 0 | 0 | 0 | 2 | 0 | 0 | 3.78382 | False |
| 36 | 4.699 | 204.351 | 0 | 0 | 0 | 2 | 0 | 0 | 3.78382 | False |
| 33 | 4.753 | 204.351 | 0 | 0 | 0 | 2 | 0 | 0 | 3.79333 | False |
| 34 | 4.798 | 204.351 | 0 | 0 | 1 | 2 | 0 | 0 | 3.7951 | False |
| 39 | 4.939 | 204.351 | 0 | 0 | 1 | 2 | 0 | 0 | 3.82029 | False |
| 32 | 4.123 | 204.351 | 0 | 0 | 3 | 4 | 0 | 0 | 3.8803 | False |
| 13 | 3.252 | 134.218 | 0 | 0 | 1 | 1 | 0 | 0 | 3.5249 | False |
| 6 | 3.254 | 136.234 | 0 | 0 | 1 | 1 | 0 | 0 | 3.52297 | False |
| 40 | 3.511 | 222.366 | 1 | 0 | 0 | 3 | 0 | 0.036 | 3.50425 | False |
| 2 | 2.926 | 136.234 | 0 | 0 | 1 | 2 | 0 | 0 | 3.59824 | False |
| 5 | 2.872 | 136.234 | 0 | 0 | 0 | 2 | 0 | 0 | 3.59722 | False |
| 23 | 3.524 | 168.233 | 2 | 0 | 7 | 0 | 0 | 0.122 | 3.59792 | False |
| 44 | 2.772 | 238.28 | 4 | 0 | 6 | 1 | 1 | 0.13 | 3.5717 | False |
| 22 | 3.054 | 150.218 | 1 | 0 | 3 | 0 | 0 | 0.084 | 3.55714 | False |
| 42 | 2.788 | 208.254 | 3 | 0 | 5 | 1 | 1 | 0.111 | 3.63239 | False |
| 15 | 2.056 | 134.175 | 1 | 0 | 1 | 1 | 1 | 0.107 | 3.5737 | False |
| 45 | 3.913 | 222.366 | 1 | 1 | 1 | 2 | 0 | 0.077 | 3.57082 | False |
| 46 | 3.86 | 222.366 | 1 | 1 | 1 | 2 | 0 | 0.073 | 3.16893 | False |
| 19 | 2.891 | 152.233 | 1 | 0 | 0 | 1 | 0 | 0.091 | 3.16858 | False |
| 43 | 3.846 | 222.366 | 1 | 1 | 1 | 2 | 0 | 0.077 | 3.15832 | False |
| 29 | 3.021 | 210.313 | 2 | 0 | 3 | 2 | 0 | 0.105 | 3.13708 | False |
| 20 | 2.697 | 152.233 | 1 | 0 | 1 | 1 | 0 | 0.092 | 3.12885 | False |
| Targets Screened | Tested Compounds | RMSD Value (Å) | Docking (Affinity) Score (kcal/mol) | Interactions | |
|---|---|---|---|---|---|
| H.B | Pi -Interaction | ||||
| EPSP synthase target site | cis-ρ-Mentha-2,8-dien-1-ol | 0.80 | −5.47 | 2 | 2 |
| Terpineol | 1.23 | −5.69 | 1 | 1 | |
| cis-ρ-Mentha-1(7),8-dien-2-ol | 1.17 | −5.66 | 2 | 3 | |
| trans-p-Mentha-8-thiol-3-one | 1.69 | −5.56 | 2 | 2 | |
| trans-p-Menth-6-en-2,8-diol | 1.26 | −5.54 | 3 | 1 | |
| Apiole | 0.81 | −6.85 | 2 | 3 | |
| Glyphosate | 0.19 | −6.55 | 6 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Calderon, O.; Saleh, A.M.; Mahmood, A.A.R.; Khalaf, M.A.; Calva, J.; Loyola-Gonzales, E.; Tataje-Napuri, F.E.; Chávez, H.; Almeida-Galindo, J.S.; Chavez-Espinoza, J.H.; et al. The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants 2023, 12, 2288. https://doi.org/10.3390/plants12122288
Herrera-Calderon O, Saleh AM, Mahmood AAR, Khalaf MA, Calva J, Loyola-Gonzales E, Tataje-Napuri FE, Chávez H, Almeida-Galindo JS, Chavez-Espinoza JH, et al. The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants. 2023; 12(12):2288. https://doi.org/10.3390/plants12122288
Chicago/Turabian StyleHerrera-Calderon, Oscar, Abdulrahman M. Saleh, Ammar A. Razzak Mahmood, Mohamed A. Khalaf, James Calva, Eddie Loyola-Gonzales, Freddy Emilio Tataje-Napuri, Haydee Chávez, José Santiago Almeida-Galindo, Javier Hernán Chavez-Espinoza, and et al. 2023. "The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide" Plants 12, no. 12: 2288. https://doi.org/10.3390/plants12122288
APA StyleHerrera-Calderon, O., Saleh, A. M., Mahmood, A. A. R., Khalaf, M. A., Calva, J., Loyola-Gonzales, E., Tataje-Napuri, F. E., Chávez, H., Almeida-Galindo, J. S., Chavez-Espinoza, J. H., & Pari-Olarte, J. B. (2023). The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants, 12(12), 2288. https://doi.org/10.3390/plants12122288











