Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants
Abstract
1. Introduction
2. Results
2.1. Growth Parameters
2.2. Plant Biomass
2.3. Photosynthetic Parameters
2.4. SPAD Value
2.5. Fruit Yield Attributes
2.6. Fruit Quality Parameters
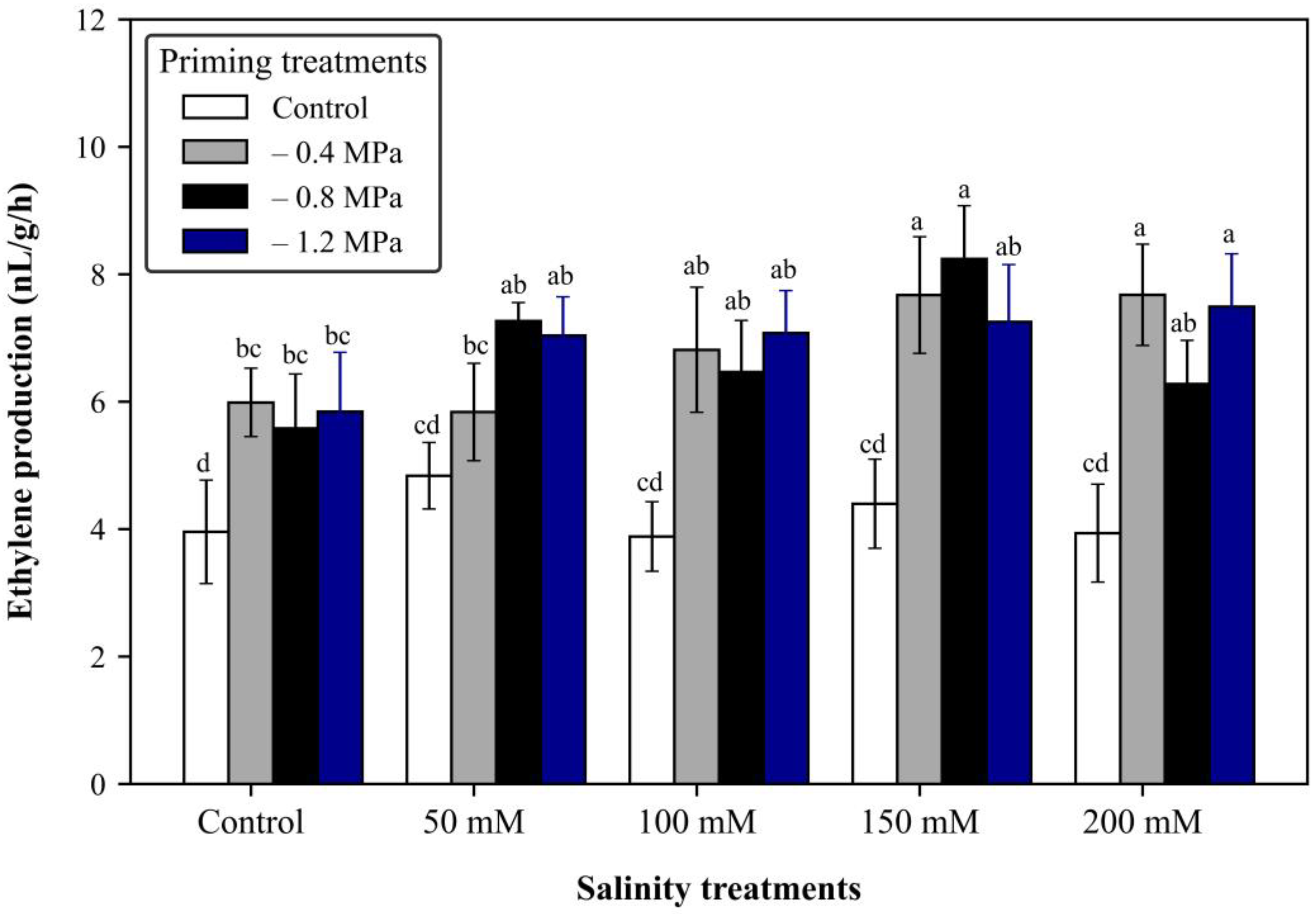
2.7. Fruit Ethylene Production
2.8. Leaf Biochemical Content
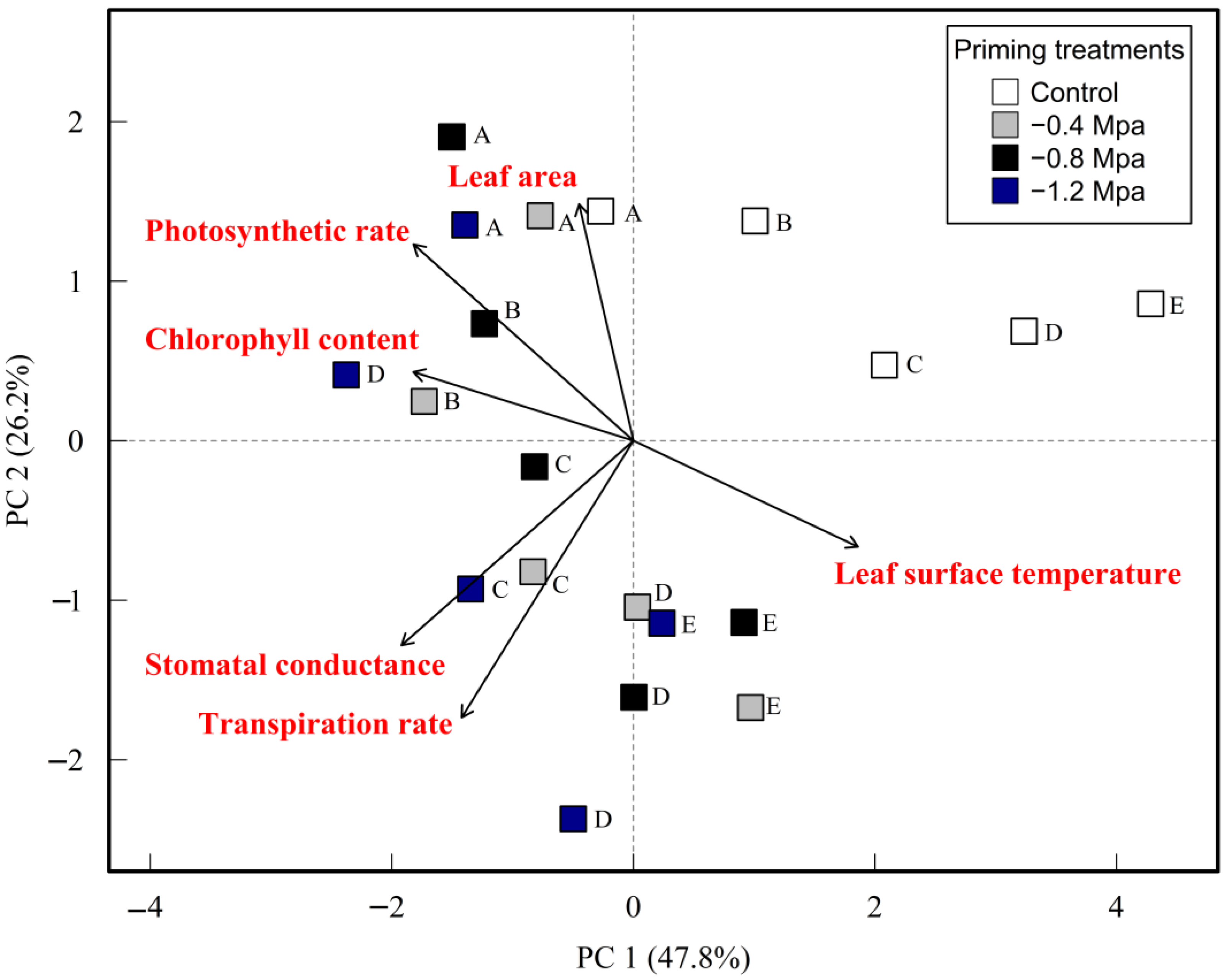
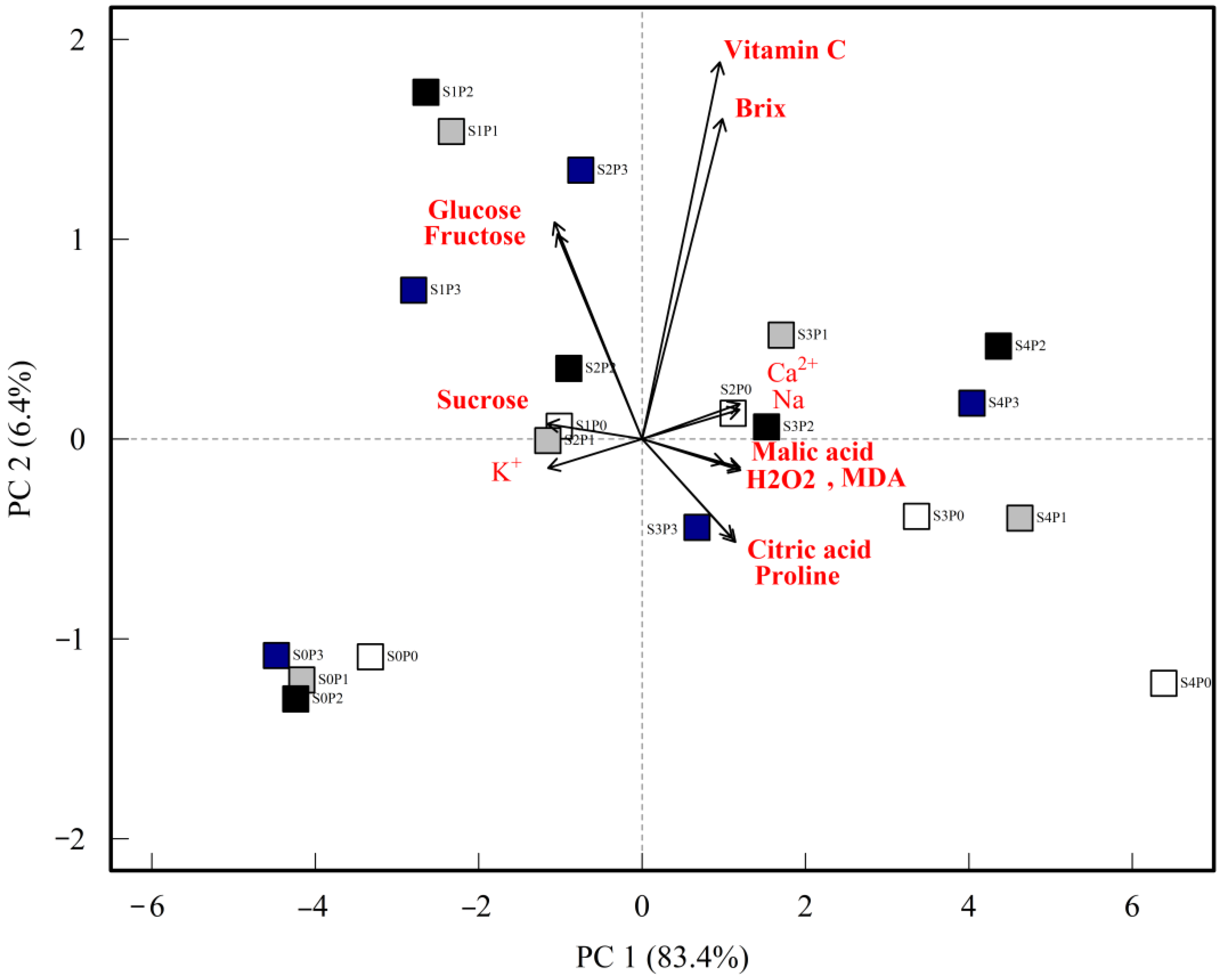
2.9. Principal Component Analysis
2.10. Regression Analysis
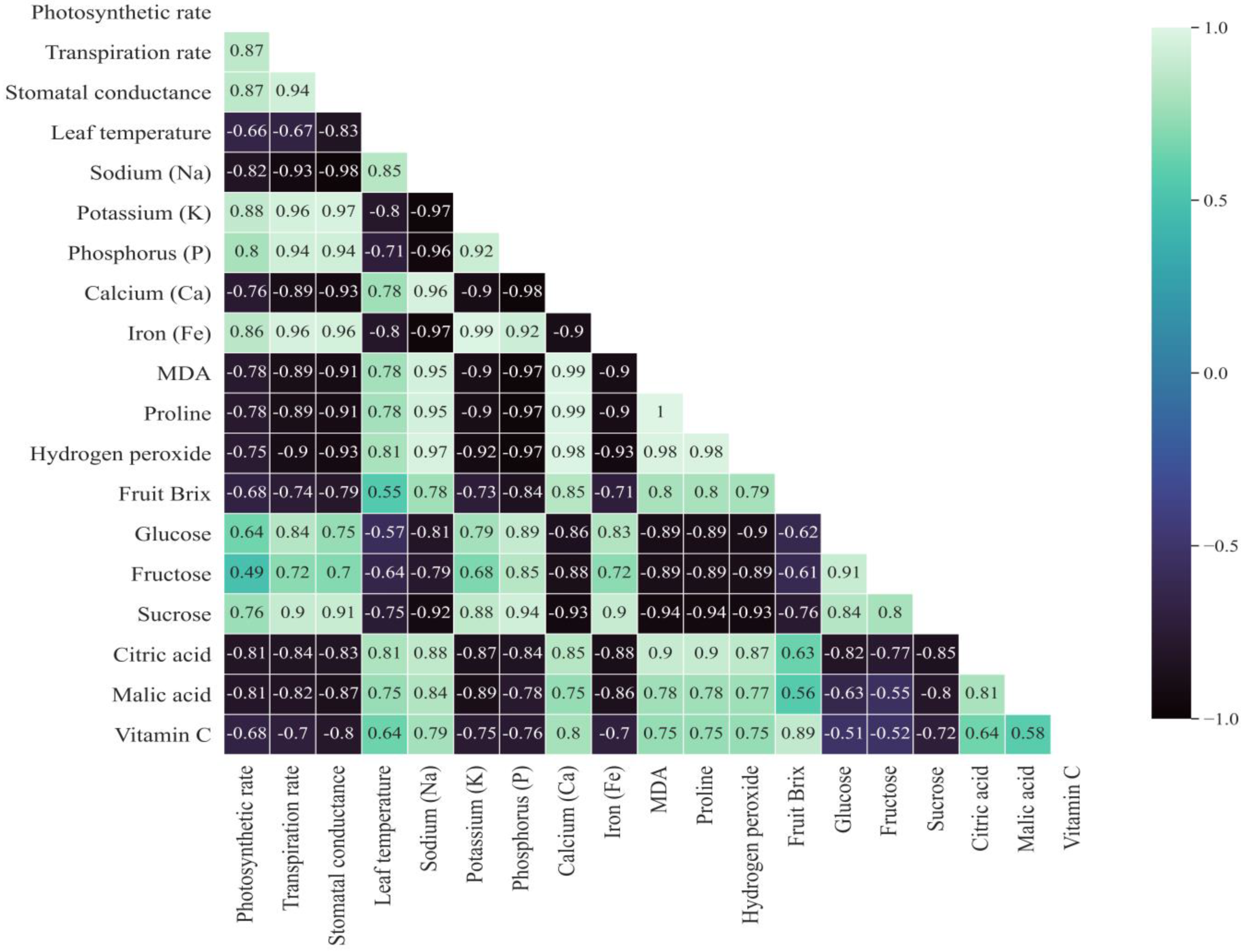
2.11. Correlation Analysis
3. Discussion
3.1. Growth Parameters
3.2. Photosynthesis Attributes
3.3. Fruit Quality and Biochemical Attributes
4. Materials and Methods
4.1. Experimental Site and Design
4.2. Application of Treatments
4.3. Growth Parameters
4.4. Photosynthetic Parameters
4.5. Fruit Yield Parameters
4.6. Quantification of Biochemical Parameters
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanower, H.M.; Sultan, A.S. Present scenario of global salt affected soils, its management and importance of salinity. Int. Res. J. Biol. Sci. Pers. 2019, 2, 263–276. [Google Scholar]
- Jaime, D.P.V. Analytical model for the global consumption of tomatoes. Afr. J. