Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years
Abstract
1. Introduction
2. Results
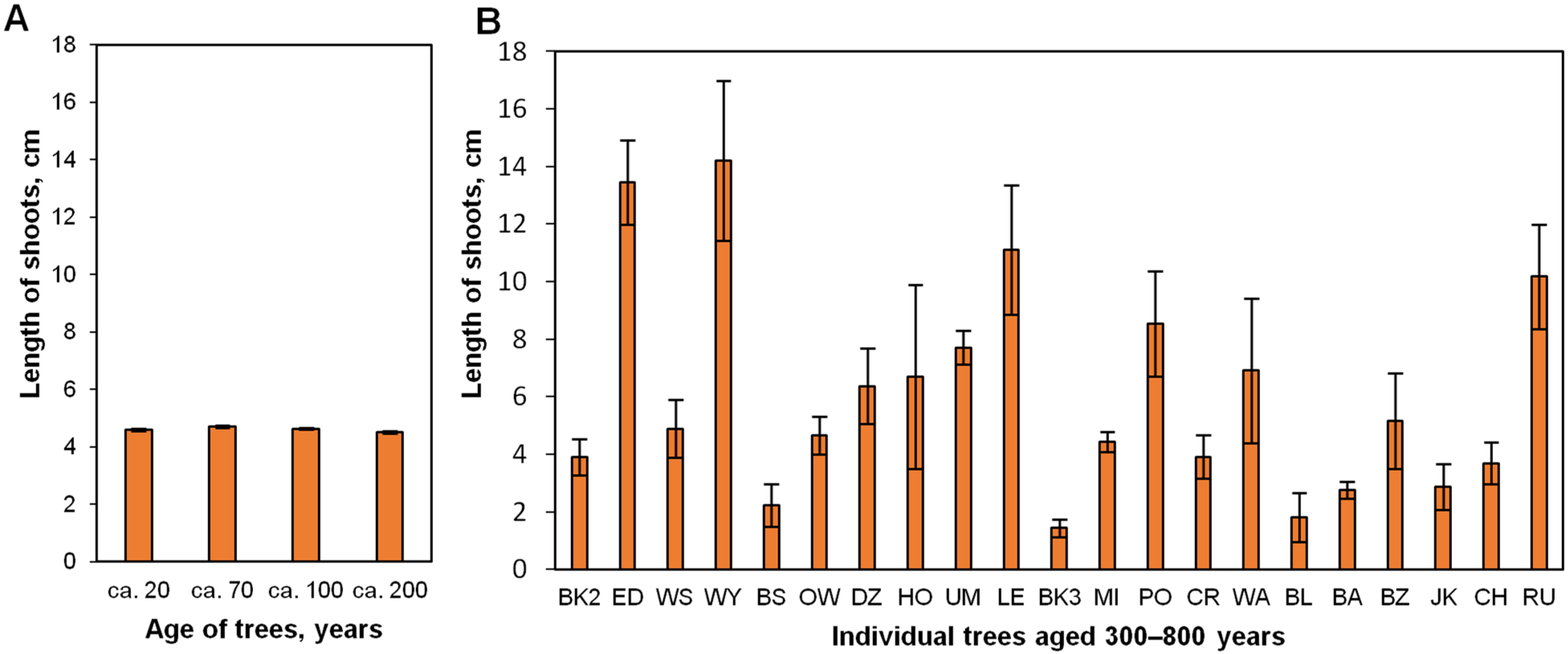
2.1. Ex Vitro Cultivation of Branches in Pots
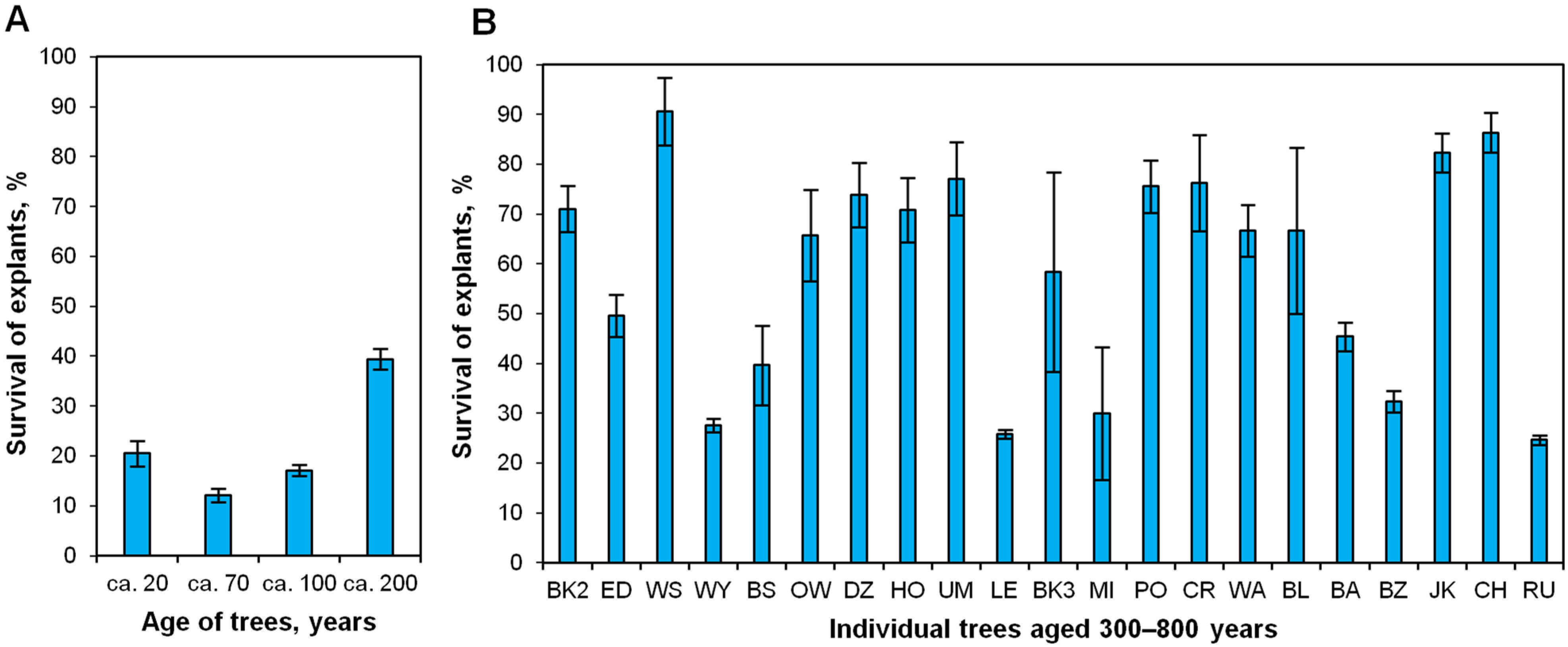
2.2. Effect of Donor Tree Age on Explant Survival after the First Month of In Vitro Culture
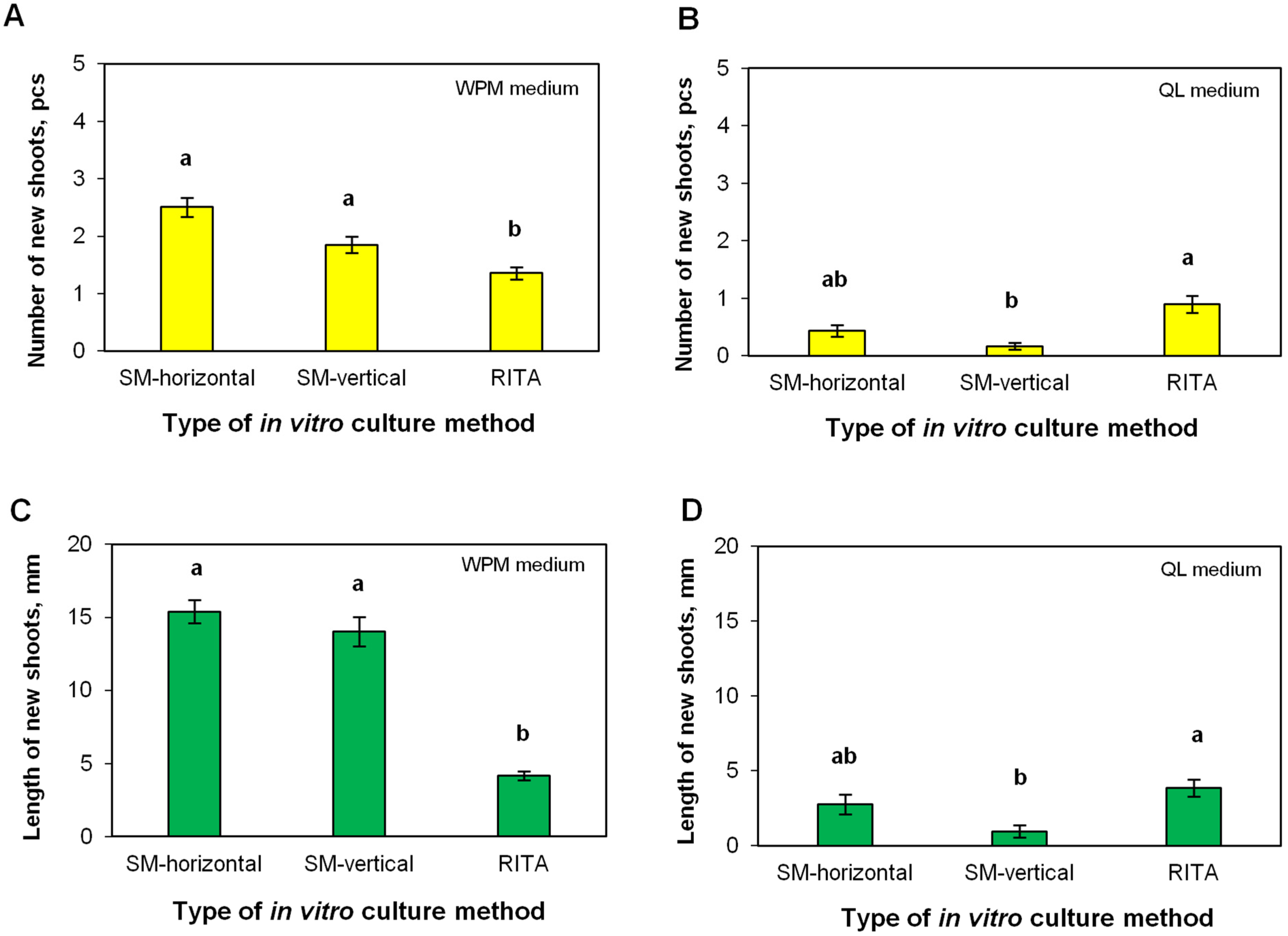
2.3. Effect of a Donor Tree and the Number of Subcultures (up to 21 Months) on In Vitro Shoot Proliferation
2.4. Efficiency of Shoot Multiplication in the RITA® Bioreactor
3. Discussion
3.1. Epicormic Shoot Formation in Pot Culture
3.2. Donor Tree Age, Genotype, and In Vitro Shoot Regeneration
3.3. Multiplication of Shoots in the RITA® Bioreactor
3.4. Shoot Positions on Agar Media
4. Materials and Methods
4.1. Plant Material
4.2. Initiation of Epicormic Shoots in Pot Culture (Ex Vitro)
4.3. Establishment of In Vitro Culture from Explants and Further Shoot Multiplication
4.4. In Vitro Multiplication of Shoots Originating from Trees of Different Ages
4.5. In Vitro Multiplication in the RITA® Bioreactor System and Agar Medium
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heywood, V.H.; Iriondo, J.M. Plant Conservation: Old Problems, New Perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the Storage Life of Seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity Conservation and Conservation Biotechnology Tools. Vitro Cell. Dev. Biol.-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Pavese, V.; Ruffa, P.; Abbà, S.; Costa, R.L.; Corredoira, E.; Silvestri, C.; Torello Marinoni, D.; Botta, R. An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars. Plants 2022, 11, 3308. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.V.; Shukla, M.R.; McIntosh, T.; Saxena, P.K. In Vitro and Cryobiotechnology Approaches to Safeguard Lupinus rivularis Douglas Ex Lindl., an Endangered Plant in Canada. Agronomy 2021, 11, 37. [Google Scholar] [CrossRef]
- Abbas, M.K.; Mahood, H.E.; Alhasan, A.S. Production of Synthetic Seeds in Vegetable Crops: A Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 012099. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-Growth Forests as Global Carbon Sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Liu, J.; Xia, S.; Zeng, D.; Liu, C.; Li, Y.; Yang, W.; Yang, B.; Zhang, J.; Slik, F.; Lindenmayer, D.B. Age and Spatial Distribution of the World’s Oldest Trees. Conserv. Biol. 2022, 36, e13907. [Google Scholar] [CrossRef]
