Analysis of Important Volatile Organic Compounds and Genes Produced by Aroma of Pepper Fruit by HS-SPME-GC/MS and RNA Sequencing
Abstract
:1. Introduction
2. Results
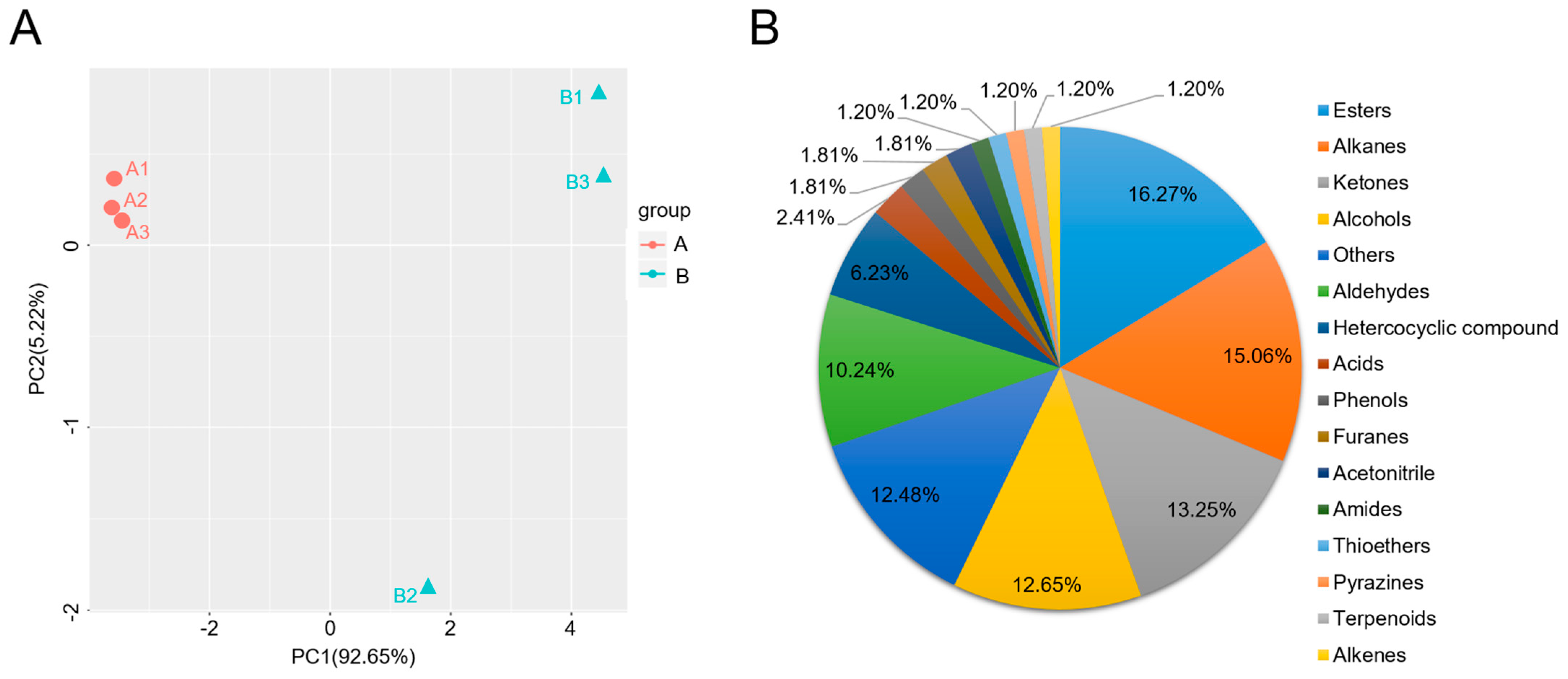
2.1. Volatile Organic Compounds (VOCs) in Non-Spicy (A) and Spicy (B) Pepper Fruits
2.2. Differential VOCs in the Non-Spicy (A) and Spicy (B) Pepper Fruits
2.3. Overview of Transcriptomic Data for 17-03 and H1023 Non-Spicy (A) and Spicy (B) Pepper Fruits
2.4. Identification of Differentially Expressed Genes (DEGs)
2.5. KEGG Functional Annotations of DEGs
2.6. Analysis of DEGs Related to Fatty Acid and Terpene Biosynthesis
3. Discussion
3.1. The Contents of Fatty Acids and Terpenes Affect the Aroma Production of Capsicum Fruit
3.2. The Key DEGs in the Aroma of Spicy Pepper Fruits
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Sample Preparation and GC-MS Analysis
4.3. RNA Extraction and RNA-Seq Analysis
4.4. Quantitative Real-Time RT-PCR
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.; Piperno, D.; Berman, M.; Cooke, R.; Rademaker, K.; Ranere, A.; et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Park, M.; Yeom, S.; Kim, Y.; Lee, J.; Lee, H.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-Mas, M.; Nitz, S.; Fernando, N. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the annuum-chinense-frutescens complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef]
- Moreno, E.; Fita, A.; González-Mas, M.; Rodríguez-Burruezo, A. HS-SPME study of the volatile fraction of Capsicum accessions and hybrids in different parts of the fruit. Sci. Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
- Forero, M.; Quijano, C.E.; Pino, J.A. Volatile compounds of chile pepper (Capsicum annuum L. var. glabriusculum) at two ripening stages. Flavour Fragr. J. 2010, 24, 25–30. [Google Scholar] [CrossRef]
- Culleré, L.; San-Juan, F.; Cacho, J. Characterisation of aroma active compounds of Spanish saffron by gas chromatography–olfactometry: Quantitative evaluation of the most relevant aromatic compounds. Food Chem. 2011, 127, 1866–1871. [Google Scholar] [CrossRef]
- Zhao, Y.; Ariefandie Febrianto, N.; Zhu, F. Characterization of physicochemical properties, flavor volatiles and phenolic compounds of feijoa fruit varieties. Food Chem. 2023, 419, 136074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, X.; Zhang, C.; Li, X.; Yue, N.; Shao, H.; Wang, J.; Jin, F. Discrimination and Characterization of Volatile Flavor Compounds in Fresh Oriental Melon after Forchlorfenuron Application Using Electronic Nose (E-Nose) and Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS). Foods 2023, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Popova, A.; Dincheva, I. Pattern Recognition of Varieties of Peach Fruit and Pulp from Their Volatile Components and Metabolic Profile Using HS-SPME-GC/MS Combined with Multivariable Statistical Analysis. Plants 2022, 11, 3219. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Cho, Y.; Kim, M.; Kim, Y. Determination of Volatiles and Carotenoid Degradation Compounds in Red Pepper Fermented by Lactobacillus parabuchneri. J. Food Sci. 2018, 83, 2083–2091. [Google Scholar] [CrossRef]
