Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden
Abstract
1. Introduction
2. Results
2.1. Spontaneously Settled Species in the Study Area
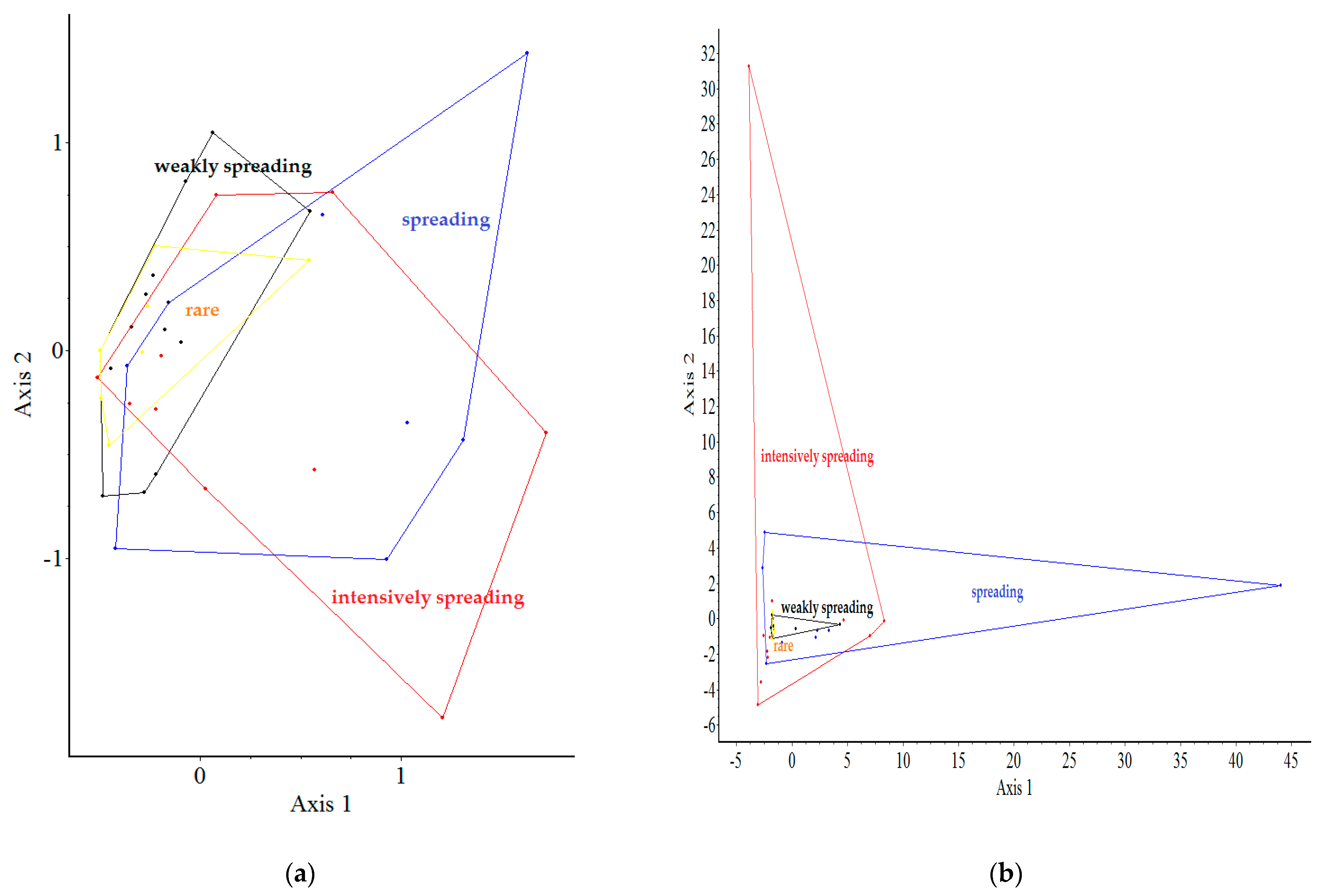
2.2. The Spontaneous Emergence Categories
2.2.1. Category I
2.2.2. Category II
2.2.3. Category III
2.2.4. Category IV
2.2.5. Category V
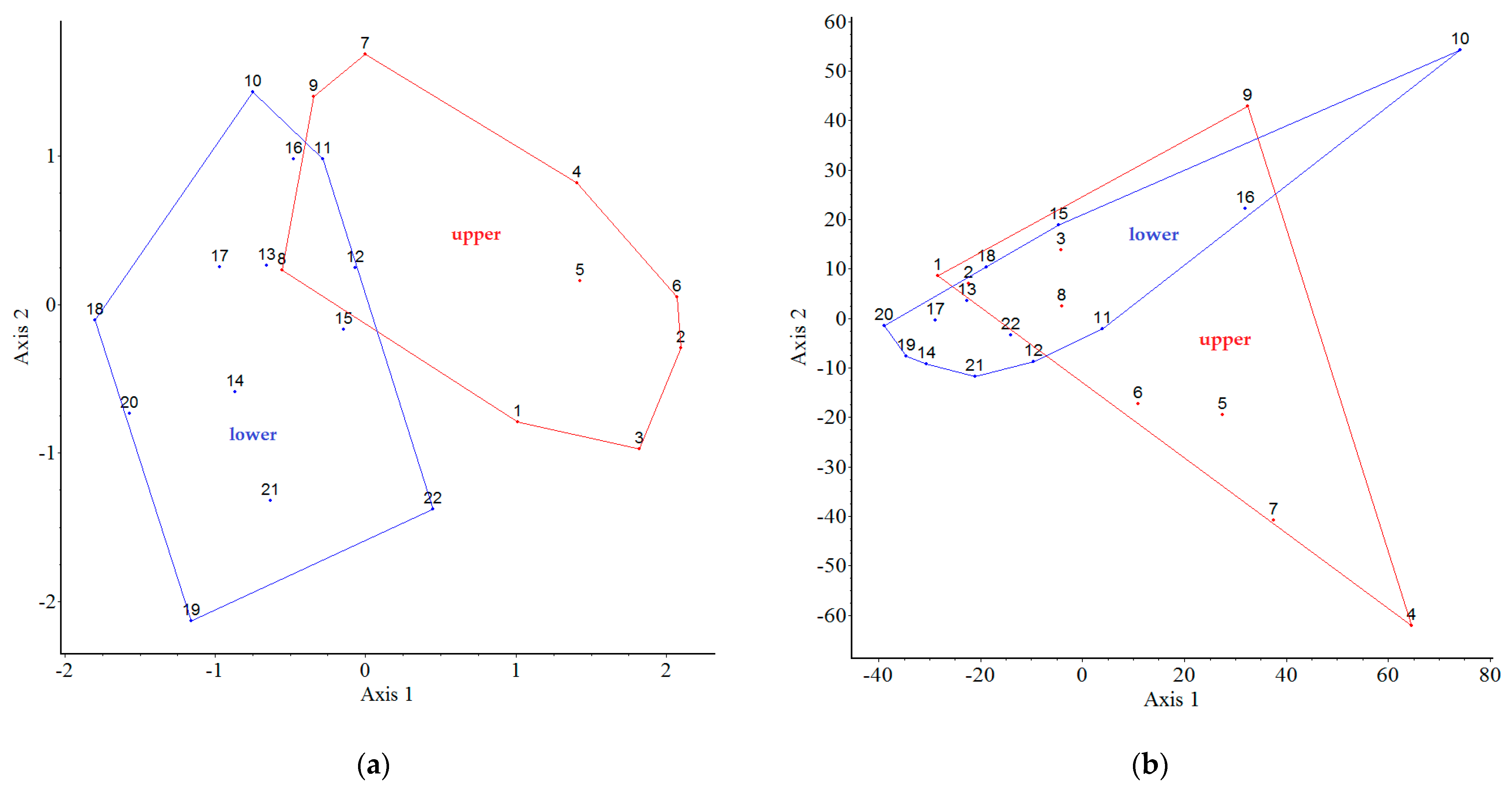
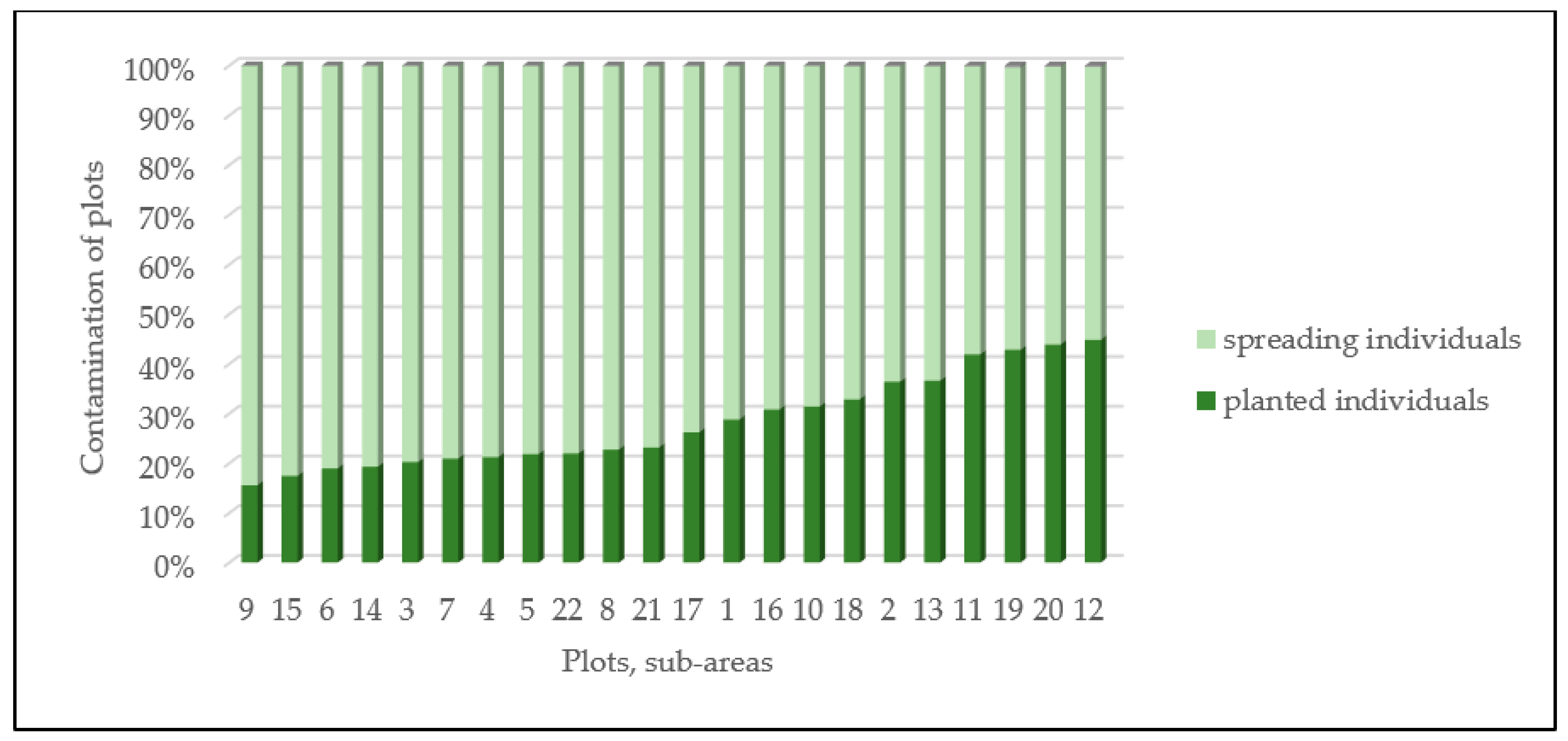
2.3. Correlation between Spontaneously Emerging and Established Individuals Per Plot
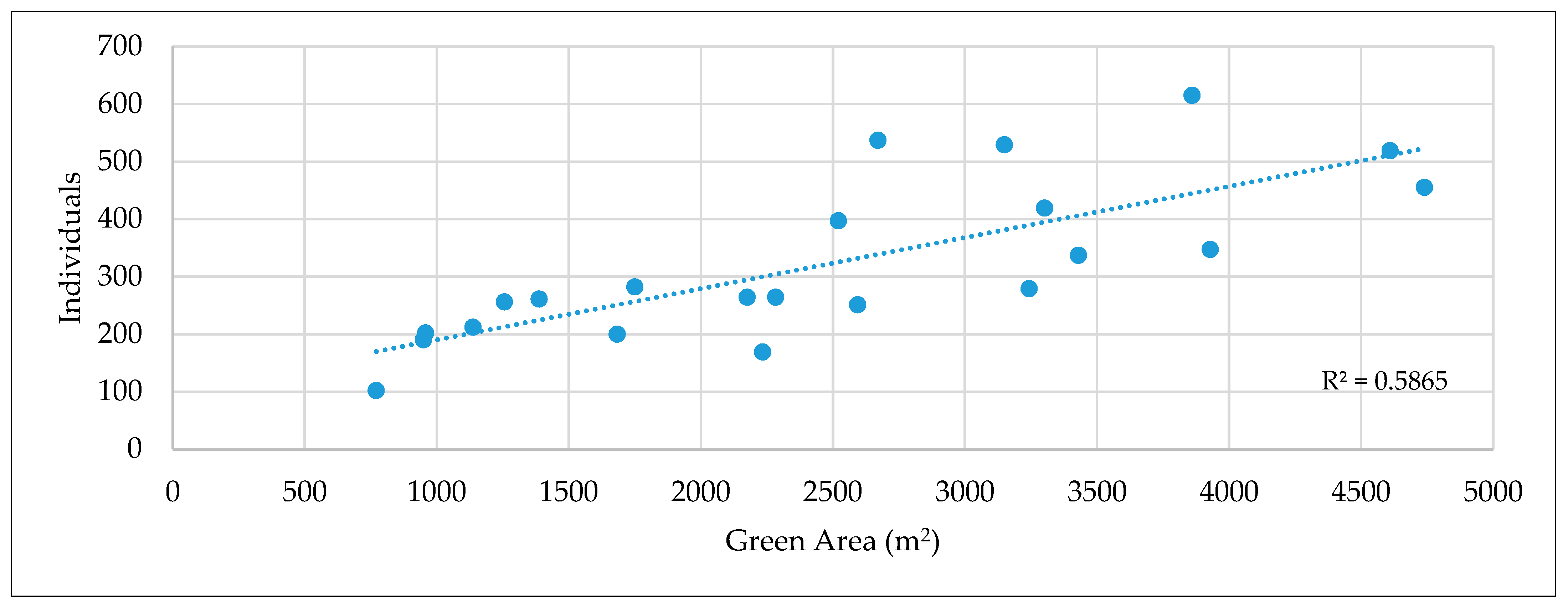
2.4. Effect of the Area on the Number of Individuals
2.5. Comparison of Plots Using a Multivariate Analysis
3. Discussion
3.1. Woody Weeds in the Garden and Other Habitats
3.2. Potential Weeds in Public Open Spaces
3.3. Proposal for Reclassification: A Prelude to Change
3.4. Spontaneous Spreading Abilities of Several Planted Individuals
3.5. Diversity Analysis and Other Additional Correlations
4. Materials and Methods
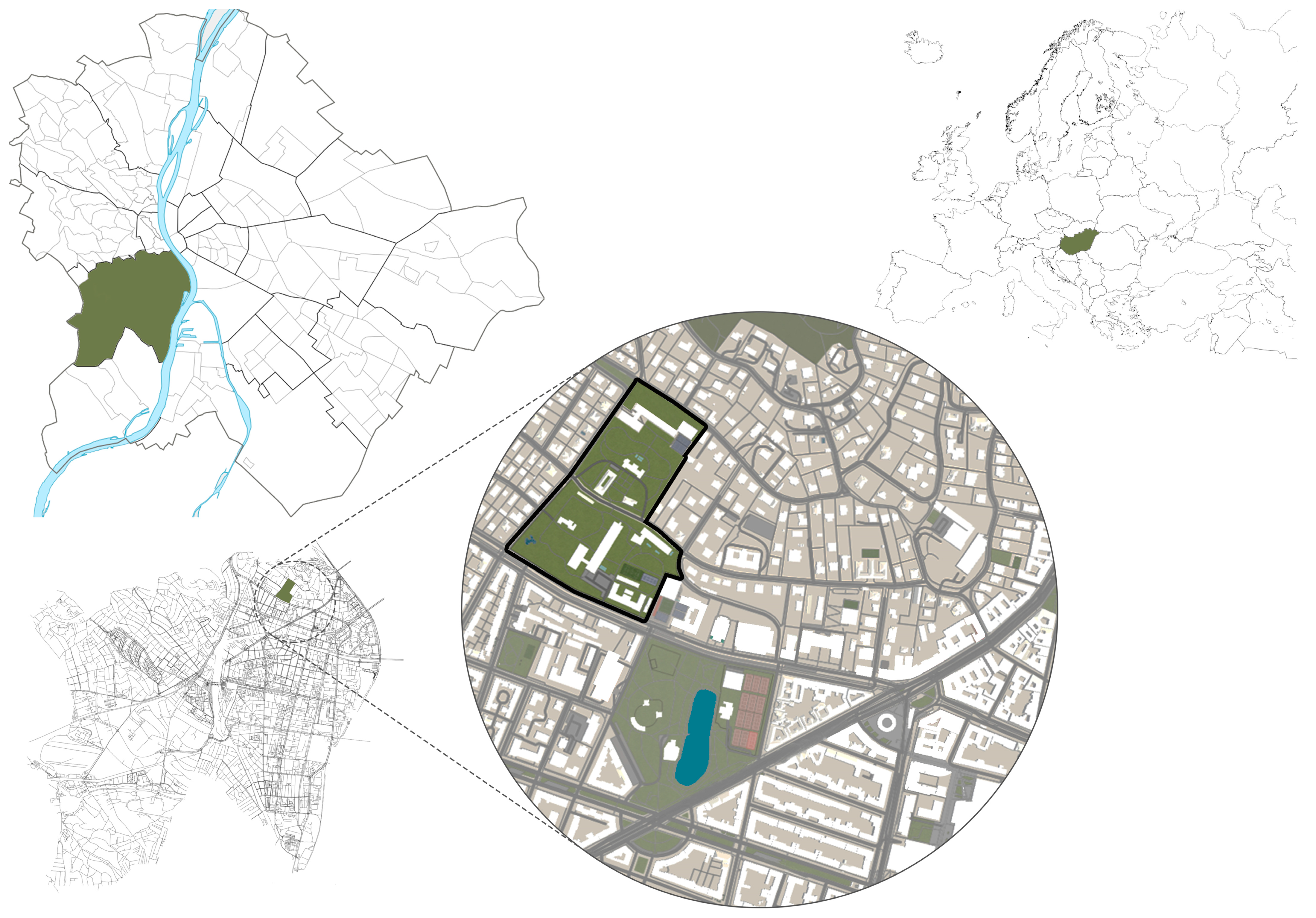
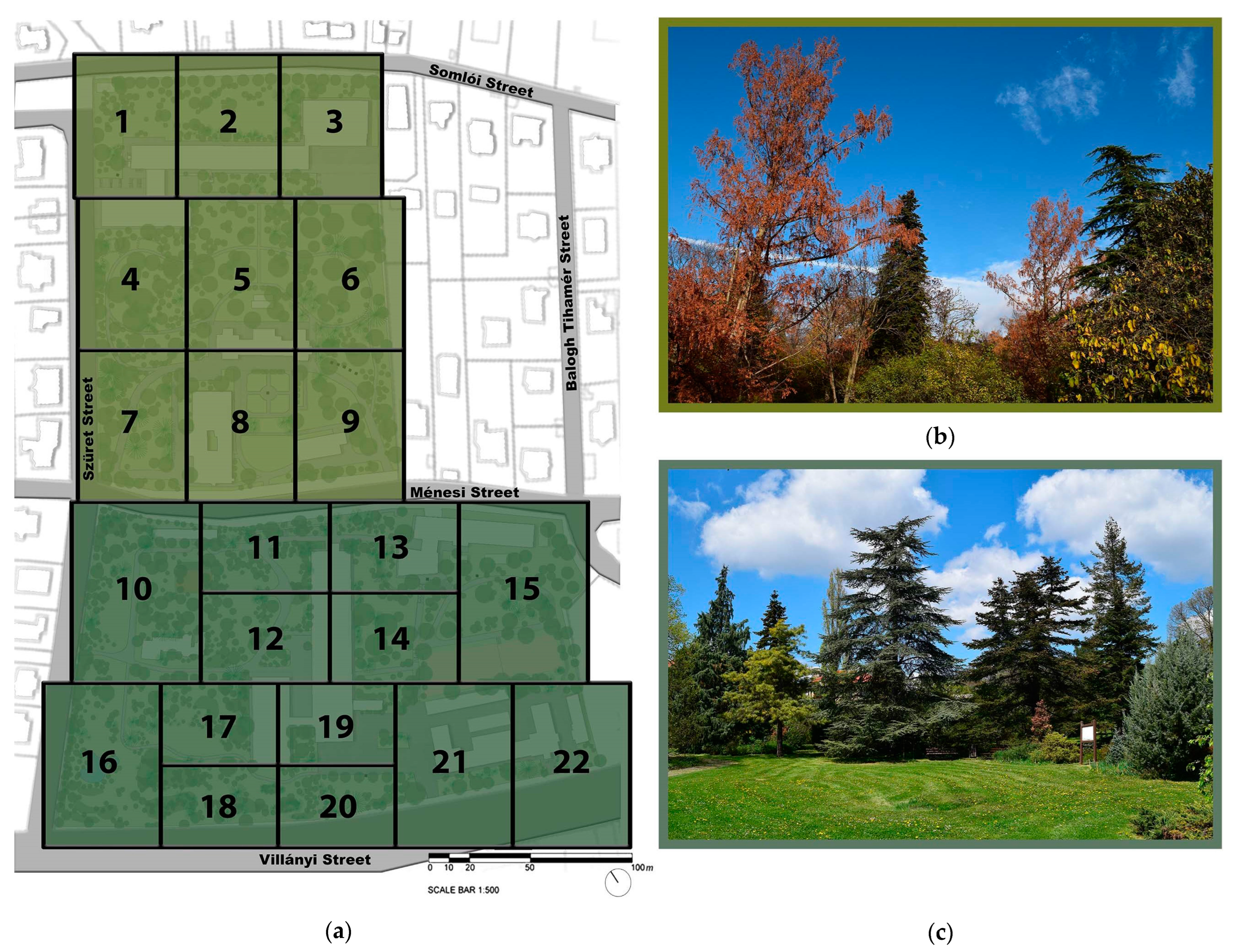
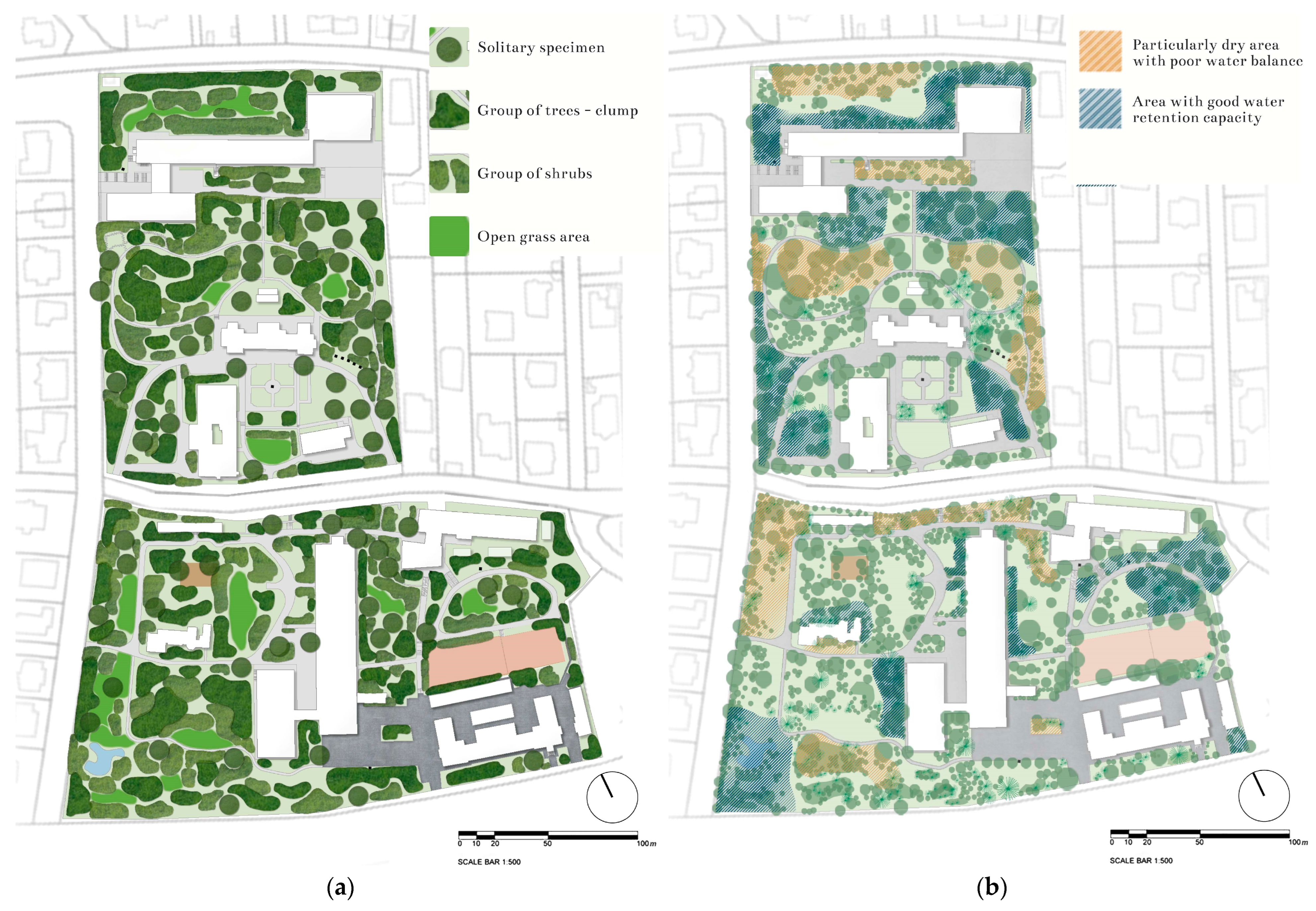
4.1. Research Area—Buda Arboretum
4.2. Field Sampling and Data Collection
- Category I—Intensively spreading taxa (> 100);
- Category II—Spreading taxa (number of individuals 50–99);
- Category III—Weakly spreading, and taxa with a low distribution (10–49);
- Category IV—Just emerging (rare) (number of individuals 1–9);
- Category V—Colonization species (species that reproduce only vegetatively and form large colonies)—not included in statistical evaluations.
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dehnen-Schmutz, K.; Touza, J.; Perrings, C.; Williamson, M. A Century of the Ornamental Plant Trade and Its Impact on Invasion Success. Divers. Distrib. 2007, 13, 527–534. [Google Scholar] [CrossRef]
- Hulme, P.E. Addressing the Threat to Biodiversity from Botanic Gardens. Trends Ecol. Evol. 2011, 26, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Resolving Whether Botanic Gardens Are on the Road to Conservation or a Pathway for Plant Invasions: Botanic Garden Collections and Plant Invasions. Conserv. Biol. 2015, 29, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Sharrock, S.L. The Biodiversity Benefits of Botanic Gardens. Trends Ecol. Evol. 2011, 26, 433. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V. Botanic Gardens and Taxonomy–Their Economic Role. Bull Bot Surv. India 1985, 25, 134–147. [Google Scholar]
- Heywood, V.H. Patterns, Extents and Modes of Invasions by Terrestrial Plants. In Biological Invasions: A Global Perspective; Drake, J.A., Mooney, H.A., di Castri, F., Groves, R.H., Kruger, F.J., Rejmânek, M., Williamson, M., Eds.; John Wiley & Sons: Chichester, UK, 1989; pp. 31–60. [Google Scholar]
- Heywood, V.H. The Role of Botanic Gardens as Resource and Introduction Centres in the Face of Global Change. Biodivers. Conserv. 2010, 20, 221. [Google Scholar] [CrossRef]
- Primack, R.B.; Miller-Rushing, A.J. The Role of Botanical Gardens in Climate Change Research. New Phytol. 2009, 182, 303–313. [Google Scholar] [CrossRef]
- Pyšek, P. On the Terminology Used in Plant Invasion Studies. In Plant Invasions-General Aspects and Special Problems; SPB Academic Pulblishing: Amsterdam, The Netherlands, 1995; pp. 71–81. [Google Scholar]
- Mojzes, A. Ökofiziológiai sajátságok a növényi invázió és a klímaváltozásra adott növényi válaszok hátterében. Ph.D. Thesis, ELTE Biológiai Intézet, Természettudományi Kar, Növényrendszertani és Ökológiai Tanszék, Budapest, Hungary, 2010. [Google Scholar]
- Richardson, D.M.; Pysek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and Invasion of Alien Plants: Concepts and Definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Brundu, G.; Brock, J.; Camarda, I.; Child, L.; Wade, M. Plant Invasions: Species Ecology and Ecosystem Management. In Plant Invasions: Species Ecology and Ecosystem Management; Backhuys Publishers: Leiden, The Netherlands, 2001. [Google Scholar]
- Hilton-Taylor, C.; Brackett, D. 2000 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2000; ISBN 978-2-8317-0564-4. [Google Scholar]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying Threats to Imperiled Species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Mihály, B.; Botta-Dukát, Z. Özönnövények-Biológiai Inváziók Magyarországon; Természetbúvár Alapítvány K: Budapest, Hungary, 2004; ISBN 2399984753148. [Google Scholar]
- GISD. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 19 March 2023).
- Petrosyan, V.; Osipov, F.; Feniova, I.; Dergunova, N.; Warshavsky, A.; Khlyap, L.; Dzialowski, A. The TOP-100 Most Dangerous Invasive Alien Species in Northern Eurasia: Invasion Trends and Species Distribution Modelling. NB 2023, 82, 23–56. [Google Scholar] [CrossRef]
- Balogh, L.; Dancza, I.; Király, G. A Magyarországi Neofitonok Időszerű Jegyzéke És Besorolásuk Inváziós Szempontból [Actual List of Neophytes in Hungary and Their Classification According to Their Success]. In Biologiai invaziok Magyarorszagon: Ozonnovenyek [Biological Invasions in Hungary: Invasive Plants]; TermészetBÚVÁR: Budapest, Hungary, 2004; pp. 61–92. ISBN 978-963-86107-5-1. [Google Scholar]
- Csiszár, Á. Inváziós Növényfajok Magyarországon; Nyugat-magyarországi Egyetem K: Sopron, Hungary, 2012; ISBN 978-963-334-050-9. [Google Scholar]
- Act LIII of 1996-Nature Conservation Act, Decree 13/2001 KöM, Regulation (EU) No 1143/2014 of the European Parliament and the Council and the Related Commission Implementing Regulation 2016/1141. 2016. Available online: https://net.jogtar.hu/jogszabaly?docid=99600053.tv (accessed on 29 March 2023).
