PhCHS5 and PhF3′5′H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number
Abstract
:1. Introduction
2. Results
2.1. Phylogenetic Analysis of CHS5 and F3′5′H in Phalaenopsis Species
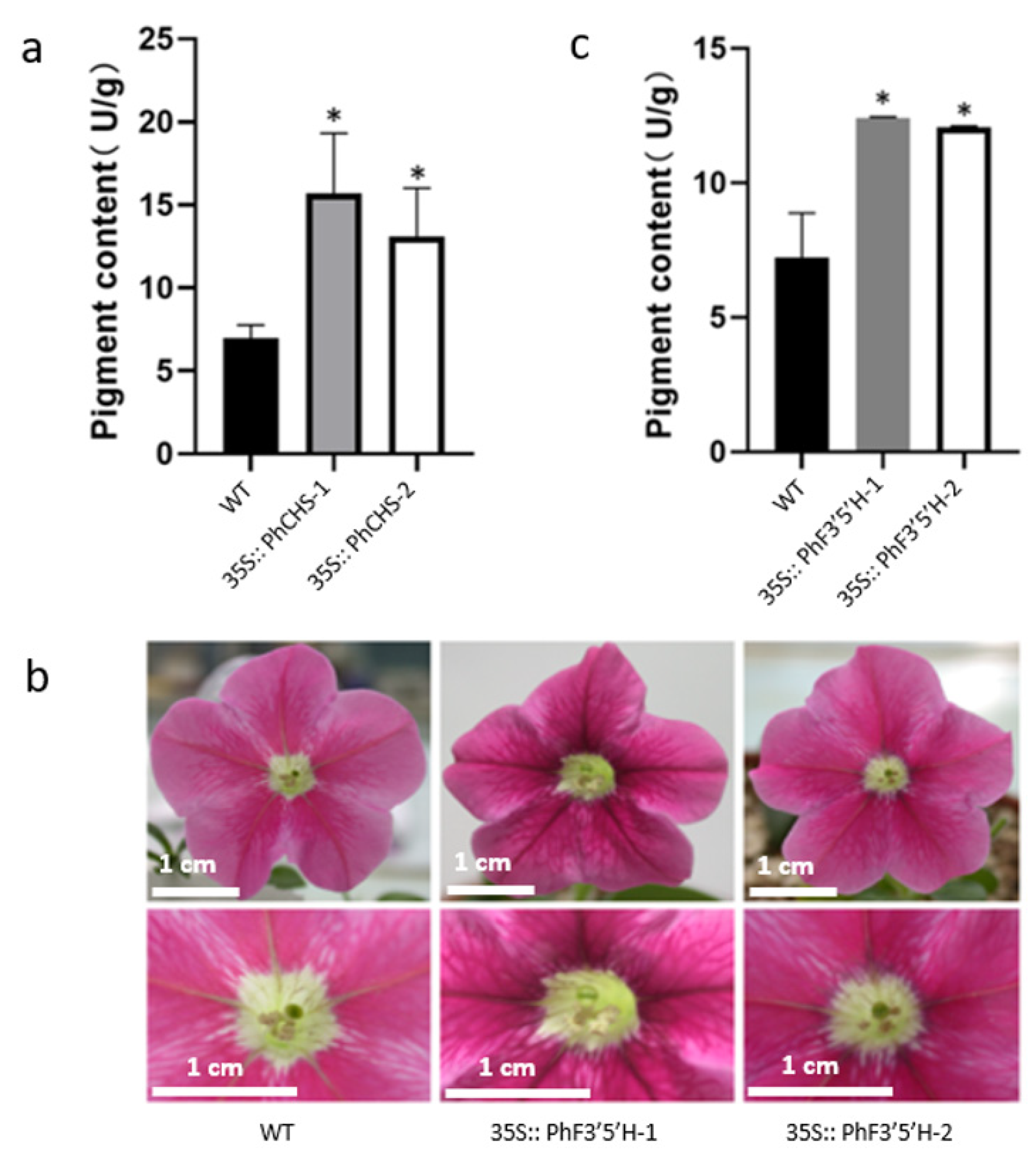
2.2. Analyses of the Phenotypes and Anthocyanin Contents of Genetically Modified Petunia hybrida
2.3. Induction and Cultivation of Phalaenopsis Protocorms
2.4. Screening of PhCHS5 and PhF3′5′H in Transformed Phalaenopsis Protocorms
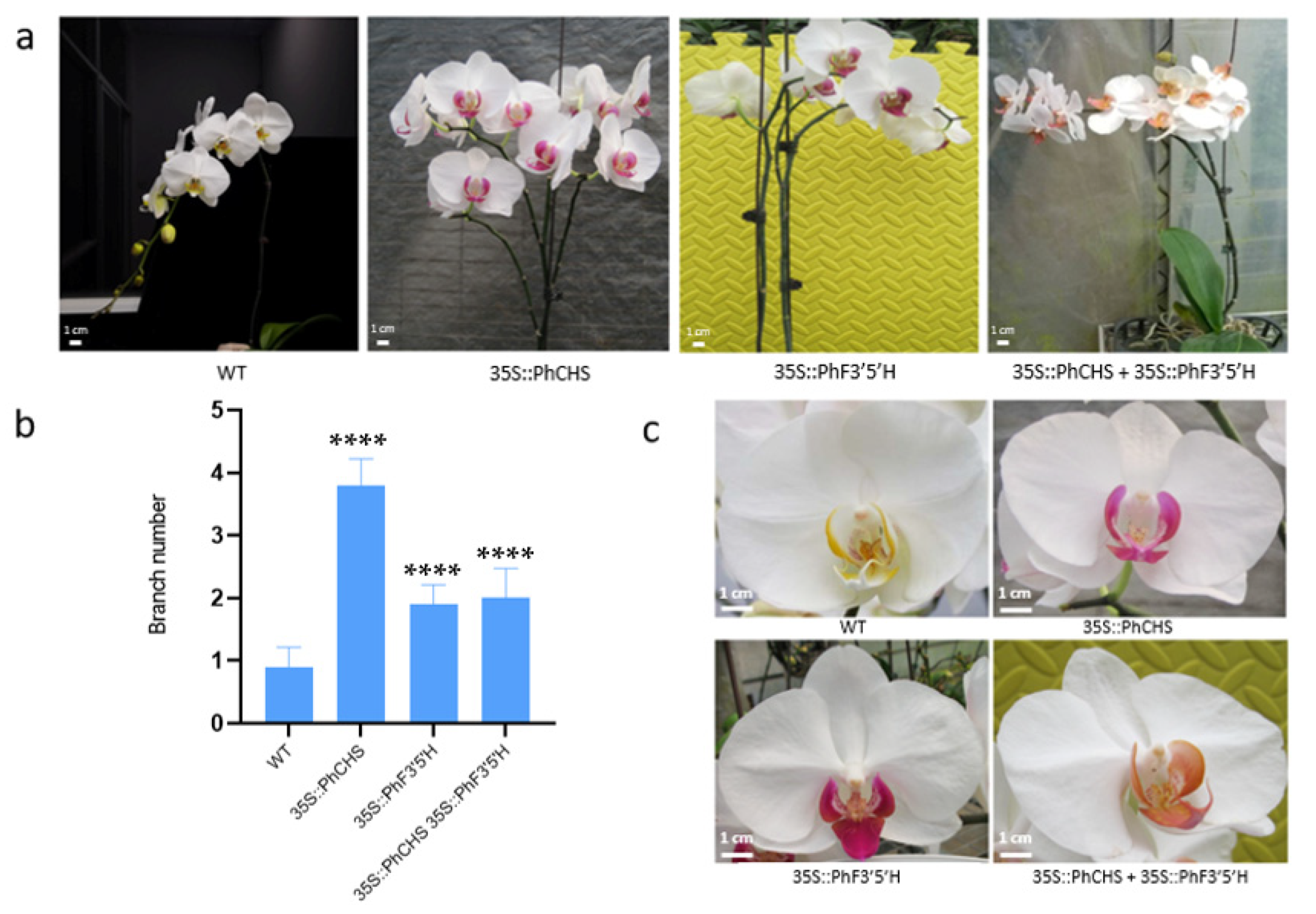
2.5. Regulatory Effects of PhCHS5 and PhF3′5′H Expression on the Branching of Phalaenopsis Stems and the Color of Phalaenopsis Lips
3. Materials and Methods
3.1. Materials and Growth Conditions
3.2. Preliminary Treatment and Cultivation of Phalaenopsis Tissue Culture
3.3. Construction of PhCHS5 and PhF3′5′H Prokaryotic Expression Vectors
3.4. Agrobacterium-Mediated Leaf Disc Method Transgenic to Obtain Transgenic Petunia Expressing PhCHS5 and PhF3′5′H Gene
3.5. Construction of Transgenic Phalaenopsis by Gene Gun
3.6. Construction of Transgenic Phalaenopsis by Agrobacterium Infection
3.7. Analysis of Anthocyanin in the Petals of Transgenic Petunia
3.8. Molecular Identification and Phenotype Analysis of Transgenic Phalaenopsis Strains
3.9. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Azadi, P.; Bagheri, H.; Nalousi, A.M.; Nazari, F.; Chandler, S.F. Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 2016, 34, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Chen, L.; Xiao, Y.; Wu, J.; Zeng, L.; Li, H.; Wu, Q.; Hu, Z. Recent Advanced Metabolic and Genetic Engineering of Phenylpropanoid Biosynthetic Pathways. Int. J. Mol. Sci. 2021, 22, 9544. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, E.; Ohtomo, S.; Asai, K.; Ohta, Y.; Takiue, Y.; Hasumi, A.; Nishihara, M.; Nakatsuka, T. Additional betalain accumulation by genetic engineering leads to a novel flower color in lisianthus (Eustoma grandiflorum). Plant Biotechnol. 2021, 38, 323–330. [Google Scholar] [CrossRef]
- Cardona, J.; Lara, C.; Ornelas, J.F. Pollinator divergence and pollination isolation between hybrids with different floral color and morphology in two sympatric Penstemon species. Sci. Rep. 2020, 10, 8126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Cheng, J.; Yang, M.; Cui, J.; Chen, Y.M.; Deng, Z.H.; Zhao, X.H. Study on foodborne deceptive pollination of qinliwandai LAN. J. Beijing For. Univ. 2015, 37, 100–106. [Google Scholar]
