1. Introduction
Due to the development of new growing areas, where traditional varieties cannot be adapted, the need to produce new varieties of ornamental plants with improved properties has increased [
1]. At the beginning of the 20th century, plant biologists established that the frequency and efficiency of genetic modifications in treated seeds could be increased by using chemical and radiation technology [
2]. Subsequently, various mutagens, such as physical or chemical mutagens, were used to induce a wide range of genetic variability that led to and contributed to current plant breeding [
3]. Radiation-induced mutation breeding is thus a remarkable method that can produce superior mutant varieties, in contrast to traditional breeding such as selection and crossing, which is time consuming and labor intensive with limited genetic trait changes [
4,
5,
6].
Mutation breeding, including natural and artificial mutations, is an excellent method in the field of ornamental plant breeding because many species can be easily propagated, which facilitates the cultivation of spontaneous and induced mutants. The breeding of mutant varieties with the help of an ion beam has already been attempted on many ornamental plants, and some species have also been used to investigate the mutagenesis process. In addition, progress is being made in clarifying the genetic mechanism of the expression of important traits, which will probably result in the development of more efficient mutation breeding methods in ornamental plant breeding [
7]. There are several literature references regarding this. In the case of gamma radiation applied to chrysanthemum plants, doses of 10 Gy and 20 Gy resulted in a change in flower color. It was found that gamma radiation affected leaf length, leaf width, stem diameter, stem length, and inflorescence diameter. In addition to morphological changes, it also resulted in histological changes, as changes also occurred in the structure of the leaves [
8]. Changes in the morphological characters of
Dendrobium odoardi Kraenzl. were also observed in individuals [
9].
Tulipa sp. (L.) gamma radiation (5 Gy) stimulated the sprouting of the bulbs, and the survival rate of the bulbs was also higher. Too-high doses (20–100 Gy) inhibited growth. As the gamma radiation increased, the anthocyanin and flavonoid content decreased. The morphological properties of leaf stomata also changed [
10]. In another bulbous ornamental plant,
Narcissus tazetta (L.) var.
chinensis, it was observed that gamma radiation given at a low dose (10 Gy) results in a change in growth, while gamma radiation applied at a higher dose brings about other morphological changes in the plants [
11]. In the ornamental version of
Capsicum, gamma radiation caused larger flowers, male sterility, and changed fruit color in the second generation [
12]. When the rhizomes of
Cyperus alternifolius L. were treated with gamma radiation, the lowest (20 Gy) and the highest (100 Gy) dose increased the germination capacity, while the doses between the two caused distorted growth. However, the distortions disappeared in the M2 generation [
13]. At the same time, it can also be used to reduce seed germination. In the case of
Pennisetum alopecuroides (L.) Spreng, the 60 Gy dose reduced the seed yield [
14]. Gamma radiation does not always bring the expected result. In the case of
Camellia sinensis (L.) Kuntze, lower radiation doses increased the germination power, but the seedlings died en masse at the age of five months. When gamma radiation was applied at a higher dose, the germination capacity decreased, but the morphological characteristics became more favorable (larger leaf size, larger flower size, higher height) [
15], as in
Adenium obesum (Forssk.) Roem. and Schult. [
16]. In the case of
Lilium, the number of leaves and the content of chlorophyll changed as a result of gamma radiation [
17]. In the case of
Echinacea purpurea (L.) Moench, the flower color changed and the size of the inflorescence increased, and the shape of the inflorescence and the height of the plant were altered as a result of gamma rays [
18]. When
Philodendron erubescens (K. Koch and Augustin) ‘Gold’ plants were treated with gamma radiation, the plant size, the number of branches, and the color of the leaves changed, which could be the basis for the breeding of a new variety [
19].
Tests show that low doses of gamma radiation improve the morphological and biochemical properties of some plants. Gamma ray treatments carried out in the early stages of seed germination promote the synthesis of RNA and protein, thus increasing the growth of seedlings as well as increasing the antioxidant capacity of cells, helping cells to fight against daily stress [
20]. Environmental factors can be broadly divided into biotic and abiotic components [
21]. Biotic stress is one of the most common causes of the death of ornamental plants, which can be changed by modifying the genetic codes [
22]. Biotic factors caused by pests, bacteria, viruses, fungi, nematodes, etc., represent a serious threat to the growth and development of plants, thereby adversely affecting quality. The breeding of stress-resistant plant varieties is becoming very important in the current agricultural system. Mutagenesis is one of the most common techniques for controlling plant stress. To induce mutagenesis, plant breeders use technologies such as physical (gamma radiation, ultraviolet radiation, etc.), chemical (ethyl methanesulfonate, methyl methanesulfonate, sodium azide, etc.) and genetic (ZFN, TALEN, and CRISPR) technologies [
21]. These technologies are also increasingly used in ornamental plant breeding.
Osmathus fragrans Lour. is very sensitive to salt stress. However, with gamma radiation, salt tolerance can be achieved in the species, which becomes more and more noticeable as the dose increases. In addition, it moderated the MDA (melonaldide) level, which was associated with a significant increase in superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activity. The accumulation of proline can also contribute to increasing the tolerance against salt stress [
23]. In the case of
Paeonia x
suffruticosa Andrews, it was established that 30 and 40 Gy gamma radiation affected the physiological and biochemical state of the plants. As a result of the treatment, the activity of antioxidant enzymes, including superoxide dismutase, peroxidase, and catalase, gradually increased up to the value of 40 Gy. Total soluble protein content progressively decreased, while proline and malondialdehyde content increased significantly [
24]. An increase in salt stress resistance due to gamma radiation can also be observed in
Musa L. individuals under in vitro conditions [
25]. Gamma radiation improved powdery mildew resistance in
Gerbera jamesonii ‘Harley’ [
26]. Gamma radiation can also change the histological properties.