- Pacyniak, C. Wiek najstarszych i niektórych pomnikowych dębów w Polsce. In Dęby; Bogucki Wydawnictwo Naukowe: Poznań-Kórnik, Polska, 2006; pp. 850–876. [Google Scholar]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; p. e01c6df+. Available online: https://forest.jrc.ec.europa.eu/media/atlas/Quercus_robur_petraea.pdf (accessed on 1 June 2023).
- Palmer, S.C.F.; Mitchell, R.J.; Truscott, A.-M.; Welch, D. Regeneration Failure in Atlantic Oakwoods: The Roles of Ungulate Grazing and Invertebrates. For. Ecol. Manag. 2004, 192, 251–265. [Google Scholar] [CrossRef]
- Woziwoda, B.; Dyderski, M.K.; Kobus, S.; Parzych, A.; Jagodziński, A.M. Natural Regeneration and Recruitment of Native Quercus robur and Introduced Q. rubra in European Oak-Pine Mixed Forests. For. Ecol. Manag. 2019, 449, 117473. [Google Scholar] [CrossRef]
- Brown, L.B.; Allen-Diaz, B. Forest Stand Dynamics and Sudden Oak Death: Mortality in Mixed-Evergreen Forests Dominated by Coast Live Oak. For. Ecol. Manag. 2009, 257, 1271–1280. [Google Scholar] [CrossRef]
- Sanchez, M.C.; San-Jose, M.C.; Ballester, A.; Vieitez, A.M. Requirements for In Vitro Rooting of Quercus robur and Q. rubra Shoots Derived from Mature Trees. Tree Physiol. 1996, 16, 673–680. [Google Scholar] [CrossRef]
- Vidal, N.; Arellano, G.; San-José, M.C.; Vieitez, A.M.; Ballester, A. Developmental Stages during the Rooting of In-Vitro-Cultured Quercus robur Shoots from Material of Juvenile and Mature Origin. Tree Physiol. 2003, 23, 1247–1254. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. Methods Mol. Biol. 2018, 1815, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Chalupa, V. Vegetative Propagation of Oak (Quercus robur and Q. petraea) by Cutting and Tissue Culture. Ann. For. Sci. 1993, 50, 295s–307s. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Concepcion Sánchez, M.; Amo-Marco, J.B.; Ballester, A. Forced Flushing of Branch Segments as a Method for Obtaining Reactive Explants of Mature Quercus robur Trees for Micropropagation. Plant Cell Tissue Organ Cult. 1994, 37, 287–295. [Google Scholar] [CrossRef]