- Heywood, V. Sharrock, with European Code of Conduct or Botanic Gardens on Invasive Alien Species; Council of Europe Publishing: Strasbourg, France, 2013; ISBN 978-1-905164-48-6. [Google Scholar]
- Gouveia, A.; Marchante, E. Are Botanic Gardens Still Actively Dispersing Invasive Plant Seeds? EMAPI 2014. Book of Abstracts. 2017; p. 74. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/http://www.invasep.eu/book_of_abstracts_emapi14_lisboa.pdf (accessed on 29 March 2023).
- Inváziós Növényfajok Országos Térinformatikai Adatbázisa-National GIS Database of Invasive Plant Spieces. Available online: https://aszaly.geo.u-szeged.hu/portal/apps/webappviewer/index.html?id=1530024ff6b7454aa828ce8e97cab899 (accessed on 19 March 2023).
- Bartha, D. Fekete Lista: Magyarország Inváziós fa- és Cserjefajai = Black List: Invasive Tree and Shrub Species of Hungary; University of Sopron: Sopron, Hungary, 2020; ISBN 978-963-334-357-9. [Google Scholar]
- Genovesi, P.; Shine, C. Stratégie Européenne Relative aux Espèces Exotiques Envahissantes: Convention Relative à la Conservation de la vie Sauvage et du Milieu Naturel de l’Europe (Convention de Berne); Sauvegarde de la nature; Conseil de l’Europe: Strasbourg, France, 2004; ISBN 978-92-871-5488-0. [Google Scholar]
- Whittemore, A. Exotic Species of Celtis (Cannabaceae) in the Flora of North America. J. Botan. Res. Inst. Texas 2008, 2, 627–632. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.; Sarker, T.; Stinca, A.; Motti, R.; Cesarano, G.; Teobaldelli, M.; Saulino, L.; Cona, F.; et al. Windstorm Disturbance Triggers Multiple Species Invasion in an Urban Mediterranean Forest. iForest 2018, 11, 64–71. [Google Scholar] [CrossRef]
- Csontos, P.; Tamás, J. Spread of Invasive Phanerophytes and Further Records to the Distribution of Woody Species in Hungary. Tájökológiai Lapok/J. Landsc. Ecol. 2006, 4, 127–138. [Google Scholar]
- Szilágyi, K. Szabó Krisztina Eszterháza Központi Kertjeinek Faállomány Vizsgálata És a Barokk Fasorok Megújítása. In Eszterháza Kertművészete Fehéren Feketén: Eszterháza Kertművészeti Örökségének Kutatása, Fejlesztése, Tervezése a Kezdetektől Napjainkig; Ormos Imre Alapítvány: Budapest, Hungary, 2021; ISBN 978-615-81628-1-4. [Google Scholar]
- Csepely-Knorr, L.; Sárospataki, M. A Gellérthegyi Paradicsom-a Budai Arborétum Felső Kertjének Építéstörténete a II. Világháborúig. 4d-Tájépítészeti És Kertművészeti Folyóirat 2009, 14, 13–16. [Google Scholar]
- Zalainé, d.K.Á. 150 Év a Kertészettudományi Élelmiszertudományi És Tájépítészeti Oktatás Szolgálatában 1853–2003 (Budapest, 2003)|Könyvtár|Hungaricana. Available online: https://library.hungaricana.hu/hu/view/BCE_KEE_Sk_2003_150_ev/?pg=0&layout=s (accessed on 19 March 2023).
- Schmidt, G. Budai Arborétum; KÉE-KK Dísznövénytermesztési és Dendrológiai Tanszék: Budapest, Hungary, 1994. [Google Scholar]
- Budai Arborétum-MATE. Available online: https://budaiarboretum.uni-mate.hu/ (accessed on 19 March 2023).
- Az Arborétum Története-Budai Arborétum-MATE. Available online: https://budaiarboretum.uni-mate.hu/az-arboretum-tortenete (accessed on 19 March 2023).
- Schild, D.; Kardos, B. Ménesi út 44-48.-Budapest100. Available online: https://budapest100.hu/en/house/menesi-ut-44-48/ (accessed on 19 March 2023).
- Schmidt, G. A Budapesti Corvinus Egyetem Budai Arborétuma; Budapest Corvinus Egyetem, Kertészettudományi Kar, Dísznövénytermesztési és Dendrológia Tanszék: Budapest, Hungary, 2013. [Google Scholar]
- Osváth, Z. Másfél Évszázada a Gellért-Hegy Déli Lejtőjén. Az Épített Örökség Intézménytörténeti Tudathordozó Funkciója a Budapesti Cor-Vinus Egyetem Budai Campusán. PAAA 2014, 92–116. [Google Scholar] [CrossRef]
- Szilágyi, K. A Kerttörténet És a Kertművészei Oktatásának És Kutatásának Száz Éve a Kertészeti Tanintézettől a Tájépítészeti Karig. 4d-Tájépítészeti És Kertművészet Folyóirat 2013, 29, 29–32. [Google Scholar]
- Rigó, A.; Kovács, A.; Németh, C. A Budai Arborétum Mohaflórája. Bot. Közlemények 2019, 106, 217–235. [Google Scholar] [CrossRef]
- Védett Növények-Budai Arborétum-MATE. Available online: https://budaiarboretum.uni-mate.hu/v%C3%A9dett-n%C3%B6v%C3%A9nyek (accessed on 19 March 2023).
- Czigány, K.; Honfi, P.; Kohut, I.; Schmidt, G.; Sütöriné, D. A megújult Budai Arborétum. Available online: https://www.regikonyvek.hu/kiadas/a-megujult-budai-arboretum-2012-budapesti-corvinus-egyetem-kerteszettudomanyi-karanak-disznovenytermesztesi-es-dendrologiai-tanszeke (accessed on 19 March 2023).
- Jámbor, I. Education from Garden Design to Landscape Architecture in Hungary. 4d-Tájépítészeti És Kertművészet Folyóirat 2012, 27, 25–28. [Google Scholar]
- Nádasy, L.Z.; Illyés, Z.; Gergely, A. The Perceptual Value of Individual Trees as Cityscape Elements–A Case Study in Albertfalva, Budapest. In Proceedings of the Fábos Conference on Landscape and Greenway Planning, Budapest, Hungary, 30 June–3 July 2022. [Google Scholar] [CrossRef]
- Podani, J. SYN-TAX 2000 Computer Programs for Data Analysis in Ecology and Systematics; Scientia Publishing: Budapest, Hungary, 2001. [Google Scholar]
- Hammer, Ø.; Harper, D.T. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Mack, R.N. Predicting the Identity of Plant Invaders: Future Contributions from Horticulture. Hort Sci. 2005, 40, 1168–1174. [Google Scholar] [CrossRef]
- Czúcz, B. A Budai Vár Fásszárú Adventív Flórája. Available online: https://kitaibelia.unideb.hu/?download&aid=360 (accessed on 19 March 2023).
- Udvardy, L.; Facsar, G. Vascular Adventives Signing Changes in the Global and Local Climate in Hungary. In Invázie a Invázne Organizmy, Proceedings of the Vedeckej Konferencie, Nitra, Slovakia, 18–20 November 1998; Eliáš, P., Ed.; Slovenský Národný Komitét Scope: Bratislava, Slovakia, 1999. [Google Scholar]
- Csapody Vera Növénybarát Kör. Available online: https://csapodykor.egzotaklub.hu/ (accessed on 19 March 2023).
- Primack, R.B.; Ellwood, E.R.; Gallinat, A.S.; Miller-Rushing, A.J. The Growing and Vital Role of Botanical Gardens in Climate Change Research. New Phytol. 2021, 231, 917–932. [Google Scholar] [CrossRef]
- Caño, L.; Campos, J.A.; García-Magro, D.; Herrera, M. Replacement of Estuarine Communities by an Exotic Shrub: Distribution and Invasion History of Baccharis Halimifolia in Europe. Biol. Invasions 2013, 15, 1183–1188. [Google Scholar] [CrossRef]
- Fried, G.; Caño, L.; Brunel, S.; Beteta, E.; Charpentier, A.; Herrera, M.; Starfinger, U.; Panetta, F.D. Monographs on Invasive Plants in Europe: Baccharis halimifolia L. Bot. Lett. 2016, 163, 127–153. [Google Scholar] [CrossRef]
- Csecserits, A.; Barabás, S.; Csabai, J.; Devescovi, K.; Hanyecz, K.; Höhn, M.; Kósa, G.; Németh, A.; Orlóci, L.; Papp, L.; et al. Summary of the Experiences of Hungarian Botanical Gardens with Terrestrial Plant Species Included in the European Union’s List of Invasive Alien Species. Bot. Közlemények 2018, 105, 143–154. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Sax, D.F.; Olden, J.D. The Potential Conservation Value of Non-Native Species: Conservation Value of Non-Native Species. Conserv. Biol. 2011, 25, 428–437. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. The Biogeography of Naturalization in Alien Plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Foster, J.; Sandberg, L.A. Friends or Foe? Invasive Species and Public Green Space in Toronto. Geogr. Rev. 2004, 94, 178–198. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Crawley, M.J. Insect Herbivores and Plant Population Dynamics. Annu. Rev. Entomol. 1989, 34, 531–562. [Google Scholar] [CrossRef]
- Reichard, S.H.; White, P. Horticulture as a Pathway of Invasive Plant Introductions in the United States. BioScience 2001, 51, 103. [Google Scholar] [CrossRef]






| Spontaneously Spreading Plants | ||
|---|---|---|
| Taxa | Individuals | |
| Native | 38 | 2836 |
| Non-native | 76 | 4186 |
| Total | 114 | 7022 |
| Species in BA | Risks of Biodiversity | Additional Criteria | Bio-Eco Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interspecific Competition | Hybridization | Transfer Pathogens | Negative Effect on Ecosystem | Current Distribution | Emergency Measure | Occurs in Important Habitats | Reproduction Cap. | Spread Cap. | Current Spread History | Monopolization of Resources | Facilitation of Climate Change | |
| Acer negundo | yes | no | questionable | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Ailanthus altissima | yes | no | no | yes | large-scale | yes | high | high | expansive | yes | yes | |
| Amorpha fruticosa | yes | no | questionable | yes | large-scale | yes | high | high | expansive | yes | yes | |
| Celtis occidentalis | yes | no | no | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Elaeagus angustifolia | probable | no | no | yes | large-scale | yes | high | high | expansive | no | yes | |
| Fraxinus pennsylvanica | yes | no | questionable | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Lycium barbarum | yes | no | no | yes | large-scale | yes | high | low | stable | yes | unknown | |
| Parthenocissus inserta | yes | no | no | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Prunus serotina | yes | no | no | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Robinia pseudoacacia | yes | no | questionable | yes | large-scale | yes | high | high | expansive | yes | yes | |
| Syringa vulgaris | yes | no | probable | yes | large-scale | yes | high | high | expansive | yes | yes | |
| Ulmus pumila | yes | yes | yes | probable | large-scale | no | high | high | expansive | unknown | yes | |
| Vitis vulpina | yes | yes | yes | yes | large-scale | yes | high | high | expansive | yes | unknown | |
| Elaeagnus commutata | questionable | no | no | yes | small-scale | available | unknown | high | high | unknown | yes | yes |
| Hedera crebrescens | probable | probable | questionable | yes | small-scale | available | no | high | high | expansive | yes | unknown |
| Ptelea trifoliata | questionable | no | no | yes | small-scale | available | yes | high | high | expansive | unknown | unknown |
| Akebia quinata | probable | no | no | yes | absent | available | yes | high | high | unknown | yes | yes |
| Baccaris halimifolia | yes | no | no | questionable | absent | available | yes | high | high | expansive | unknown | yes |
| Eucalyptus sp. | probable | no | no | yes | absent | available | no | high | high | expansive | yes | yes |
| Ligustrum sinense | probable | no | questionable | yes | absent | available | unknown | high | high | expansive | unknown | yes |
| Pinus pinaster | yes | no | probable | yes | absent | available | yes | high | high | expansive | yes | yes |
| Toona sinensis | yes | no | probable | probable | absent | unknown | unknown | high | high | unknown | yes | yes |
| Taxa in BA | Operative Group | Watch List | Spontaneous Appearance in Urban Environments |
|---|---|---|---|
| For Natural Habitats | |||
| Acer pseudoplatanus cv. Atropurpureum | x | x | |
| Acer opalus | x | ||
| Broussonetia papyrifera | x | x | |
| Buddleja davidii | x | x | |
| Celtis australis | x | x | |
| Cotoneaster divaricatus | x | ||
| Cotoneaster horizontalis | x | ||
| Cytisus scoparius | x | ||
| Diospyros lotus | x | ||
| Euonymus fortunei | x | x | |
| Fallopia baldschuanica | x | x | |
| Gleditsia triacanthos | x | x | |
| Juglans nigra | x | x | |
| Koelreuteria paniculata | x | x | |
| Lonicera fragrantissima | x | ||
| Lonicera × purpusii | x | ||
| Lonicera standishii | x | ||
| Mahonia aquifolium | x | x | |
| Mahonia repens | x | ||
| Morus alba | x | x | |
| Parthenocissus quinquefolia | x | x | |
| Paulownia tomentosa | x | x | |
| Phyllostachys viridiglaucescens | x | x | |
| Pinus nigra | x | x | |
| Populus × euramericana | x | x | |
| Prunus cerasus | x | x | |
| Prunus mahaleb | x | x | |
| Prunus cerasifera | x | x | |
| Pterocarya fraxinifolia | x | ||
| Rhus typhina | x | x | |
| Robinia viscosa | x | ||
| Rosa rugosa | x | x | |
| Rubus phoenicolasius | x | ||
| Yucca filamentosa | x | ||
| Non-Native/Non-Indigenous Intensively spreading taxa (> 100) Category I | Ailanthus altissima | 0.24 |
| Celtis occidentalis | 0.16 | |
| Cotoneaster spp. | 0.59 | |
| Diospyros spp. | 0.75 | |
| Koelreuteria paniculata | 0.40 | |
| Mahonia spp. | 0.11 | |
| Morus alba | 0.02 | |
| Parthenocissus spp. | 0.13 | |
| Prunus cerasifera | 0.76 | |
| Robinia spp. | 0.34 | |
| Non-Native/Non-Indigenous Spreading taxa (number of individuals (50–99) Category II | Acer negundo | 0.56 |
| Crataegus spp. | 0.48 | |
| Fraxinus spp. | 0.80 | |
| Ligustrum spp. (evergreen) | 0.17 | |
| Ligustrum ovalifolium | 0.43 | |
| Smilax spp. | 0.39 | |
| Viburnum spp. | 0.18 | |
| Non-Native/Non-Indigenous Weakly spreading, taxa with a low distribution (10–49) Category III | Aesculus hippocastanum | 0.12 |
| Berberis julianae | 0.14 | |
| Cercis siliquastrum | 0.31 | |
| Cladrastris kentukea | 0.58 | |
| Cotoneaster multiflorus | 0.01 | |
| Fontanesia phillyreoides | 0.99 | |
| Gymnocladus dioicus | 1.00 | |
| Lonicera japonica | 0.83 | |
| Parrotia persica | 0.52 | |
| Populus spp. | 0.40 | |
| Prunus spp. (mahaleb) | 0.28 | |
| Sophora japonica | 0.01 | |
| Symphoricarpos spp. | 0.24 | |
| Tetradium daniellii | 0.44 | |
| Toona sinensis | 1.00 | |
| Vitis spp. | 0.46 | |
| Wisteria spp. | 0.92 | |
| Native/Indigenous | Acer campestre | 0.44 |
| Category I–III | Acer platanoides | 0.55 |
| Acer pseudoplatanus | 0.75 | |
| Acer tataricum | 0.73 | |
| Corylus avellana | 0.28 | |
| Crataegus spp. | 0.48 | |
| Fraxinus excelsior | 0.27 | |
| Fraxinus ornus | 0.16 | |
| Ligustrum vulgare | 0.07 | |
| Lonicera tatarica | 0.12 | |
| Prunus avium | 0.50 | |
| Prunus padus | 0.15 | |
| Quercus ssp. (deciduous) | 0.31 | |
| Sambucus nigra | 0.03 | |
| Taxus baccata | 0.33 | |
| Tilia spp. | 0.52 | |
| Tilia tomentosa | 0.42 | |
| Ulmus spp. | 0.72 | |
| Viburnum spp. | 0.18 |
| Ranking | Species | Spontaneous Number of Individuals | Planted Number of Individuals | Rate |
|---|---|---|---|---|
| 1 | Celtis occidentalis | 561 | 3 | 187 |
| 2 | Ailanthus altissima | 432 | 3 | 144 |
| 3 | Koelreuteria paniculata | 258 | 5 | 52 |
| 4 | Acer campestre | 216 | 5 | 43 |
| 5 | Diospyrus lotus | 157 | 4 | 39 |
| 6 | Acer platanoides | 647 | 18 | 36 |
| 7 | Morus alba | 108 | 3 | 36 |
| 8 | Acer pseudoplatanus | 193 | 6 | 32 |
| 9 | Prunus cerasifera | 337 | 20 | 17 |
| 10 | Acer negundo | 52 | 4 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, K.; Gergely, A.; Tóth, B.; Szilágyi, K. Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden. Plants 2023, 12, 1989. https://doi.org/10.3390/plants12101989
Szabó K, Gergely A, Tóth B, Szilágyi K. Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden. Plants. 2023; 12(10):1989. https://doi.org/10.3390/plants12101989
Chicago/Turabian StyleSzabó, Krisztina, Attila Gergely, Barnabás Tóth, and Kinga Szilágyi. 2023. "Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden" Plants 12, no. 10: 1989. https://doi.org/10.3390/plants12101989
APA StyleSzabó, K., Gergely, A., Tóth, B., & Szilágyi, K. (2023). Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden. Plants, 12(10), 1989. https://doi.org/10.3390/plants12101989