- Korkmaz, A.; Hayaloglu, A.A.; Atasoy, A.F. Evaluation of the volatile compounds of fresh ripened Capsicum annuum and its spice pepper (dried red pepper flakes and isot). LWT-Food Sci. Technol. 2017, 84, 842–850. [Google Scholar] [CrossRef]
- Luning, P.A.; Carey, A.T.; Roozen, J.P.; Wichers, H.J. Characterization and Occurrence of Lipoxygenase in Bell Peppers at Different Ripening Stages in Relation to the Formation of Volatile Flavor Compounds. J. Agric. Food Chem. 1995, 43, 1493–1500. [Google Scholar] [CrossRef]
- Luning, P.A.; Ebbenhorst-Seller, T.; De Rijk, T.; Roozen, J.P. Effect of Hot-Air Drying on Flavour Compounds of Bell Peppers (Capsicum annuum). J. Sci. Food Agric. 1995, 68, 355–365. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Tikunov, Y.; Portis, E.; Bovy, A. The Genetic Basis of Tomato Aroma. Genes 2021, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Deng, Y.; Luo, W.; Luo, X.; Wang, C.; Quan, C.; Guo, Z.; Wang, Y. Bacterial diversity and its correlation with sensory quality of two types of zha-chili from Shennongjia region, China. Food Res. Int. 2023, 168, 112789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, H.; Hu, S.; Zong, Y.; Xia, H.; Li, H. Transcriptomic profiling of the floral fragrance biosynthesis pathway of Liriodendron and functional characterization of the LtuDXR gene. Plant Sci. 2022, 314, 111124. [Google Scholar] [CrossRef]
- Qiao, D.; Mi, X.; An, Y.; Xie, H.; Cao, K.; Chen, H.; Chen, M.; Liu, S.; Chen, J.; Wei, C. Integrated metabolic phenotypes and gene expression profiles revealed the effect of spreading on aroma volatiles formation in postharvest leaves of green tea. Food Res. Int. 2021, 149, 110680. [Google Scholar] [CrossRef]
- Liu, X.; Hao, N.; Feng, R.; Meng, Z.; Li, Y.; Zhao, Z. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Bauchet, G.; Grenier, S.; Samson, N.; Segura, V.; Kende, A.; Beekwilder, J.; Cankar, K.; Gallois, J.; Gricourt, J.; Bonnet, J.; et al. Identification of major loci and genomic regions controlling acid and volatile content in tomato fruit: Implications for flavor improvement. New Phytol. 2017, 215, 624–641. [Google Scholar] [CrossRef] [Green Version]
- Mozūraitis, R.; Hall, D.; Trandem, N.; Ralle, B.; Tunström, K.; Sigsgaard, L.; Baroffio, C.; Fountain, M.; Cross, J.; Wibe, A.; et al. Composition of Strawberry Floral Volatiles and their Effects on Behavior of Strawberry Blossom Weevil, Anthonomus rubi. J. Chem. Ecol. 2020, 46, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Hernández, A.; Aranda, E.; Casquete, R.; Velázquez, R.; Bartolomé, T.; Córdoba, M. Impact of volatile composition on the sensorial attributes of dried paprikas. Food Res. Int. 2017, 100, 691–697. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Gasparrini, C.; Di Martino, L.; Di Cecco, V.; Manzi, A.; Di Santo, M.; D’Archivio, A.A. Geographical Discrimination of Bell Pepper (Capsicum annuum) Spices by (HS)-SPME/GC-MS Aroma Profiling and Chemometrics. Molecules 2021, 26, 6177. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef]
- Yue, X.; Liu, S.; Wei, S.; Fang, Y.; Zhang, Z.; Ju, Y. Transcriptomic and Metabolic Analyses Provide New Insights into the Effects of Exogenous Sucrose on Monoterpene Synthesis in “Muscat Hamburg” Grapes. J. Agric. Food Chem. 2021, 69, 4164–4176. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Chai, Y.; Liu, W.; Chen, H.; Sun, L.; Tang, X.; Luo, C.; Chen, D.; Cheng, X.; et al. Opisthopappus taihangensis Terpenoids and their gene regulatory networks in ‘Taihang Mingzhu’ as detected by transcriptome and metabolome analyses. Front. Plant Sci. 2022, 13, 1014114. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Song, Y.; Zhang, P.; Chen, D.; Guan, L.; Liu, S. Comprehensive study of volatile compounds and transcriptome data providing genes for grape aroma. BMC Plant Biol. 2023, 23, 171. [Google Scholar] [CrossRef]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.; McCarthy, D.; Smyth, G. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, C.; Wang, J.; Yang, Y.; Han, K.; Bakpa, E.; Li, J.; Lyu, J.; Yu, J.; Xie, J. Capsicum annuum Comprehensive fruit quality assessment and identification of aroma-active compounds in green pepper (Capsicum annuum L.). Front. Nutr. 2022, 9, 1027605. [Google Scholar] [CrossRef] [PubMed]
- Trovato, E.; Vento, F.; Creti, D.; Dugo, P.; Mondello, L. Elucidation of Analytical-Compositional Fingerprinting of Three Different Species of Chili Pepper by Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry Analysis, and Sensory Profile Evaluation. Molecules 2022, 27, 2355. [Google Scholar] [CrossRef] [PubMed]
- Garruti, D.; Mesquita, W.; Magalhes, H.; Arajo, D.; Pereira, R. Odor-contributing volatile compounds of a new Brazilian tabasco pepper cultivar analyzed by HS-SPME-GC-MS and HS-SPME-GC-O/FID. Food Sci. Technol. 2021, 41, 696–701. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Li, M.; Qu, Y.; Long, H.; Yi, J. Evaluation of the physiochemical and aromatic qualities of pickled Chinese pepper (Paojiao) and their influence on consumer acceptability by using targeted and untargeted multivariate approaches. Food Res. Int. 2020, 137, 109535. [Google Scholar] [CrossRef]
- Pino, J.; Gonzalez, M.; Ceballos, L.; Centurion-Yah, A.R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Bogusz Junior, S.; Marco, P.H.; Valderrama, P.; Damasceno, F.C.; Aranda, M.S.; Zini, C.A.; Caramao, E.B.; Tavares Melo, A.M.; Teixiera Filho, J.; Godoy, H.T. Analysis of volatile compounds in Capsicum spp. by headspace solid-phase microextraction and GC × GC-TOFMS. Anal. Methods 2015, 7, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Fall, R.; Karl, T.; Hansel, A.; Jordan, A.; Lindinger, W. Volatile organic compounds emitted after leaf wounding: On-line analysis by proton-transfer-reaction mass spectrometry. J. Geophys. Res. Atmos. 1999, 104, 15963–15974. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent Advance in Aromatic Volatile Research in Tomato Fruit: The Metabolisms and Regulations. Food Bioprocess Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- ul Hassan, M.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, J.; Cao, X.; Wei, C.; Kuang, J.; Chen, K.; Zhang, B. Peach fruit PpNAC1 activates PpFAD3-1 transcription to provide ω-3 fatty acids for the synthesis of short-chain flavor volatiles. Hortic. Res. 2022, 9, uhac085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Shan, Q.; Shi, Z.; Li, J.; Zhao, X.; Chang, C.; Yu, J. Lonicera japonicaIntegrated volatile metabolomic and transcriptomic analysis provides insights into the regulation of floral scents between two contrasting varieties of Lonicera japonica. Front. Plant Sci. 2022, 13, 989036. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Dong, S.; Zhang, S.; Wei, X.; Xie, Q.; Ding, Q.; Xia, R.; Zhang, X. Chromosome-level reference genome assembly provides insights into aroma biosynthesis in passion fruit (Passiflora edulis). Mol. Ecol. Resour. 2021, 21, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, W.; Shen, L.; Cao, J.; Liu, C.; Hu, J.; Guan, D.; He, S. A CaCDPK29-CaWRKY27b module promotes CaWRKY40-mediated thermotolerance and immunity to Ralstonia solanacearum in pepper. New Phytol. 2022, 233, 1843–1863. [Google Scholar] [CrossRef]




| NO. | Names | CAS | R.T. (min) | Relative Amount | Up and Down | |
|---|---|---|---|---|---|---|
| A | B | |||||
| Fatty derivatives | ||||||
| 1 | Butanal, 3-methyl- | 590-86-3 | 3.361 | 0.0285 | 0.0052 | down |
| 2 | Hexanal | 66-25-1 | 7.096 | 0.0761 | 2.5828 | up |
| 3 | 2-Pentenal, (E)- | 1576-87-0 | 8.422 | 0.0059 | 0.1034 | up |
| 4 | 2-Hexenal, (E)- | 6728-26-3 | 11.088 | 0.1170 | 6.2553 | up |
| 5 | Octanal | 124-13-0 | 13.206 | 0.0017 | 0.0109 | up |
| 6 | Nonanal | 124-19-6 | 16.117 | 0.0059 | 0.0163 | up |
| 7 | 5-Ethylcyclopent-1-enecarboxaldehyde | 16.645 | 0.0032 | 0.0194 | up | |
| 8 | 2-Dodecenal, (E)- | 20407-84-5 | 19.78 | 0.0012 | 0.0179 | up |
| 9 | 2,4-Di-tert-butylphenol | 96-76-4 | 35.852 | 0.0257 | 0.0041 | down |
| 10 | Acetone | 67-64-1 | 2.345 | 0.2485 | 0.0158 | down |
| 11 | 3-Octanone | 106-68-3 | 12.191 | 0.0037 | 0.0318 | up |
| 12 | 3-Hepten-2-one | 1119-44-4 | 13.525 | 0.0061 | 0.0276 | up |
| 13 | 3-Octanone, 2-methyl- | 923-28-4 | 14.288 | 0.0351 | 1.1169 | up |
| 14 | 5-Hepten-2-one, 6-methyl- | 110-93-0 | 14.578 | 0.0117 | 0.1538 | up |
| 15 | 2-Cyclohexen-1-one, 3,4,4-trimethyl- | 17299-41-1 | 17.506 | 0.0028 | 0.0418 | up |
| 16 | 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | 3796-70-1 | 27.131 | 0.0031 | 0.0399 | up |
| 17 | 3-Buten-2-one, 4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)- | 23267-57-4 | 29.89 | 0.0065 | 0.1235 | up |
| 18 | 1H-Pyrrole-2,5-dione, 3-ethyl-4-methyl- | 20189-42-8 | 34.977 | 0.0007 | 0.0029 | up |
| 19 | 1-Penten-3-ol | 616-25-1 | 9.706 | 0.0217 | 1.0939 | up |
| 20 | 1-Pentanol | 71-41-0 | 12.326 | 0.0152 | 0.1110 | up |
| 21 | 1-Hexanol | 111-27-3 | 15.257 | 0.0156 | 1.3680 | up |
| 22 | 2-Hexen-1-ol, (E)- | 928-95-0 | 16.629 | 0.0055 | 0.4177 | up |
| 23 | 1-Octen-3-ol | 3391-86-4 | 17.783 | 0.0019 | 0.0223 | up |
| 24 | 4,4,6-Trimethyl-cyclohex-2-en-1-ol | 21592-95-0 | 31.422 | 0.0009 | 0.0032 | up |
| 25 | Methyl 2-ethyldecanoate | 32.694 | 0.0073 | 0.0019 | down | |
| 26 | 2-Propanol, 1-chloro-, phosphate (3:1) | 13674-84-5 | 42.542 | 0.0003 | 0.0002 | down |
| 27 | Methyl salicylate | 119-36-8 | 25.255 | 0.0038 | 0.0414 | up |
| 28 | Cyclohexen-1-carbonitrile | 1855-63-6 | 20.345 | 0.0074 | 0.0027 | down |
| 29 | Hexanoic acid, 2-ethyl- | 149-57-5 | 29.281 | 0.1168 | 0.0340 | down |
| 30 | Octanoic acid | 124-07-2 | 31.511 | 0.0209 | 0.0049 | down |
| Aromatic derivatives | ||||||
| 31 | Mequinol | 150-76-5 | 27.082 | 0.0054 | 0.0306 | up |
| Nitrogen and oxygen heterocyclic compounds | ||||||
| 32 | Furan, 3-methyl- | 930-27-8 | 3.061 | 0.0139 | 0.0028 | down |
| 33 | Furan, 2-ethyl- | 3208-16-0 | 3.982 | 0.0030 | 0.0240 | up |
| 34 | Furan, 2-pentyl- | 3777-69-3 | 11.485 | 0.0117 | 0.0578 | up |
| 35 | 2(4H)-Benzofuranone,5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- | 17092-92-1 | 36.24 | 0.0018 | 0.0232 | up |
| Terpenoid derivatives | ||||||
| 36 | 1-Cyclohexene-1-carboxaldehyde,2,6,6-trimethyl- | 432-25-7 | 21.822 | 0.0011 | 0.0309 | up |
| 37 | Hydroxypivalic acid | 4835-90-9 | 32.258 | 0.0120 | 0.0019 | down |
| Samples | Clean Reads | GbClean Bases | GC Content | %≥Q30 |
|---|---|---|---|---|
| A1 | 16,205,787 | 4,808,073,634 | 42.55% | 93.12% |
| A2 | 21,019,032 | 6,251,941,422 | 42.36% | 93.22% |
| A3 | 23,177,163 | 6,915,966,400 | 42.27% | 93.54% |
| B1 | 17,803,462 | 5,305,305,850 | 42.54% | 93.37% |
| B2 | 7,404,704 | 2,209,849,414 | 42.48% | 92.87% |
| B3 | 11,323,565 | 3,377,464,642 | 42.70% | 93.53% |
| Samples | Total Reads | Mapped Reads | Uniq Mapped Reads | Multiple Map Reads | Reads Map to ‘+’ | Reads Map to ‘−’ |
|---|---|---|---|---|---|---|
| A1 | 32,411,574 | 30,153,480 (93.03%) | 29,180,709 (90.03%) | 972,771 (3.00%) | 14,970,003 (46.19%) | 15,006,995 (46.30%) |