- Chmielarz, P.; Michalak, M.; Pałucka, M.; Wasileńczyk, U. Successful Cryopreservation of Quercus robur Plumules. Plant Cell Rep. 2011, 30, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Manzanera, J.A.; Bueno, M.A.; Pardos, J.A. Quercus robur L. (Pedunculate oak). In Trees, 4th ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 321–341. [Google Scholar]
- Gatti, E.; Sgarbi, E. Micropropagation of Quercus robur: Explant Sources and Cultural Conditions Affect In Vitro Responses Differently. Acta Hortic. 2015, 1083, 303–310. [Google Scholar] [CrossRef]
- Quorin, M.; Lepoivre, P. Improved Media for In Vitro Culture of Prunus sp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Chalupa, V. Large Scale Micropropagation Of Quercus robur L. Using Adenine-Type Cytokinins and Thidiazuron to Stimulate Shoot Proliferation. Biol. Plant 1988, 30, 414–421. [Google Scholar] [CrossRef]
- Barragán-Zúñiga, J.; Rocha-Guzmán, N.E.; Montoya-Ayón, J.B.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Sigala-Rodríguez, J.Á.; Pulido-Díaz, C.; Chávez-Simental, J.A.; González-Laredo, R.F. Propagación In Vitro de Quercus sideroxyla a Partir de Bellotas Maduras. Agrociencia 2020, 54, 129–145. [Google Scholar]
- Welander, M.; Sayegh, A.; Hagwall, F.; Kuznetsova, T.; Holefors, A. Technical Improvement of a New Bioreactor for Large Scale Micropropagation of Several Vaccinium Cultivars. Acta Hortic. 2017, 1180, 387–392. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The Effect of PlantformTM Bioreactor on Micropropagation of Quercus robur in Comparison to a Conventional In Vitro Culture System on Gelled Medium, and Assessment of the Microenvironment Influence on Leaf Structure. Plant Biosyst.—Int. J. Deal. Asp. Plant Biol. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Wawrzyniak, M.K.; Ley-López, J.M.; Kalemba, E.M.; Mendes, M.M.; Chmielarz, P. 6-Benzylaminopurine and Kinetin Modulations during In Vitro Propagation of Quercus robur (L.): An Assessment of Anatomical, Biochemical, and Physiological Profiling of Shoots. Plant Cell Tissue Organ Cult. 2022, 151, 149–164. [Google Scholar] [CrossRef]
- Zamani, M.; Zare, A.G.; Emam, M.; Tork, D.B. Effects of Ectomycorrhizal Fungi on the Acclimatization of Micropropagated Quercus castaneifolia Plantlets in Greenhouse. Iran Nat. 2021, 5, 47–54. [Google Scholar] [CrossRef]
- Hackett, W.P. Juvenility, Maturation, and Rejuvenation in Woody Plants. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1985; pp. 109–155. ISBN 978-1-118-06073-5. [Google Scholar]
- Aftab, F. Progress and Prospects for Efficient Micropropagation of Woody Plants. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 363–377. ISBN 978-94-007-4115-7. [Google Scholar]
- Vahdati, K.; Sadeghi-Majd, R.; Sestras, A.F.; Licea-Moreno, R.J.; Peixe, A.; Sestras, R.E. Clonal Propagation of Walnuts (Juglans spp.): A Review on Evolution from Traditional Techniques to Application of Biotechnology. Plants 2022, 11, 3040. [Google Scholar] [CrossRef]
- Kotlarski, S.; Michalak, M.; Chmielarz, P. Klonowanie Najstarszych Dębów Pomnikowych Rosnących w Polsce z Wykorzystaniem Metody In Vitro [Cloning of the Oldest Monumental Oaks Growing in Poland with the Use of In Vitro Method]. Rocz. Pol. Tow. Dendrol. 2019, 67, 53–60. [Google Scholar]
- Zimnoch-Guzowska, E.; Chmielarz, P.; Wawrzyniak, M.K.; Plitta-Michalak, B.P.; Michalak, M.; Pałucka, M.; Wasileńczyk, U.; Kosek, P.; Kulus, D.; Rucińska, A.; et al. Polish Cryobanks: Research and Conservation of Plant Genetic Resources. Acta Soc. Bot. Pol. 2022, 91, 9121. [Google Scholar] [CrossRef]
- Beck, S.L.; Dunlop, R.; van Staden, J. Rejuvenation and Micropropagation of Adult Acacia mearnsii Using Coppice Material. Plant Growth Regul. 1998, 26, 149–153. [Google Scholar] [CrossRef]
- Preece, J.E. A Century of Progress with Vegetative Plant Propagation. HortScience 2003, 38, 1015–1025. [Google Scholar] [CrossRef]
- Raihan, T.; Geneve, R.L.; Perry, S.E.; Rodriguez Lopez, C.M. The Regulation of Plant Vegetative Phase Transition and Rejuvenation: miRNAs, a Key Regulator. Epigenomes 2021, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Sambeek, J.W.V.; Preece, J.E.; Coggeshall, M.V. Forcing Epicormic Sprouts on Branch Segments of Adult Hardwoods for Softwood Cuttings. Int. Plant Propagator’s Soc. 2003, 52, 417–424. [Google Scholar]
- Sotelo, M.; Monza, J. Micropropagation of Eucalyptus maidenii Elite Trees. Agrociencia Urug. 2007, 11, 81–89. [Google Scholar] [CrossRef]
- Han, K.H.; Shin, D.I.; Keathley, D.E. Tissue Culture Responses of Explants Taken from Branch Sources with Different Degrees of Juvenility in Mature Black Locust (Robinia pseudoacacia) Trees. Tree Physiol. 1997, 17, 671–675. [Google Scholar] [CrossRef]
- Basto, S.; Serrano, C.; Jaramillo, E.H. de Effects of Donor Plant Age and Explants on In Vitro Culture of Cedrela montana Moritz ex Turcz. Univ. Sci. 2012, 17, 263–271. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Zuo, E.-H.; Qi, J.-F.; Liu, Y.; Lin, C.-T.; Deng, X. A Role of Age-Dependent DNA Methylation Reprogramming in Regulating the Regeneration Capacity of Boea hygrometrica Leaves. Funct. Integr. Genom. 2020, 20, 133–149. [Google Scholar] [CrossRef]
- San-José, M.C.; Ballester, A.; Vieitez, A.M. Factors Affecting In Vitro Propagation of Quercus robur L. Tree Physiol. 1988, 4, 281–290. [Google Scholar] [CrossRef]
- Aumond, M.L.; de Araujo, A.T.; de Oliveira Junkes, C.F.; de Almeida, M.R.; Matsuura, H.N.; de Costa, F.; Fett-Neto, A.G. Events Associated with Early Age-Related Decline in Adventitious Rooting Competence of Eucalyptus globulus Labill. Front. Plant Sci. 2017, 8, 1734. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.R.; Santos, E.R.; Rodrigues, L.C.A.; Gontijo, A.B.P.L.; Falqueto, A.R. Effects of 6-Benzylaminopurine on Photosystem II Functionality and Leaf Anatomy of In Vitro Cultivated Aechmea blanchetiana. Biol. Plant. 2018, 62, 793–800. [Google Scholar] [CrossRef]
- Martins, J.P.R.; de Rodrigues, L.C.A.; Silva, T.d.S.; Gontijo, A.B.P.L.; Falqueto, A.R. Modulation of the Anatomical and Physiological Responses of In Vitro Grown Alcantarea imperialis Induced by NAA and Residual Effects of BAP. Ornam. Hortic. 2020, 26, 283–297. [Google Scholar] [CrossRef]
- Juncker, B.; Favre, J.M. Clonal Effects in Propagating Oak Trees via In Vitro Culture. Plant Cell Tissue Organ Cult. 1989, 19, 267–276. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary Immersion Systems in Plant Micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary Immersion Systems in Plant Biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A Novel Temporary Immersion Bioreactor System for Large Scale Multiplication of Banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- McAlister, B.; Finnie, J.; Watt, M.P.; Blakeway, F. Use of the Temporary Immersion Bioreactor System (RITA®) for Production of Commercial Eucalyptus Clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- Polzin, F.; Sylvestre, I.; Déchamp, E.; Ilbert, P.; Etienne, H.; Engelmann, F. Effect of Activated Charcoal on Multiplication of African Yam (Dioscorea cayenensis-rotundata) Nodal Segments Using a Temporary Immersion Bioreactor (RITA®). Vitro Cell. Dev. Biol.-Plant 2014, 50, 210–216. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Li, X.-Y.; Welander, M. Optimisation of Growing Conditions for the Apple Rootstock M26 Grown in RITA Containers Using Temporary Immersion Principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A Temporary Immersion System for Micropropagation of Axillary Shoots of Hybrid Chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of Axillary Shoots of Salix viminalis Using a Temporary Immersion System. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Mallón, R.; Covelo, P.; Vieitez, A.M. Improving Secondary Embryogenesis in Quercus robur: Application of Temporary Immersion for Mass Propagation. Trees 2012, 26, 731–741. [Google Scholar] [CrossRef]
- Watt, M.P. The Status of Temporary Immersion System (TIS) Technology for Plant Micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Martínez, M.T.; Corredoira, E.; Vieitez, A.M.; Cernadas, M.J.; Montenegro, R.; Ballester, A.; Vieitez, F.J.; San José, M.C. Micropropagation of Mature Quercus ilex L. Trees by Axillary Budding. Plant Cell Tissue Organ Cult. 2017, 131, 499–512. [Google Scholar] [CrossRef]
- Li, Q.; Gu, M.; Deng, M. In Vitro Propagation of Oriental White Oak Quercus aliena Blume. Forests 2019, 10, 463. [Google Scholar] [CrossRef]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, C.W.; Yang, W.; Turnbull, C.; Meyerowitz, E.M.; Locke, J.C.W.; Jönsson, H. Nitrate Modulates Stem Cell Dynamics in Arabidopsis Shoot Meristems through Cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 1382–1387. [Google Scholar] [CrossRef]
- Wesoły, W.; Hauke-Kowalska, M.; Lassociński, W.; Olszewska, A. Micropropagation of Oaks from Seedling Fragments. Acta Sci. Pol. Silv. Colendar. Rat. Ind. Lignar 2004, 3, 51–62. [Google Scholar]
- McCown, B.H. Special Symposium: In Vitro Plant Recalcitrance, Recalcitrance of Woody and Herbaceous Perennial Plants: Dealing with Genetic Predeterminism. Vitro Cell. Dev. Biol.-Plant 2000, 36, 149–154. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Ballester, A.; Muñoz, F.; Durán, J.; Ibarra, M. In Vitro Regeneration of the Important North American Oak Species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tissue Organ Cult. 2009, 98, 135–145. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Pintos, F.; San-José, M.C.; Ballester, A. In Vitro Shoot Proliferation Determined by Explant Orientation of Juvenile and Mature Quercus rubra L. Tree Physiol. 1993, 12, 107–117. [Google Scholar] [CrossRef] [PubMed]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM)-a Mineral Nutrient Formulation for Microculture of Woody Plant Species. J. Am. Soc. Hortic. Sci. 1981, 16, 453. [Google Scholar]
- Puddephat, I.J.; Alderson, P.G.; Wright, N.A. Influence of Explant Source, Plant Growth Regulators and Culture Environment on Culture Initiation and Establishment of Quercus robur L. Vitro J. Exp. Bot. 1997, 48, 951–962. [Google Scholar] [CrossRef]
- Guilford, J.P. Fundamental Statistics in Psychology and Education. In Science Education; McGraw-Hill Book Company: New York, NY, USA, 1965; Volume 41, p. 244. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielarz, P.; Kotlarski, S.; Kalemba, E.M.; Martins, J.P.R.; Michalak, M. Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years. Plants 2023, 12, 2230. https://doi.org/10.3390/plants12122230
Chmielarz P, Kotlarski S, Kalemba EM, Martins JPR, Michalak M. Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years. Plants. 2023; 12(12):2230. https://doi.org/10.3390/plants12122230
Chicago/Turabian StyleChmielarz, Paweł, Szymon Kotlarski, Ewa Marzena Kalemba, João Paulo Rodrigues Martins, and Marcin Michalak. 2023. "Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years" Plants 12, no. 12: 2230. https://doi.org/10.3390/plants12122230
APA StyleChmielarz, P., Kotlarski, S., Kalemba, E. M., Martins, J. P. R., & Michalak, M. (2023). Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years. Plants, 12(12), 2230. https://doi.org/10.3390/plants12122230