Catharanthus roseus (L.) G. Don. gamma radiation had an effect on the callus biomass growth of individuals, and it also had an impact on the vincristine and vinblastine content under in vitro conditions. Callus growth was maximal at 20 Gy but decreased at 100 Gy [
27]. In the case of
Dimocarpus longan J. de Lour., gamma radiation modified the morphological features and the rate of photosynthesis [
28].
Rudbeckia Hirta as a Genetic Resource
The genus
Rudbeckia consists of approximately 30 species native to North America [
29]. The genus includes annual, biennial, and perennial species [
30]. The annual species
Rudbeckia hirta includes diploid (2n = 2x = 38) and tetraploid (2n = 4x = 76) cultivars with varied flower color and flower shape, typically flowering in late summer and autumn.
Rudbeckia is a popular ornamental taxon, suitable for urban settings due to its ornamental diversity, low maintenance, and heat and drought tolerance [
31]. In Hungary, Dr. Zoltán Kováts dealt with the breeding of the species [
32].
Rudbeckias are highly adaptable species with valuable flowering as ornamental plants. Complementing the current varieties with new cultivars would be important in trade. Interspecific hybridization and induced polyploidy could be a significant improvement of the genus from an ornamental horticultural point of view. When comparing diploid and tetraploid individuals, induced polyploidy significantly reduced overwintering ability [
33]. Due to the aesthetic value of perennial herbaceous flowering plants, such as colorful flowers, more attention is paid to these plant groups in the field of urban planting [
34].
Rudbeckia hirta is a very popular species worldwide, several Hungarian varieties of which are used in public areas. ‘Mackó’, ‘Aranyálom’, ‘Kokárdás’, ‘Sárgarigó’, and ‘Őszifény’ are Hungarian cultivars found in many cities. These products were bred by Dr. Zoltán Kováts in the 1980s and 1990s at the Horticultural Research Institute, which today operates as part of MATE. The climate at that time was not characterized by the summer drought typical of current years, and heat waves lasting several weeks, as well as many diseases, were not present in those decades. These breeds can no longer cope with the current environmental problems. Another important factor is that the market is looking for compact, resistant varieties with many flowers and special flower colors or flower shapes. The old Rudbeckia hirta varieties cannot satisfy this demand. Although the classic crossbreeding and selection breeding methods are effective, producing a new variety using these methods is costly and time consuming. Mutational breeding, on the other hand, can lay a new foundation for the faster and more cost-effective production of climate-tolerant and market-demanded varieties. Treating strains of Rudbeckia hirta ‘Őszifény’ produced by selective breeding with gamma rays can be a suitable method to increase genetic variability.
After several years of selective breeding of the Rudbeckia hirta individuals used, they were treated with a dose of gamma radiation, and the M1 and M2 generations were also examined. We studied the effect of the applied radiation doses on the given breed. As a result of the treatments, we aimed to determine a dose or doses with which we can continue to work in the future. When determining this, it is important to compare the morphological and physiological results. Another goal of ours was to select the groups and doses of the cultivated plants with which we can pursue the breeding program and make them resistant to the current climate (abiotic and biotic stress effects). Our goal is to examine whether gamma mutation breeding can be used for the applied breed candidate, and if so, the question of how this process takes place becomes important.
During our studies, we specifically measured the differences between the M1 and M2 generations as well as the effect of different radiation doses belonging to the same generation on the morphological and physiological properties. Recognizing the difference between generations, the goal was to measure the changes in the characteristics of Rudbeckia hirta varieties produced through sexual intercourse between generations. This can be an important clue in subsequent gene conservation and breeding tasks.
3. Discussion
In the course of our work, we dealt with the gamma irradiation treatment of
Rudbeckia hirta and determined if the breeding stock strain we created is suitable for this type of treatment. If so, the breeding process can be facilitated by this. The effects of gamma radiation were measured and evaluated using histological and physiological methods. This revealed the effects of gamma radiation on organs, cells, and tissues, as well as the magnitude of the stress level (POD, APTI). Since POD isoforms are diverse both in their location as well as their function, alterations in the isoform composition reflects the stress defense and the cell wall composition properties of the plants [
37]. The measurements were carried out with seeds irradiated with different doses, and the measurements of two consecutive generations were also compared.
It was found that the treatment of seeds can be a suitable method in the breeding process, as Oladosu et al. [
2] established. A change in phenotypic properties was observed in all of the treated plants, which was also evident in the M2 generation in many cases, in connection with the findings of Beyaz and Yildiz [
4].
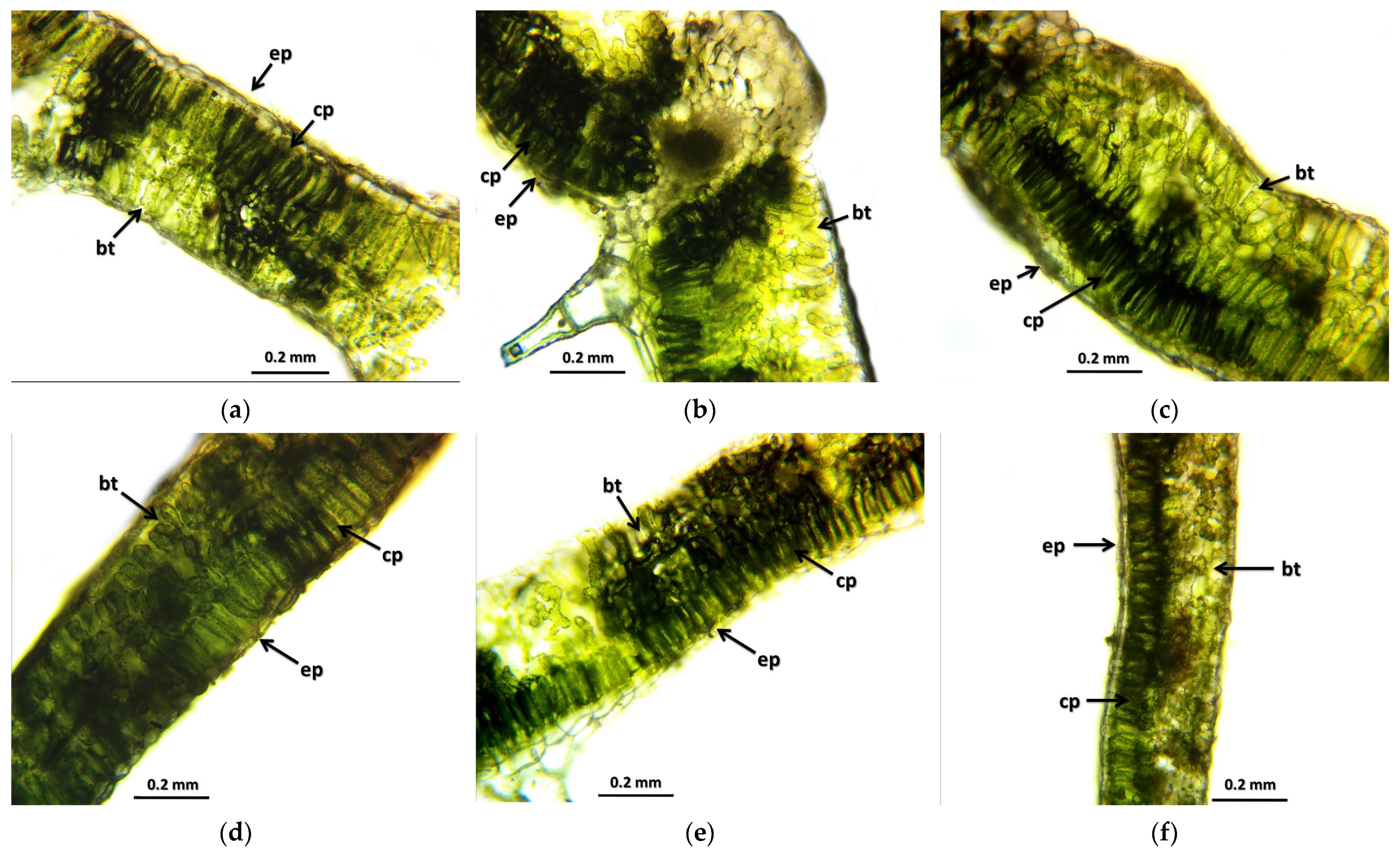
The results of the leaf and stem cross-sections showed that by increasing the radiation dose, the leaf and the stem retain their tissue youth for a longer time. The central axis of the intestinal tissue of the stem is less or not hollow, the cells of the epidermis and dermis are stronger, and the cell walls are uniform. The degree of secondary thickening in the stem increases as the dose increases. There is also a difference between the M1 and M2 generations. The described characteristics also appeared in the M2 generation in the case of the leaf cross-section, not as strongly as in the case of M1 but perceptibly. This is similar to the findings of Susila et al. [
8], Hu et al. [
11], and Li et al. [
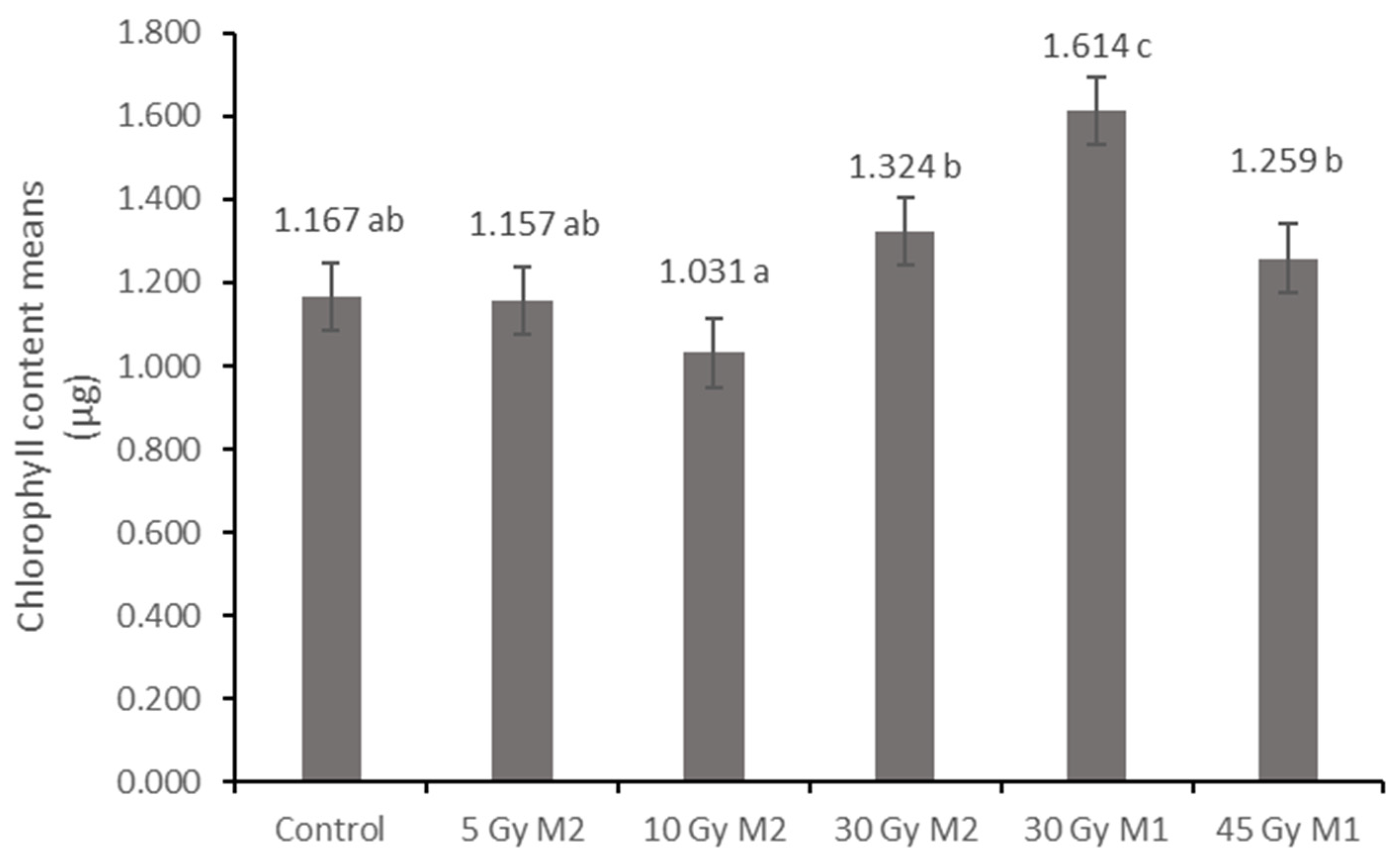
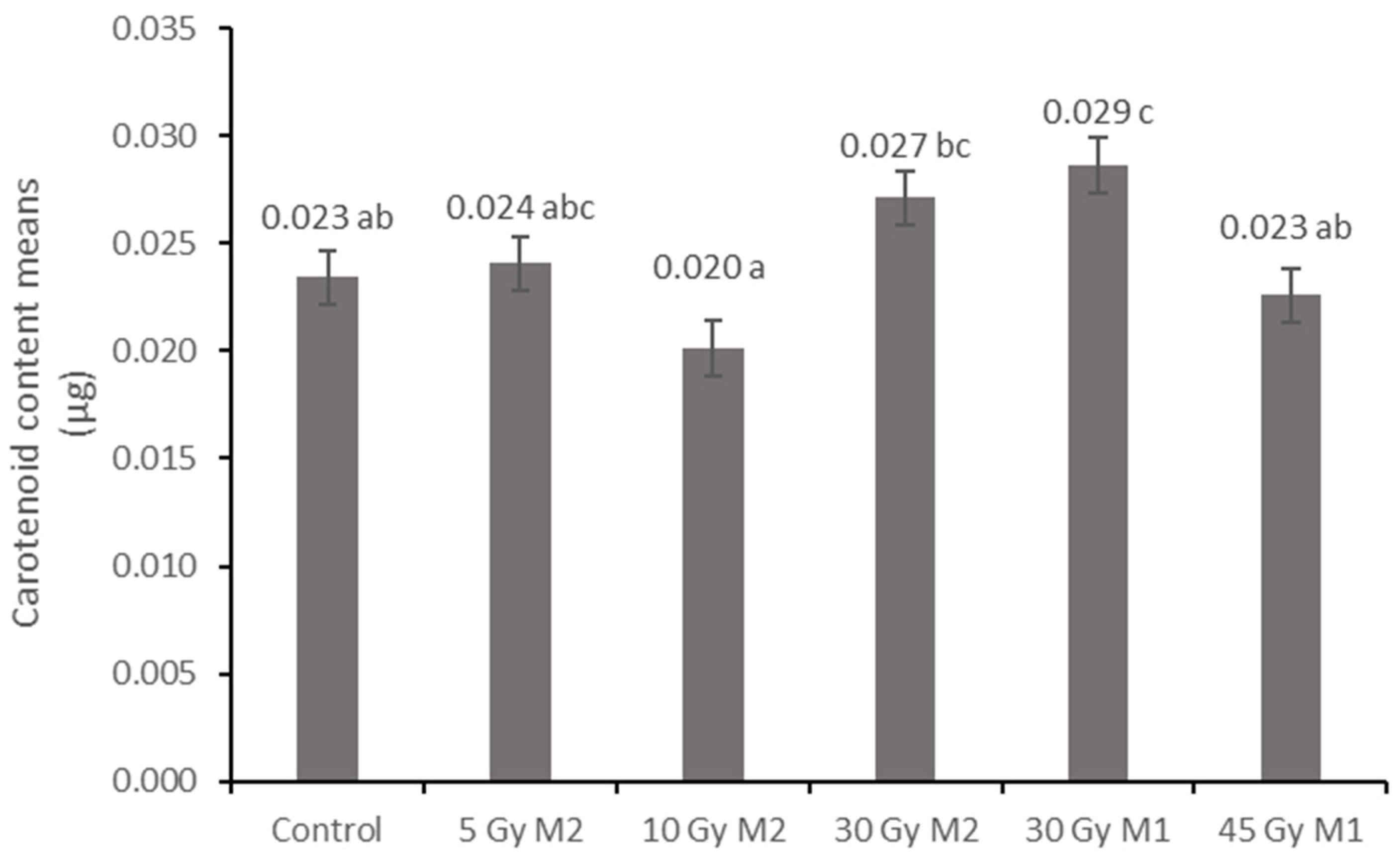
10]. The columnar parenchyma cell groups full of chloroplasts visible in the cross-section of the leaf can also show a very close relationship between the amounts of chlorophyll