Duplex Real-Time PCR Assays for the Simultaneous Detection and Quantification of Botryosphaeriaceae Species Causing Canker Diseases in Woody Crops
Abstract
1. Introduction
2. Results
2.1. Design of Primers and Probes
2.2. Analytical Specificity—Inclusivity and Exclusivity—And Limit of Detection
2.3. Detection of Botryosphaeria Species in Naturally and Artificially Infected Plants
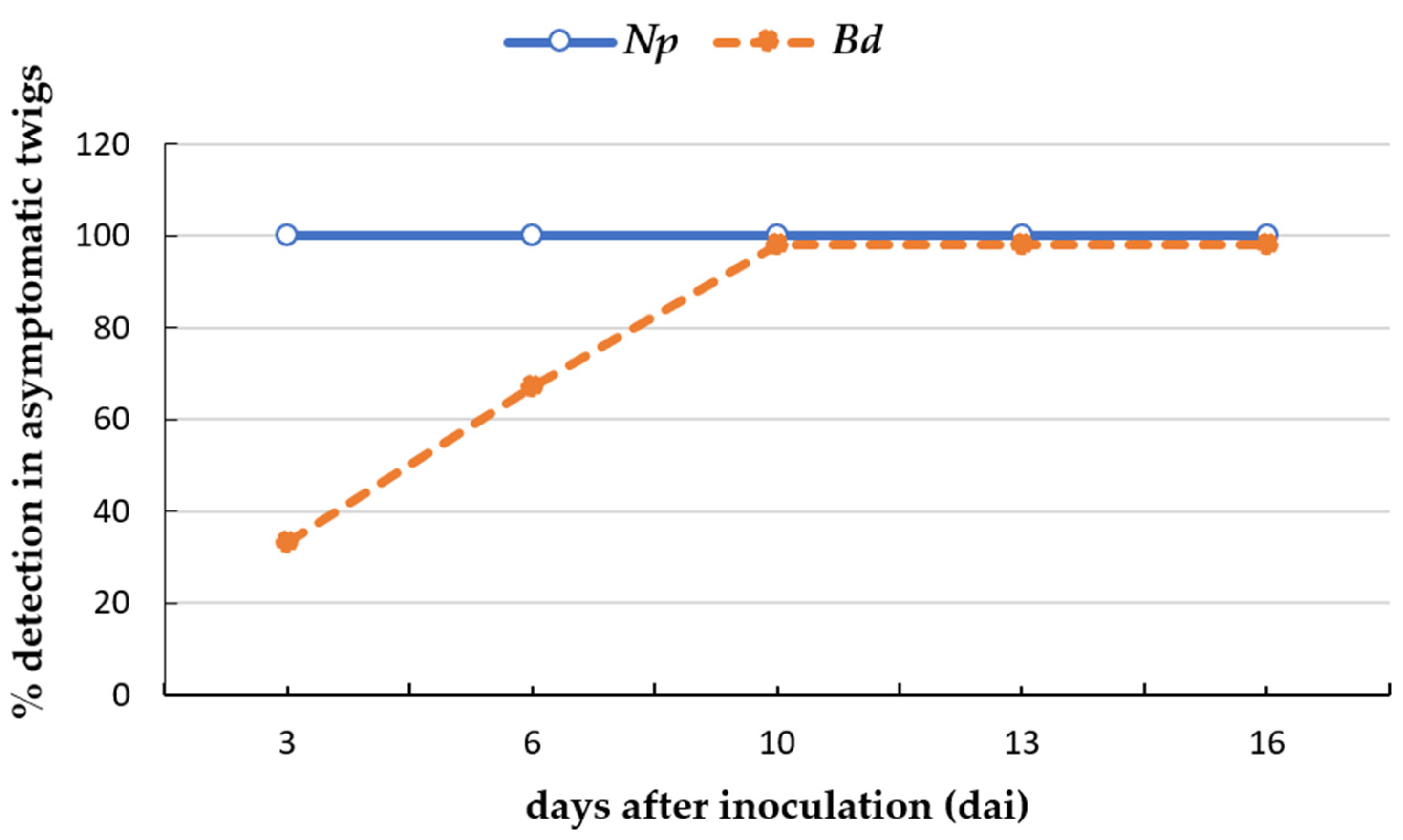
2.4. Detection of Botryosphaeriaceae Species on Asymptomatic Plant Tissues
3. Discussion
4. Materials and Methods
4.1. Surveys of Almond Orchards and Fungal Isolation
4.2. Fungal DNA Extraction and Identification by Sequencing
4.3. Design of qPCR Protocols
4.3.1. Design of Specific Primers and Probes
4.3.2. Optimization of qPCR Conditions
4.4. Analytical Specificity and Analytical Sensitivity of the qPCR Reactions
4.5. Plant DNA Extraction and Direct Sample Preparation Method
4.6. Validation of the Assays in Naturally and Artificially Infected Tissues
4.7. Detection of Botryosphaeriaceae Fungi in Asymptomatic Tissues
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias, I.; Foiles, P.; Oliveira, C. El almendro en España y Portugal: Situación, innovación tecnológica, costes, rentabilidad y perspectivas. Fruticultura 2021, 81, 6–49. [Google Scholar]
- Doll, D.A.; Michailides, T.J.; Rolshausen, P.E. Botryosphaeriaceae associated with almond trunk cankers: A threat to the almond industry? Phytopathology 2013, 103, 13. [Google Scholar]
- Velasquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Fernandez, O.; Lemaitre-Guillier, C.; Songy, A.; Robert-Siegwald, G.; Lebrun, M.H.; Schmitt-Kopplin, P.; Larignon, P.; Adrian, M.; Fontaine, F. The Combination of Both Heat and Water Stresses May Worsen Botryosphaeria Dieback Symptoms in Grapevine. Plants 2023, 12, 753. [Google Scholar] [CrossRef]
- Gramaje, D.; Agusti-Brisach, C.; Perez-Sierra, A.; Moralejo, E.; Olmo, D.; Mostert, L.; Damm, U.; Armengol, J. Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia 2012, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Urbez-Torres, J.R.; Peduto, F.; Vossen, P.M.; Krueger, W.H.; Gubler, W.D. Olive Twig and Branch Dieback: Etiology, Incidence, and Distribution in California. Plant Dis. 2013, 97, 231–244. [Google Scholar] [CrossRef]
- Carlucci, A.; Cibelli, F.; Lops, F.; Raimondo, M.L. Characterization of Botryosphaeriaceae Species as Causal Agents of Trunk Diseases on Grapevines. Plant Dis. 2015, 99, 1678–1688. [Google Scholar] [CrossRef]
- Arjona-Girona, I.; Ruano-Rosa, D.; Lopez-Herrera, C.J. Identification, pathogenicity and distribution of the causal agents of dieback in avocado orchards in Spain. Span. J. Agric. Res. 2019, 17, e1003. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic Diversity and Pathogenicity of Botryosphaeriaceae Species Associated with Symptomatic Citrus Plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef]
- Aviles, M.; de los Santos, B.; Borrero, C. Increase of canker disease severity in blueberries caused by Neofusicoccum parvum or Lasiodiplodia theobromae due to interaction with Macrophomina phaseolina root infection. Eur. J. Plant Pathol. 2021, 159, 655–663. [Google Scholar] [CrossRef]
- Zezlina, I.; Rot, M.; Kac, M.; Trdan, S. Causal Agents of Stone Fruit Diseases in Slovenia and the Potential for Diminishing Their Economic Impact—A Review. Plant Prot. Sci. 2016, 52, 149–157. [Google Scholar] [CrossRef]
- Hernandez, D.; Garcia-Perez, O.; Perera, S.; Gonzalez-Carracedo, M.A.; Rodriguez-Perez, A.; Siverio, F. Fungal Pathogens Associated with Aerial Symptoms of Avocado (Persea americana Mill.) in Tenerife (Canary Islands, Spain) Focused on Species of the Family Botryosphaeriaceae. Microorganisms 2023, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Bostock, R.M.; Trouillas, F.P.; Michailides, T.J. A six locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 2010, 102, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.T.; Lawrence, D.P.; Yaghmour, M.A.; Michailides, T.J.; Trouillas, F.P. Neoscytalidium dimidiatum Causing Canker, Shoot Blight and Fruit Rot of Almond in California. Plant Dis. 2018, 102, 1638–1647. [Google Scholar] [CrossRef]
- Jimenez Luna, I.; Doll, D.; Ashworth, V.E.T.M.; Trouillas, F.P.; Rolshausen, P.E. Comparative Profiling of Wood Canker Pathogens from Spore Traps and Symptomatic Plant Samples Within California Almond and Walnut Orchards. Plant Dis. 2022, 106, 2182–2190. [Google Scholar] [CrossRef]
- Holland, L.A.; Trouillas, F.P.; Nouri, M.T.; Lawrence, D.P.; Crespo, M.; Doll, D.A.; Duncan, R.A.; Holtz, B.A.; Culumber, C.M.; Yaghmour, M.A.; et al. Fungal Pathogens Associated with Canker Diseases of Almond in California. Plant Dis. 2021, 105, 346–360. [Google Scholar] [CrossRef]
- Luo, Y.; Niederholzer, F.J.A.; Lightle, D.M.; Felts, D.; Lake, J.; Michailides, T.J. Limited Evidence for Accumulation of Latent Infections of Canker-Causing Pathogens in Shoots of Stone Fruit and Nut Crops in California. Phytopathology 2021, 111, 1963–1971. [Google Scholar] [CrossRef]
- Sohrabi, M.; Mohammadi, H.; Leon, M.; Armengol, J.; Banihashemi, Z. Fungal pathogens associated with branch and trunk cankers of nut crops in Iran. Eur. J. Plant Pathol. 2020, 157, 327–351. [Google Scholar] [CrossRef]
- Ozer, G.; Turkolmez, S.; Dervis, S. First report of Lasiodiplodia theobromae causing dieback on almond (Prunus dulcis) in Turkey. J. Plant Pathol. 2022, 104, 445–446. [Google Scholar] [CrossRef]
- Goura, K.; Lahlali, R.; Bouchane, O.; Baala, M.; Radouane, N.; Kenfaoui, J.; Ezrari, S.; El Hamss, H.; El Alami, N.; Amiri, S.; et al. Identification and Characterization of Fungal Pathogens Causing Trunk and Branch Cankers of Almond Trees in Morocco. Agronomy 2023, 13, 130. [Google Scholar] [CrossRef]
- Olmo, D.; Armengol, J.; Leon, M.; Gramaje, D. Characterization and Pathogenicity of Botryosphaeriaceae Species Isolated from Almond Trees on the Island of Mallorca (Spain). Plant Dis. 2016, 100, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.M.; Lerma, M.L.; Castillo, P.; Tolosa, V.M.; Olmo, D.; Trapero, A.; Agusti-Brisach, C. First report of Lasiodiplodia theobromae causing crown canker of almond in Spain. J. Plant Pathol. 2022, 104, 411–412. [Google Scholar] [CrossRef]
- Agusti-Brisach, C.; Moldero, D.; Raya, M.d.C.; Lorite, I.J.; Orgaz, F.; Trapero, A. Water Stress Enhances the Progression of Branch Dieback and Almond Decline under Field Conditions. Plants 2020, 9, 1213. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Trapero, A.; Michailides, T.J. Ecology and Epidemiology of Diseases of Nut Crops and Olives Caused by Botryosphaeriaceae Fungi in California and Spain. Plant Dis. 2019, 103, 1809–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Groenewald, J.Z.; Lombard, L.; Schumacher, R.K.; Phillips, A.J.L.; Crous, P.W. Evaluating species in Botryosphaeriales. Persoonia 2021, 46, 63–115. [Google Scholar] [CrossRef]
- Moral, J.; Munoz-Diez, C.; Gonzalez, N.; Trapero, A.; Michailides, T.J. Characterization and Pathogenicity of Botryosphaeriaceae Species Collected from Olive and Other Hosts in Spain and California. Phytopathology 2010, 100, 1340–1351. [Google Scholar] [CrossRef]
- Lopez-Moral, A.; Lovera, M.; del Carmen Raya, M.; Cortes-Cosano, N.; Arquero, O.; Trapero, A.; Agusti-Brisach, C. Etiology of Branch Dieback and Shoot Blight of English Walnut Caused by Botryosphaeriaceae and Diaporthe Species in Southern Spain. Plant Dis. 2020, 104, 533–550. [Google Scholar] [CrossRef]
- Ali, H.; Alam, S.; Attanayake, R.; Rahman, M.; Chen, W. Population Structure and Mating Type Distribution of the Chickpea Blight Pathogen Ascochyta Rabiei from Pakistan and the United States. J. Plant Pathol. 2012, 94, 99–108. [Google Scholar]
- Chen, S.; Morgan, D.P.; Hasey, J.K.; Anderson, K.; Michailides, T.J. Phylogeny, Morphology, Distribution, and Pathogenicity of Botryosphaeriaceae and Diaporthaceae from English Walnut in California. Plant Dis. 2014, 98, 636–652. [Google Scholar] [CrossRef]
- Gusella, G.; Giambra, S.; Conigliaro, G.; Burruano, S.; Polizzi, G. Botryosphaeriaceae species causing canker and dieback of English walnut (Juglans regia) in Italy. For. Pathol. 2021, 51, e12661. [Google Scholar] [CrossRef]
- Luna, I.J.; Besoain, X.; Saa, S.; Peach-Fine, E.; Morales, F.C.; Riquelme, N.; Larach, A.; Morales, J.; Ezcurra, E.; Ashworth, V.E.; et al. Identity and pathogenicity of Botryosphaeriaceae and Diaporthaceae from Juglans regia in Chile. Phytopathol. Mediterr. 2021, 61, 69–74. [Google Scholar]
- Antony, S.; Billones-Baaijens, R.; Stodart, B.J.; Steel, C.C.; Lang, M.D.; Savocchia, S. Incidence and distribution of Botryosphaeriaceae species associated with dieback in walnut orchards in Australia. Plant Pathol. 2023, 72, 610–622. [Google Scholar] [CrossRef]
- Nouri, M.T.; Lawrence, D.P.; Holland, L.A.; Doll, D.A.; Kallsen, C.E.; Culumber, C.M.; Trouillas, F.P. Identification and Pathogenicity of Fungal Species Associated with Canker Diseases of Pistachio in California. Plant Dis. 2019, 103, 2397–2411. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moral, A.; del Carmen Raya, M.; Ruiz-Blancas, C.; Medialdea, I.; Lovera, M.; Arquero, O.; Trapero, A.; Agusti-Brisach, C. Aetiology of branch dieback, panicle and shoot blight of pistachio associated with fungal trunk pathogens in southern Spain. Plant Pathol. 2020, 69, 1237–1269. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Rathnayaka, A.R.; Chethana, K.W.T.; Phillips, A.J.L.; Liu, J.K.; Samarakoon, M.C.; Jones, E.B.G.; Karunarathna, S.C.; Zhao, C.L. Re-Evaluating Botryosphaeriales: Ancestral State Reconstructions of Selected Characters and Evolution of Nutritional Modes. J. Fungi 2023, 9, 184. [Google Scholar] [CrossRef]
- Crous, P.W.; Slippers, B.; Groenewald, J.Z.; Wingfield, M.J. Botryosphaeriaceae: Systematics, pathology, and genetics. Fungal Biol. 2017, 121, 305–306. [Google Scholar] [CrossRef]
- Avenot, H.F.; Jaime-Frias, R.; Travadon, R.; Holland, L.A.; Lawrence, D.P.; Trouillas, F.P. Development of PCR-Based Assays for Rapid and Reliable Detection and Identification of Canker-Causing Pathogens from Symptomatic Almond Trees. Phytopathology 2022, 112, 1710–1722. [Google Scholar] [CrossRef]
- Ni, H.-F.; Yang, H.-R.; Chen, R.-S.; Hung, T.-H.; Liou, R.-F. A nested multiplex PCR for species-specific identification and detection of Botryosphaeriaceae species on mango. Eur. J. Plant Pathol. 2012, 133, 819–828. [Google Scholar] [CrossRef]
- Ridgway, H.J.; Amponsah, N.T.; Brown, D.S.; Baskarathevan, J.; Jones, E.E.; Jaspers, M.V. Detection of botryosphaeriaceous species in environmental samples using a multi-species primer pair. Plant Pathol. 2011, 60, 1118–1127. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Rolshausen, P.E.; Schiavon, M.; Bol, S.; Travadon, R.; Lawrence, D.P.; Baumgartner, K.; Ashworth, V.E.; Comont, G.; Corio-Costet, M.-F.; et al. A Method to Detect and Quantify Eutypa late and Diplodia seriata-Complex DNA in Grapevine Pruning Wounds. Plant Dis. 2017, 101, 1470–1480. [Google Scholar] [CrossRef]
- Haidar, R.; Yacoub, A.; Pinard, A.; Roudet, J.; Fermaud, M.; Rey, P. Synergistic effects of water deficit and wood-inhabiting bacteria on pathogenicity of the grapevine trunk pathogen Neofusicoccum parvum. Phytopathol. Mediterr. 2020, 59, 473–484. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Urbez-Torres, J.R.; Liu, M.; Ayres, M.; Sosnowski, M.; Savocchia, S. Molecular Methods to Detect and Quantify Botryosphaeriaceae Inocula Associated with Grapevine Dieback in Australia. Plant Dis. 2018, 102, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gu, S.; Felts, D.; Puckett, R.D.; Morgan, D.P.; Michailides, T.J. Development of qPCR systems to quantify shoot infections by canker-causing pathogens in stone fruits and nut crops. J. Appl. Microbiol. 2017, 122, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Capote, N.; Pastrana, P.; Aguado, A.; Sánchez-Torres, P. Molecular tools for detection of plant pathogenic fungi and fungicide resistance. In Plant Pathology; InTech-Open Access Publisher: London, UK, 2012; pp. 151–202. [Google Scholar]
- Garcia, J.F.; Lawrence, D.P.; Morales-Cruz, A.; Travadon, R.; Minio, A.; Hernandez-Martinez, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Phylogenomics of Plant-Associated Botryosphaeriaceae Species. Front. Microbiol. 2021, 12, 652802. [Google Scholar] [CrossRef] [PubMed]
- Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, L.; Marangi, P.; Cristella, N.; Faggioli, F.; Reverberi, M.; Scortichini, M.; Pilotti, M. Identification and Characterization of Neofusicoccum stellenboschiana in Branch and Twig Dieback-Affected Olive Trees in Italy and Comparative Pathogenicity with N. mediterraneum. J. Fungi 2023, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Hilario, S.; Lopes, A.; Santos, L.; Alves, A. Botryosphaeriaceae species associated with blueberry stem blight and dieback in the Centre Region of Portugal. Eur. J. Plant Pathol. 2020, 156, 31–44. [Google Scholar] [CrossRef]
- Xiao, X.E.; Wang, W.; Crous, P.W.; Wang, H.K.; Jiao, C.; Huang, F.; Pu, Z.X.; Zhu, Z.R.; Li, H.Y. Species of Botryosphaeriaceae associated with citrus branch diseases in China. Persoonia 2021, 47, 106–135. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Alves, A. What Do We Know about Botryosphaeriaceae? An Overview of a Worldwide Cured Dataset. Forests 2021, 12, 313. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Kraus, C.; Markakis, E.; Alves, A.; Armengol, J.; Eichmeier, A.; Compant, S.; Gramaje, D. Fungal trunk diseases of fruit trees in Europe: Pathogens, spread and future directions. Phytopathol. Mediterr. 2022, 61, 563–599. [Google Scholar] [CrossRef]
- Urbez-Torres, J.R.; Peduto, F.; Striegler, R.K.; Urrea-Romero, K.E.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2012, 52, 169–189. [Google Scholar] [CrossRef]
- Ru, S.; Ding, S.; Oliver, J.E.; Amodu, A. A Review of Botryosphaeria Stem Blight Disease of Blueberry from the Perspective of Plant Breeding. Agriculture 2023, 13, 100. [Google Scholar] [CrossRef]
- Halleen, F.; Crous, P.W.; Petrini, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Aroca, A.; Gramaje, D.; Armengol, J.; Garcia-Jimenez, J.; Raposo, R. Evaluation of the grapevine nursery propagation process as a source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and occurrence of trunk disease pathogens in rootstock mother vines in Spain. Eur. J. Plant Pathol. 2010, 126, 165–174. [Google Scholar] [CrossRef]
- Berlanas, C.; Ojeda, S.; Lopez-Manzanares, B.; Andres-Sodupe, M.; Bujanda, R.; del Pilar Martinez-Diz, M.; Diaz-Losada, E.; Gramaje, D. Occurrence and Diversity of Black-Foot Disease Fungi in Symptomless Grapevine Nursery Stock in Spain. Plant Dis. 2020, 104, 94–104. [Google Scholar] [CrossRef]
- van der Merwe, R.; Halleen, F.; van Dyk, M.; Jacobs, V.G.; Mostert, L. Occurrence of Canker and Wood Rot Pathogens on Stone Fruit Propagation Material and Nursery Trees in the Western Cape of South Africa. Plant Dis. 2021, 105, 3586–3599. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Lopez, J.M.; Capote, N.; Melero-Vara, J.M.; Lopez-Herrera, C.J. Control of avocado white root rot by chemical treatments with fluazinam in avocado orchards. Crop Prot. 2020, 131, 105100. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee SJ, W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Inderbitzin, P.; Harkness, J.; Turgeon, B.G.; Berbee, M.L. Lateral transfer of mating system in Stemphylium. Proc. Natl. Acad. Sci. USA 2005, 102, 11390–11395. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Mondragón-Flores, A.; Rodríguez-Alvarado, G.; Gómez-Dorantes, N.; Guerra-Santos, J.J.; Fernández-Pavía, S.P. Botryosphaeriaceae: A complex, diverse and cosmopolitan family of fungi. Rev. Mex. Cienc. Agrícolas 2021, 12. [Google Scholar]

| Target Organism | Oligo Name | Oligo Type | Sequence 5’–3’ | Target Gene 1 |
|---|---|---|---|---|
| Botryosphaeria dothidea | Bd-F1 | Forward primer | CGCCGAATTTGCCTTATCA | tef1 |
| Bd-R1 | Reverse primer | TTAGCATATGGTCGCATAGAC | ||
| Bd-P | Probe | FAM-TCACCAACG/ZEN/CTTCCAGCCACTCA-IABkFQ | ||
| Neofusicoccum parvum | Np-F1 | Forward primer | GAAGTTCGAGAAGGTAAGAAAGT | tef1 |
| Np-R1 | Reverse primer | TGAGTGCGGGAACCC | ||
| Np-P | Probe | FAM-CTGCACGCG/ZEN/CTGGGTGCCAG-IABkFQ | ||
| Neofusicoccum spp. | Nspp-F | Forward primer | GGCCTGGACGGCTCT | tub2 |
| Nspp-R1 | Reverse primer | AGTGAGAGAGTACCTCGTTGAAG | ||
| Nspp-P | Probe | SUN-GCGCGAATG/ZEN/GCAATGGCTGACC-IABkFQ | ||
| Botryosphaeriaceae family | Bot-F1 | Forward primer | GTATGGCAATCTTCTGAACG | tub2 |
| Bot-R2 | Reverse primer | GAARAGCTGGCCRAAGG | ||
| Bot-P | Probe | SUN-TCGAGCCCG/ZEN/GCACSATGGAT-IABkFQ | ||
| Almond Tree Samples 1 | Simplex qPCR 2 | Duplex qPCR 2 | Isolation and Sequencing | |||
|---|---|---|---|---|---|---|
| Np | Bd | Np + Nspp. | Np + Bot Family | Bd + Bot Family | ||
| Mar 1 | − | + | − | + | + | B. dothidea |
| Sol 2 | − | + | − | + | + | B. dothidea |
| Sol 3 | − | + | − | + | + | B. dothidea |
| Sol 5 | − | − | − | − | − | Cytospora sp. |
| Sol 6 | − | − | − | − | − | Cytospora sp. |
| Sol 7 | + | − | + | + | + | N. parvum/Cytospora sp. |
| Sol 9 | + | − | + | + | + | N. parvum |
| Sol 11 | − | + | − | + | + | B. dothidea |
| Lau 2 | − | + | − | + | + | B. dothidea |
| Lau 3 | − | − | − | − | − | Cytospora sp. |
| Lau 4 | − | − | − | − | − | Cytospora sp. |
| Lau 7 | − | + | − | + | + | B. dothidea |
| Bel 3 | − | + | − | + | + | B. dothidea |
| Bel 4 | − | + | − | + | + | B. dothidea |
| Bel 5 | − | + | − | + | + | B. dothidea |
| Bel 6 | − | + | − | + | + | B. dothidea |
| Sol 10 * | − | − | − | − | − | NI |
| Lau 6 * | − | − | − | − | − | NI |
| Target Organism | Dilution | Real-Time PCR (Cq) 1 | |
|---|---|---|---|
| DNA Extraction | Plant Crude Extracts | ||
| Neofusicoccum parvum | 1:1 | + (22.92 ± 0.10) | - |
| 1:10 | + (26.35 ± 0.04) | + (29.24 ± 0.02) | |
| 1:102 | + (29.70 ± 0.07) | + (31.52 ± 0.42) | |
| 1:103 | + (32.97 ± 0.09) | + (35.18 ± 0.98) | |
| 1:104 | + (36.08 ± 0.43) | + (38.88 ± 0.00) | |
| 1:105 | + (39.12 ± 0.00) | +/− | |
| 1:106 | +/− | − | |
| 1:107 | − | − | |
| Botryosphaeria dothidea | 1:1 | + (22.73 ± 0.07) | − |
| 1:10 | + (26.80 ± 0.13) | + (32.71 ± 0.20) | |
| 1:102 | + (30.44 ± 0.11) | + (35.90 ± 0.28) | |
| 1:103 | + (33.63 ± 0.14) | + (38.84 ± 0.16) | |
| 1:104 | + (36.91 ± 0.29) | + (42.85 ± 0.00) | |
| 1:105 | + (39.41 ± 0.45) | − | |
| 1:106 | +/− | − | |
| 1:107 | − | − | |
| Species | Isolate ID | Host | Year of Isolation | Country |
|---|---|---|---|---|
| Botryosphaeria dothidea | Bd ALM1 | Prunus dulcis | 2016 | Spain |
| Bd ALM2 | Prunus dulcis | 2016 | Spain | |
| Bd ALM3 | Prunus dulcis | 2016 | Spain | |
| Bd ALM4 | Prunus dulcis | 2021 | Spain | |
| Bd ALM6 | Prunus dulcis | 2021 | Spain | |
| Bd ALM7 | Prunus dulcis | 2021 | Spain | |
| Bd ALM8 | Prunus dulcis | 2021 | Spain | |
| Bd ALM9 | Prunus dulcis | 2021 | Spain | |
| Bd ALM10 | Prunus dulcis | 2022 | Spain | |
| Bd ALM11 | Prunus dulcis | 2022 | Spain | |
| Bd ALM12 | Prunus dulcis | 2022 | Spain | |
| Bd ALM13 | Prunus dulcis | 2022 | Spain | |
| Bd ALM14 | Prunus dulcis | 2022 | Spain | |
| Bd ALM15 | Prunus dulcis | 2022 | Spain | |
| Bd ALM16 | Prunus dulcis | 2022 | Spain | |
| Bd ALM17 | Prunus dulcis | 2022 | Spain | |
| ALM TOR1 | Prunus dulcis | 2022 | Spain | |
| Bo.13.2 | Vaccinium corymbosum | 2009 | Spain | |
| Bd1141 | Vitis vinifera | - | Spain | |
| Bd1143 | Vitis vinifera | - | Spain | |
| Botryosphaeria sp. | Bo.11 | Vaccinium corymbosum | 2009 | Spain |
| Neofusicoccum australe | Bo.8 | Vaccinium corymbosum | 2009 | Spain |
| Neofusicoccum luteum | NF 146 | Persea americana | 2013 | Spain |
| Neofusicoccum mediterraneum | Nm ALM3 | Prunus dulcis | 2020 | Spain |
| CJL 593 | Pistacia vera | 2005 | Spain | |
| Neofusicoccum parvum | Np ALM1 | Prunus dulcis | 2018 | Spain |
| Np ALM2 | Prunus dulcis | 2019 | Spain | |
| Np ALM5 | Prunus dulcis | 2022 | Spain | |
| NF 152 | Persea americana | 2013 | Spain | |
| NF 161 | Persea americana | 2013 | Spain | |
| Bo.2 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.4.1 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.4.2 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.6.1 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.7 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.9 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.10 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.13.3 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.14.2 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.16 | Vaccinium corymbosum | 2009 | Spain | |
| Bo.17.1 | Vaccinium corymbosum | 2009 | Spain | |
| Diplodia cortícola | CJL 165 | Quercus suber | 1995 | Spain |
| CJL 166 | Quercus suber | 1995 | Spain | |
| Diplodia cupresii | GIHF 321 | Vitis vinifera | 2021 | Spain |
| Diplodia mutila | CJL 456 | Fraxinus excelsior | 2003 | Spain |
| Diplodia seriata | Ds ALM1 | Prunus dulcis | 2019 | Spain |
| CJL 398 | Vitis vinifera | 2003 | Spain | |
| Dothiorella fraxini | GIHF 132 | Fraxinus angustifolia | 2016 | Spain |
| Dothiorella iberica | CJL 218 | Quercus ilex | 1999 | Spain |
| CJL 220 | Quercus ilex | 1999 | Spain | |
| Dothiorella viticola | CJL 570 | Vitis vinifera | 2004 | Spain |
| CJL 572 | Vitis vinifera | 2004 | Spain | |
| Lasiodiplodia theobromae | L.2 | Vaccinium corymbosum | 2017 | Spain |
| GIHF 272 | Vitis vinifera | 2019 | Spain | |
| Macrophomina phaseolina | Mp ALM 1 | Prunus dulcis | 2018 | Spain |
| Mp ALM 2 | Prunus dulcis | 2019 | Portugal | |
| Mp ARA11 | Vaccinium corymbosum | 2018 | Spain | |
| Mp ARA12 | Vaccinium corymbosum | 2019 | Spain | |
| TOR 872 | Vaccinium corymbosum | 2017 | Spain | |
| TOR 956 | Vaccinium corymbosum | 2020 | Spain | |
| Cytospora acaciae | Ca ALM1 | Prunus dulcis | 2022 | Spain |
| Ca ALM2 | Prunus dulcis | 2022 | Spain | |
| Ca ALM3 | Prunus dulcis | 2022 | Spain | |
| Botrytis cinerea | Bc ARA1 | Vaccinium corymbosum | 2021 | Spain |
| Bc ALM1 | Prunus dulcis | 2016 | Spain | |
| Monilia fructicola | Mf CIR1 | Prunus salicina | 2011 | Spain |
| Monilia laxa | Ml CIR1 | Prunus salicina | 2011 | Spain |
| Diaporthe amygdali | DAL-65 | Prunus dulcis | 2017 | Spain |
| Diaporthe foeniculina | DAL-69 | Prunus dulcis | 2017 | Spain |
| Diaporthe phaseolorum | DAL-222 | Prunus dulcis | 2018 | Spain |
| Collectotrichum accutatum | 20,240 | CECT | - | Spain |
| Verticilium dahliae | Vd ALM1 | Prunus dulcis | 2017 | Spain |
| Cylindrocladiella variabilis | AL139 | Prunus dulcis | 2019 | Spain |
| Dactylonectria macrodidyma | AL150 | Prunus dulcis | 2019 | Spain |
| Dactylonectria novozelandica | AL84 | Prunus dulcis | 2019 | Spain |
| Dactylonectria torresensis | AL3 | Prunus dulcis | 2019 | Spain |
| Ilyonectria liriodendri | AL79 | Prunus dulcis | 2019 | Spain |
| Neonectria quercicola | AL141 | Prunus dulcis | 2019 | Spain |
| Rhizoctonia solani | Rs ALM4 | Prunus dulcis | 2020 | Spain |
| Epicoccum nigrum | En ALM5 | Prunus dulcis | 2020 | Spain |
| Alternaria alternata | Al ALM1 | Prunus dulcis | 2020 | Spain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Cuadrado, L.; López-Herrera, C.J.; Aguado, A.; Capote, N. Duplex Real-Time PCR Assays for the Simultaneous Detection and Quantification of Botryosphaeriaceae Species Causing Canker Diseases in Woody Crops. Plants 2023, 12, 2205. https://doi.org/10.3390/plants12112205
Romero-Cuadrado L, López-Herrera CJ, Aguado A, Capote N. Duplex Real-Time PCR Assays for the Simultaneous Detection and Quantification of Botryosphaeriaceae Species Causing Canker Diseases in Woody Crops. Plants. 2023; 12(11):2205. https://doi.org/10.3390/plants12112205
Chicago/Turabian StyleRomero-Cuadrado, Laura, Carlos José López-Herrera, Ana Aguado, and Nieves Capote. 2023. "Duplex Real-Time PCR Assays for the Simultaneous Detection and Quantification of Botryosphaeriaceae Species Causing Canker Diseases in Woody Crops" Plants 12, no. 11: 2205. https://doi.org/10.3390/plants12112205
APA StyleRomero-Cuadrado, L., López-Herrera, C. J., Aguado, A., & Capote, N. (2023). Duplex Real-Time PCR Assays for the Simultaneous Detection and Quantification of Botryosphaeriaceae Species Causing Canker Diseases in Woody Crops. Plants, 12(11), 2205. https://doi.org/10.3390/plants12112205