| A2 | 42,038,064 | 39,748,585 (94.55%) | 38,393,724 (91.33%) | 1,354,861 (3.22%) | 19,700,905 (46.86%) | 19,778,992 (47.05%) |
| A3 | 46,354,326 | 41,728,496 (90.02%) | 40,347,864 (87.04%) | 1,380,632 (2.98%) | 20,723,470 (44.71%) | 20,785,507 (44.84%) |
| B1 | 35,606,924 | 32,174,009 (90.36%) | 31,015,903 (87.11%) | 1,158,106 (3.25%) | 15,969,352 (44.85%) | 16,009,903 (44.96%) |
| B2 | 14,809,408 | 13,344,748 (90.11%) | 12,922,962 (87.26%) | 421,786 (2.85%) | 6,635,327 (44.80%) | 6,652,940 (44.92%) |
| B3 | 22,647,130 | 20,308,455 (89.67%) | 19,647,834 (86.76%) | 660,621 (2.92%) | 10,092,548 (44.56%) | 10,120,926 (44.69%) |
| Gene Name | Gnen ID | Primer F: | Primer R: |
|---|---|---|---|
| FAD1 | Capana03g000452 | CCTGTAGCACTTGCAGCTCT | TCTCCAAATGCAAGCAACGC |
| FAD2 | Capana06g002292 | CTACCCAAAGCCCAGACCAG | ACTAACCCTCAATGCCCAGC |
| ADH | Capana03g001569 | TGGCCAGTGTGTGCATACAT | ACTGCATCTGAAGGAAGGCC |
| ADHL1 | Capana04g000980 | CAAGGGATGGAAGCAGCAGA | GCAACTTTCCATGCTGCTCC |
| TPS | Capana10g000571 | ACCTCACGTAGCCAAACGAG | AAGGGCGTCAACTAAGGCAA |
| LOX3.1 | Capana12g002284 | TGCGAAGTGAAGTTAGCCGT | GTTGCTTCCCTCCTCAAGCT |
| LOX1.5 | Capana01g000180 | TGCAAACGCGTGAAGAACTG | ATAGTCAGCGGTACCAGGGT |
| HPL-1 | Capana03g003512 | TTAGGGCCACTTTGGGATCG | TTGACATCCAGTACCGCCAC |
| HPL-2 | Capana03g003513 | CGCCTATCTTGATGCATGGC | AGTCTGTTTGTGCCCTCGTT |
| Actin | GQ339766 | CCTCTCAACCCTAAGGCCAACA | ACGTCCAGCAAGATCCAAACGAA |
| 18SR | EF564281 | CCGGTCCGCCTATGGTGTGCACCGG | GCAGTTGTTCGTCTTTCATAAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Li, Y.; Wu, L.; Wei, H.; Fu, J.; Chen, W.; Lin, S.; Yang, S.; Zhang, R.; Shang, W.; et al. Analysis of Important Volatile Organic Compounds and Genes Produced by Aroma of Pepper Fruit by HS-SPME-GC/MS and RNA Sequencing. Plants 2023, 12, 2246. https://doi.org/10.3390/plants12122246
Qiu Y, Li Y, Wu L, Wei H, Fu J, Chen W, Lin S, Yang S, Zhang R, Shang W, et al. Analysis of Important Volatile Organic Compounds and Genes Produced by Aroma of Pepper Fruit by HS-SPME-GC/MS and RNA Sequencing. Plants. 2023; 12(12):2246. https://doi.org/10.3390/plants12122246
Chicago/Turabian StyleQiu, Yinhui, Yongqing Li, Lidong Wu, Hang Wei, Jianwei Fu, Weiting Chen, Shuting Lin, Sheng Yang, Rui Zhang, Wei Shang, and et al. 2023. "Analysis of Important Volatile Organic Compounds and Genes Produced by Aroma of Pepper Fruit by HS-SPME-GC/MS and RNA Sequencing" Plants 12, no. 12: 2246. https://doi.org/10.3390/plants12122246
APA StyleQiu, Y., Li, Y., Wu, L., Wei, H., Fu, J., Chen, W., Lin, S., Yang, S., Zhang, R., Shang, W., Liao, C., Zeng, S., Luo, Y., & Cai, W. (2023). Analysis of Important Volatile Organic Compounds and Genes Produced by Aroma of Pepper Fruit by HS-SPME-GC/MS and RNA Sequencing. Plants, 12(12), 2246. https://doi.org/10.3390/plants12122246