and carotenoids. According to them, the 10 Gy M2 group had the lowest chlorophyll and carotenoid measurements, while the 30 Gy M1 represented the highest chlorophyll and carotenoid values. This assumes some analogy with the microscopic images of leaf cross-sections. The higher doses of gamma radiation initially affected the chlorophyll and carotenoid content, which are the determining factors for the vitality and good stress tolerance of plants. It should be mentioned in this context that the chlorophyll and carotenoid content measured in the 30 Gy M2 and 45 Gy M1 groups—although it does not show a statistical difference with most of the measured groups—is still high. For the
Rudbeckia strain, doses of 30 Gy M1 and 45 Gy M1 were found to be more effective than lower doses. These results differ from those of Li et al. [
10], in which a dosage strength above 20 had an inhibitory effect on several morphological and physiological processes in
Tulipa bulbs. This is surprising, because for perennial plants with an overwintering organ the optimal dosage strength is assumed to be higher. Likewise, our results are not similar to the findings of Rêgo et al. [
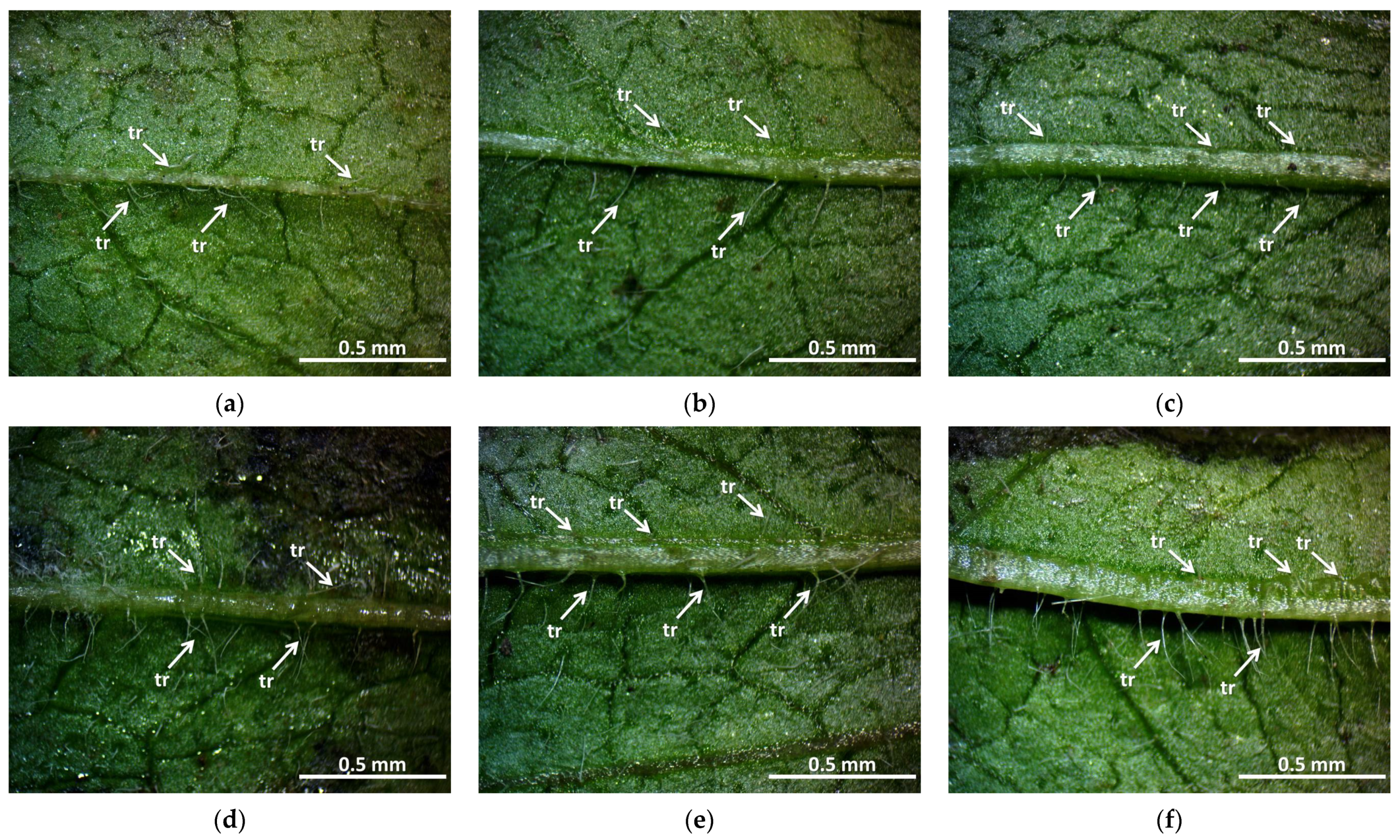
12]. This can also be explained by the density of the trichomes of the groups treated with a higher dose, which play a major role in preserving the plants’ greater vitality.
The amount and length of the trichomes also changed in the main leaf veins of the irradiated groups. This is more visible at higher doses (30 Gy M1 and 45 Gy M1). In terms of generations, it is more even in the M2 generation. In summary, it can be said that the trichomes were stronger in all treated groups than in the control group—this may be related to better drought and climate tolerance, which is a very important breeding goal and aspect. This result is similar to the findings of Bhoi et al. [
21], which state that the breeding of stress-resistant plant varieties is becoming crucial in the current agricultural system.
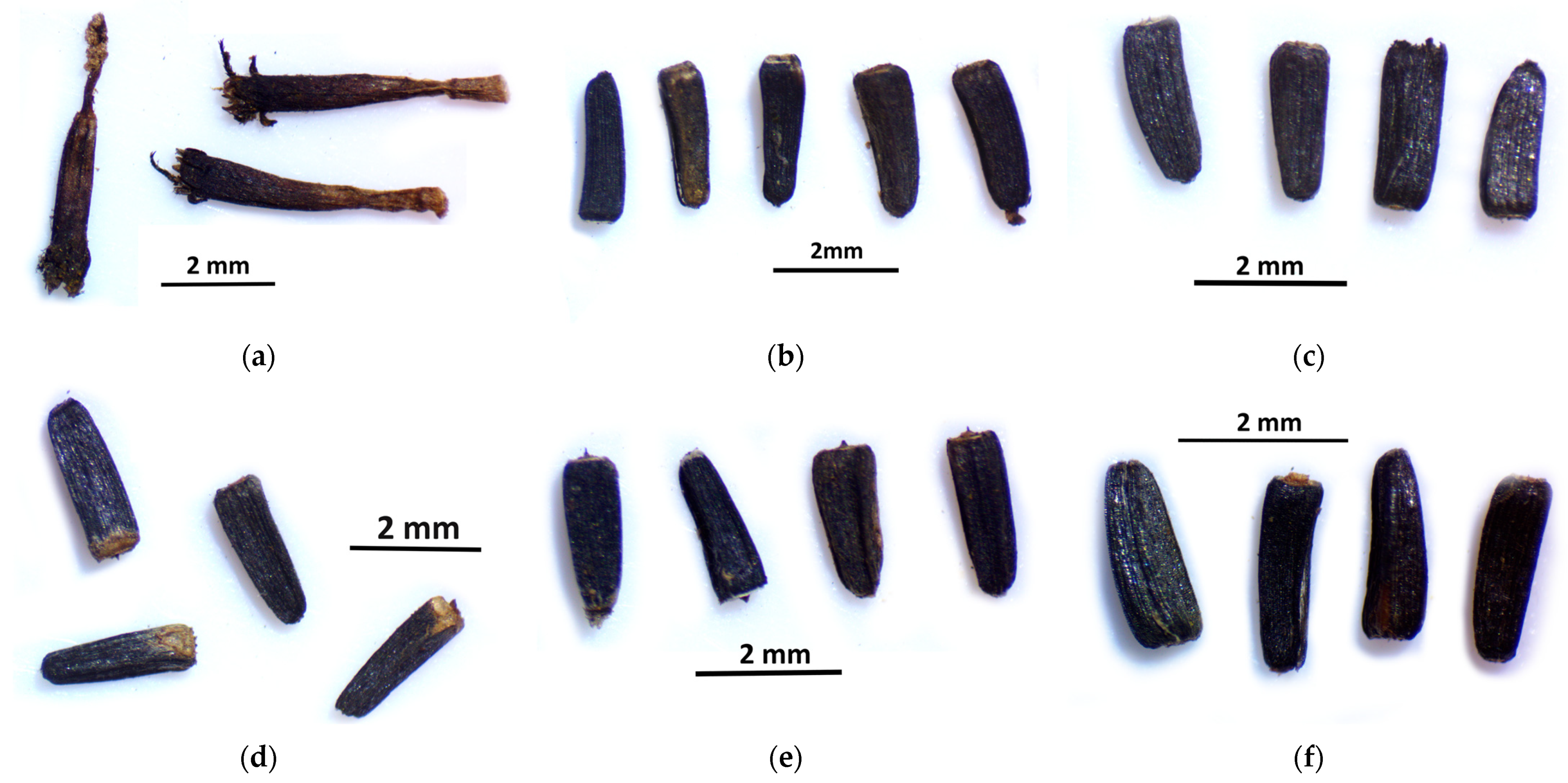
The effect of gamma radiation can also be seen in the size, morphological properties, and physiological state of the fruits. The seeds of the control group were not ripe at the time of sampling, and most of the seeds remained unripe. In the case of the groups that received gamma radiation, the fruits were uniform and healthy. In this regard, the radiation also helped vitality, increasing the survival chances of plants, as Ulukapi et al. [
20] also stated.
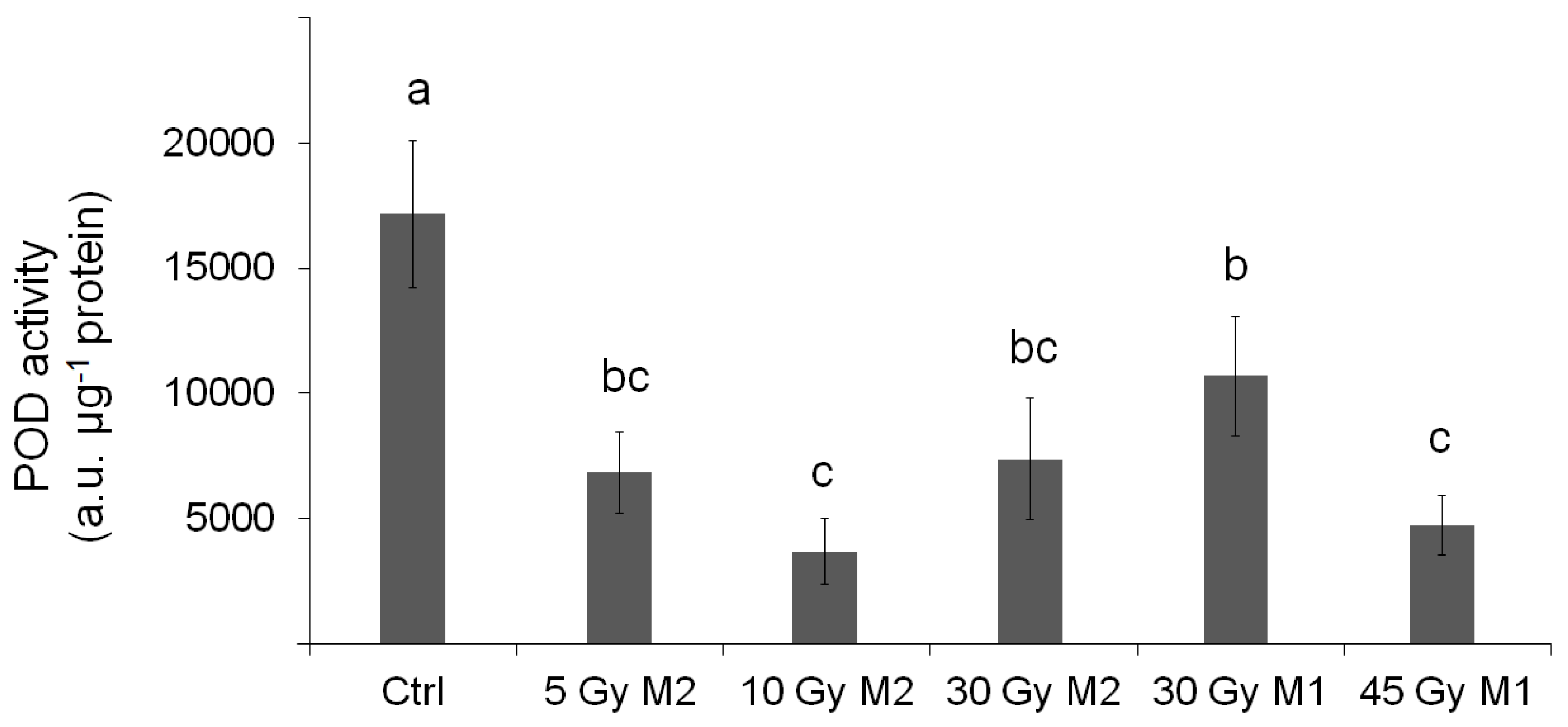
The physiological results also largely reflect the experiences and conclusions presented so far. It is clear from the results of the peroxidase enzyme activity measurements that the enzyme activity of the samples taken in the climate of the end of summer in Hungary shows significantly higher values—in connection with the findings of Geng et al. [
23], we achieved a similar result. The peroxidase enzyme is enriched when the plant is stressed. In the case of treated plants, the level of enzyme activity is significantly lower—this contradicts the findings of Wang et al. [
24]. From this, we can conclude that the statistically verifiable lower stress level of plants treated with gamma radiation is due to the effects of gamma radiation. Bhoi et al. [
21] also stated that mutagenesis is one of the most common techniques for controlling plant stress. In this case, there was no significant difference between the generations.
In this context, the APTI level did not show a statistically verifiable difference, but it is worth highlighting that the urban stress tolerance is higher at higher doses of the M2 generation.
In summary, it can be said that Rudbeckia hirta is a suitable species for use in urban green spaces. Hungarian-bred varieties no longer correspond to the effects of today’s climate and old varieties are no longer fashionable—this is essential in ornamental plant breeding. Gamma radiation had a detectable and favorable effect on the strain of Rudbeckia. Stronger doses showed more favorable phenotypic characteristics. The difference between the generations was also noticeable, and in many cases the positive effects of the treatments were preserved. During our measurements, we came to the conclusion that gamma radiation is a suitable method for the breeding of Rudbeckia hirta as it promotes the breeding of new, stress-tolerant varieties. This was confirmed by histological and physiological methods.
In the future, it will be an increasingly important factor to breed varieties for the urban environment that tolerate the changed climate and, at the same time, the urban biotic and abiotic stress effects. The Hungarian breeds that are currently in development form a very important genetic basis, but they no longer meet the challenges of today. Mutational breeding, on the other hand, can mark a new direction and a new way to create varieties from old varieties that can be used in urban public areas of the 21st century. Our current results show that generational changes and higher applied doses are an important step for us in terms of breeding. With these results, we can contribute to the creation of an environmentally conscious, more sustainable urban plant application.
4. Materials and Methods
4.1. Characteristics of the Selected Rudbeckia
Rudbeckia is similar to the basic variety but its habit is more compact (50–60 cm), it has a larger number of inflorescences, and the burgundy and brown stains are more intense and stronger, often covering the entire petal, so the yellow color does not appear (
Figure 9).
4.2. Preliminary Treatment
The seeds were treated with gamma rays at the Seibersdorf Plant Breeding and Genetics Laboratory station in 2021 and 2022. Each experiment was repeated 3 times and 30 plants were examined in all groups. The plants were grown in a random block arrangement under outdoor conditions.
The seeds were irradiated at the following doses in the year in parentheses. Thus, the investigated dose effects were examined on M1 or M2 generations. For the M2 generation, plants were grown from irradiated seeds, and we collected the seeds in 2021. They were then cultivated in 2022.
M2 (2021)—5, 10, 30 Gray
M1 (2022)—30, 45 Gray.
4.3. Making Microscopic Images
The core examinations were performed with a Delta Optical SZ-450T type trinocular stereomicroscope, which has a stepless zoom of 10–45x.
The Euromex bScope BS.1153-PLi microscope was used for the light microscopic examinations.
Leaf cross-section eyepiece: Lens: PLi 10/0.25 Eyepiece: WF120×/20.
Stem cross-section: Lens: PLi 4/0.1.
Leaf cross-sections were cut with a manual sled microtome.
The stem cross-section was obtained by manual pruning with a scalpel.
In both cases, the Levenhuk M1400 plus camera was used to take the microscopic images.
4.4. Preparation and Microscopic Examination of Leaf and Stem Cross-Sections
The cut leaf was lifted from the knife into a watch glass filled with water using a soft, wet brush. After cutting the samples belonging to 1 leaf, we lifted the leaf samples onto the slide with a wet, soft brush. Afterwards, we dropped water on it and covered it with a coverslip and examined it under the microscope.
In the case of the treated and control plants, a cross-section was made from several points of the leaf, which was examined and photographed under a light microscope. After that, secondary thickenings and changes were examined. The Topuview program was used to take the photos and the Fiji program was used for the measurements.
The stem was cut manually with a scalpel then placed on a glass slide. Water was dropped on it, it was covered with a coverslip and placed under the microscope to be examined, and photographs were taken.
4.5. Stereomicroscopic Examination of Seeds
For each group, 1 inflorescence was collected. In order to not lose seeds, this was collected by pulling the paper bag over the inflorescence, tying it, and then cutting it off the stem. Then, the dropout took place in the bag. The samples collected in this way were sorted under a stereomicroscope so that plant debris did not get between the seeds. After that, the seeds were counted to obtain the total number of seeds, and then the leech seeds and healthy seeds were sorted out, which were also counted. The seeds were examined under the stereomicroscope with 2 types of magnification so that the size and surface differences between the individual groups became clearly visible.
4.6. Stereomicroscopic Examination of Leaf Hairiness
In the case of the examined groups, different hairiness was observed on the leaf surface. To quantify this, we used the main vein of the dorsal part of the leaf since the number of trichomes on the leaf surface could not be counted. Based on the image taken from the dorsal part, it was counted manually, marking the individual trichomes. In addition, a recording was made of the hairiness of the leaf edge.
4.7. Physiological Assessment
4.7.1. Measurement of Chlorophyll and Carotenoid Content
The measurements were made from the plates of the examined leaves based on the methodology of Helrich [
38]. Until the physiological tests, the leaf samples were stored in a plastic, zippered bag in a freezer. For the chlorophyll and carotenoid analysis, 3 × 100 mg leaf samples from each group were measured. These were ground in a pestle and mortar with a small amount of quartz sand, then the crushed material was filled into a measuring cylinder and diluted to 5 mL (the diluting solution consisted of a mixture of 80% acetone and distilled water). After that, the sample was shaken (so that the rubbed substances dissolve sufficiently), then it was placed in a test tube and covered with paraffin for a day. Thus, quartz sand and larger particles that adversely affect the measurement settled to the bottom of the solution. The next day, the supernatant of each sample was placed into a cuvette using a pipette and analyzed using a Genesys 10vis spectrophotometer. The instrument measured the solution at 480, 644, and 663 nanometers. The following equations were used to calculate the total chlorophyll and carotenoid amounts from the results obtained in this way:
where: V = amount of tissue extract (10 mL); w = mass of tissue (0.1 g); A = absorbance.
4.7.2. Peroxidase Enzyme Measurement
The activity of class-III peroxidase isoforms (POD; EC 1.11.1.7) was measured according to Rao et al. [
39] and Solti et al. [
40]. Briefly, 500 mg frozen leaf material mixed from multiple individual leaves was homogenized with 1 mL isolating buffer: 50 mM Na-K-phosphate buffer, pH 7.0, 1.0 mM EDTA, and 0.1% (
w/
v) Triton X-100 and centrifuged for 20,000×
g 20 min at 4 °C. Supernatant was solubilized in 5 mM Tris-HCl, pH 6.8, 0.01% (m/V) SDS, 10% (
v/
v) glycerol, and 0.001% (m/V) bromophenol blue. Proteins were separated on 10–18% gradient polyacrylamide gels as in Solti et al. [
40]. POD activity was developed in 50 mM acetate buffer, pH 4.5, 2 mM benzidine, and 3 mM H
2O
2. The enzyme activity was terminated in 50% (
v/
v) methanol. After digitization using an Epson Perfection V750 PRO gel scanner, densities were measured using Phoretix v 4.0 (Phoretix International, Newcastle upon Tyne, UK). POD activity was normalized based on the total protein contents, determined as in Sárvári et al. [
41].
4.7.3. Calculation of Air Pollution Tolerance Index
The level of air pollution can be expressed by the air pollution tolerance index (APTI) as an indirect reaction of plants. High values of APTI indicate low sensitivity, while tree species of low APTI can be considered biological pollution indicators [
42,
43,
44] (
Table 2).
APTI values were calculated based on the ascorbic acid content in mg/g (A), total chlorophyll content in mg/g (T), pH of leaf extract (P), and relative water content (R) of the leaves. Using these parameters, we applied the equation proposed by Singh et al. [
36]:
The ascorbic acid content was measured with the redox titration method, where 2 g of leaf tissue was crushed and homogenized with 50 mL of water in 3–4 parts. After that, we collected the extract and made up 100 mL in volumetric flasks. From this extract, the leaf pH was first measured using a digital pH meter. After pH measurement, 20 mL portions of the sample were titrated in triplicate with 0.0025 mol of iodine solution in 1 mL of 0.5% starch solution. The blue color remained for 20 s. Chlorophyll was extracted from approximately 50 mg of fresh leaves using 5 mL of 96% ethanol. The absorbance of the extracts was measured at wavelengths of 653, 666, and 750 nm using spectrophotometric analysis. The total chlorophyll content (T) was calculated as follows:
where V is the volume (mL) of the leaf extract, m is the fresh weight (g) of the leaf sample, and E666 and E653 are the absorbance levels at 666 nm and 653 nm minus the absorbance level at 750 nm, respectively. For the pH measurement, 2 g of leaf tissue was crushed and homogenized in 100 mL of deionized water. To determine the relative water content, the fresh weight of individual leaves (FW) was measured. Then, the leaves were immersed in water overnight before being weighed again to determine the turgid weight (TW). Finally, the leaves were dried in an oven at 70 °C to measure the dry weight (DW). The relative water content (R) was calculated as follows:
4.8. Statistical Evaluation
Microsoft Office 365 Excel was used to document our measurement data, and Microsoft Office 365 Word was used for text editing. The processing, comparison, and examination of measurable differences in our results were carried out with the IBM SPSS Statistics 26 program using the ANOVA method. In order to achieve a normal distribution, a part of the database (fresh root mass, fresh green mass, dry root mass, dry green mass) was transformed and the Winsorization method was applied. In all cases, the measured data were analyzed at a 95% reliability (significance) level. Having evaluated the Levene test, if the Sig. > 0.05 the Tukey’s test was used, and if Sig. < 0.05, the Games–Howell post hoc test was used.