- Ray, H.A.; Stuhl, C.J.; Gillett-Kaufman, J.L. Floral fragrance analysis of Prosthechea cochleata (Orchida-ceae), an endangered native, epiphytic orchid, in Florida. Plant Signal. Behav. 2018, 13, e1422461. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.M.; Zhang, J.; Dong, X.Y.; Fu, Z.Z.; Jiang, H.; Zhang, H.C. Identification and functional analysis of anthocyanin biosynthesis genes in Phalaenopsis hybrids. Biol. Plant. 2018, 62, 45–54. [Google Scholar] [CrossRef]
- Lin, L.; Tang, H.R.; Chen, Q.; Lu, M.; Jia, H.F.; Li, Y.G.; Zhang, X.N. Cloning and sequence analysis of chalcone synthase gene in ornamental peach. Acta Hortic. Sin. 2012, 39, 581–587. [Google Scholar]
- Xu, W.; Yu, X.Y.; Chen, J.; Fu, H.Y.; Hu, B.; Chen, Y.B.; Li, D. Cloning and sequence analysis of CHS gene from Loropetalum rubrum. Chin. Agric. Sci. Bull. 2012, 29, 24–28. [Google Scholar]
- Fukusaki, E.I.; Kawasaki, K.; Kajiyama, S.I.; An, C.I.; Suzuki, K.; Tanaka, Y.; Kobayashi, A. Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. J. Biotechnol. 2004, 111, 229–240. 9. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.E.; Spelt, C.E.; Elzen, P.J.; Mol, J.N. Cloning and molecular characterization of the chalcone synthase multigene family of Petunia hybrida. Gene 1989, 81, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanin, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.C.; Gao, Y.K.; Zhang, X.M.; Sun, J.Q.; Zhao, L.L.; Wang, Y. Progress on plant genes involved in biosynthetic pathway of Anthocyanin. Bull. Bot. Res. 2011, 31, 633–640. [Google Scholar]
- Noda, N. Recent advances in the research and development of blue flowers. Breed Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.P.; Yang, X.N.; Guo, H.C. Bioinformatics and expression analysis of flavonoids-3-5-hydroxylase (F3′5′H) gene in color potato variety ‘Zhuanxinwu’. Genom. Appl. Biol. 2015, 34, 1494–1502. [Google Scholar]
- Su, V.; Hsu, B.D. Cloning and expression of a putative cytochrome P450 gene that influences the color of Phalaenopsis flowers. Biotechnol. Lett. 2003, 25, 1933–1939. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakamura, N.; Kobayashi, H.; Okuhara, H.; Kondo, M.; Koike, Y.; Hoshi, Y.; Nomizu, T. Method for Cultivating Lilies Containing Delphinidin in the Petals Thereof. European Patent WO2012036290, 22 March 2012. [Google Scholar]
- Feng, H.; Wang, J.H.; Wang, M.L.; Li, Y.; Li, Y. Study on genetic transformation of chalcone synthase gene in Petunia. J. Tianjin Agric. 2007, 14, 9–12. [Google Scholar]
- Nakatsuka, T.; Mishiba, K.; Kubota, A.; Abe, Y.; Yamamura, S.; Nakamura, N. Genetic engineering of novel flower color by suppression anthocyanin modification genes in gentian. J. Plant Physiol. 2010, 167, 231–237. [Google Scholar] [CrossRef]
- Han, Y.Y.; Ming, F.; Wang, J.W.; Wen, J.G.; Ye, M.M.; Shen, D.L. Cloning and characterization of a novel chalcone synthase gene from Phalaenopsis hybrida orchid flowers. Russ. J. Plant Physiol. 2006, 53, 250–258. [Google Scholar] [CrossRef]
- Wang, J.W.; Ming, F.; Han, Y.Y.; Shen, D.L. Flavonoid -3′5′-hydroxylase from Phalaenopsis: cDNA cloning, endogenous expression and molecular modeling. Biotechnol. Lett. 2006, 28, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, M.; Chambrier, P.; Rodrigues Bento, S.; Morel, P. Petunia, Your Next Supermodel. Front. Plant Sci. 2016, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, T.N.; Naing, A.H.; Arun, M.; Jeon, S.M.; Kim, C.K. Expression of RsMYB1 in Petunia enhances antho-cyanin production in vegetative and floral tissues. Sci. Hortic. 2017, 214, 58–65. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, F.; Du, Y.; Liu, C.; Bu, G.; Xin, Y.; Liu, B. A modified SDS-based DNA extraction method from raw soybean. Biosci. Rep. 2019, 39, BSR20182271. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Zhao, L.Y. Cloning and bioinformatics analysis of chalcone synthase CHS gene from Rose. J. Agric. 2015, 5, 91–96. [Google Scholar]
- Sun, R.; Yu, N.; Sun, P. Advances in genetic engineering of Phalaenopsis. North. Hortic. 2014, 12, 177–180. [Google Scholar]
- Katsumoto, Y.; Fukuchi, M.M.; Fukui, Y. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Nakano, M.; Mii, M.; Kobayashi, H.; Otani, M.; Yagi, M. Molecular approaches to flower breeding. Breed. Res. 2016, 18, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Lou, Q.; Quan, Y.; Liu, Y.; Wang, Y. Flower-specific expression of the Phalaenopsis flavonoid 3′,5′-hydoxylase modifies flower color pigmentation in Petunia and Lilium. Plant Cell Tissue Organ Cult. 2013, 115, 263–273. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Flower colour and cytochromes p450. Phytochem. Rev. 2013, 5, 283–291. [Google Scholar] [CrossRef]
- Van der Krol, A.; Lenting, P.; Veenstra, J.; Van der Meer, I.; Koes, R.; Gerats, A.; Mol, J.; Stuitje, A. An anti-sense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature 1988, 333, 866–869. [Google Scholar] [CrossRef]
- Coburn, R.A.; Griffin, R.H.; Smith, S.D. Genetic basis for a rare floral mutant in an Andean species of Solanaceae. Am. J. Bot. 2015, 102, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Otsuki, H.; Sakuta, M. Transcriptional control of anthocyanin biosynthetic genes in the Caryophyllales. J. Exp. Bot. 2007, 58, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Houwelingen, A. Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida. Plant J. 1998, 13, 39–50. [Google Scholar]
- Momonoi, K.; Yoshida, K.; Mano, S.; Takahashi, H.; Nakamori, C.; Shoji, K.; Nitta, A.; Nishimura, M. A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J. 2009, 59, 437–447. [Google Scholar] [CrossRef]



| Numbering | BA/NAA (ppm) Concentration | Protocorm Induction Rate (%) | Unit Axillary Buds Get the Number of Bushes |
|---|---|---|---|
| YD1 | 2/0.2 | 50 ± 1.2 b | 14 ± 1.4 b |
| YD2 | 2/2.0 | 40 ± 2.6 c | 25 ± 3.4 a |
| YD3 | 3/0.1 | 80 ± 2.0 a | 10 ± 1.6 bc |
| YD4 | 3/2.0 | 10 ± 1.8 d | 4 ± 2.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Y.; Zhang, Q.; Xu, Q.; Yu, X.; Wang, W.; Gai, R.; Ming, F. PhCHS5 and PhF3′5′H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number. Plants 2023, 12, 2204. https://doi.org/10.3390/plants12112204
Lou Y, Zhang Q, Xu Q, Yu X, Wang W, Gai R, Ming F. PhCHS5 and PhF3′5′H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number. Plants. 2023; 12(11):2204. https://doi.org/10.3390/plants12112204
Chicago/Turabian StyleLou, Yuxia, Qiyu Zhang, Qingyu Xu, Xinyu Yu, Wenxin Wang, Ruonan Gai, and Feng Ming. 2023. "PhCHS5 and PhF3′5′H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number" Plants 12, no. 11: 2204. https://doi.org/10.3390/plants12112204
APA StyleLou, Y., Zhang, Q., Xu, Q., Yu, X., Wang, W., Gai, R., & Ming, F. (2023). PhCHS5 and PhF3′5′H Genes Over-Expression in Petunia (Petunia hybrida) and Phalaenopsis (Phalaenopsis aphrodite) Regulate Flower Color and Branch Number. Plants, 12(11), 2204. https://doi.org/10.3390/plants12112204






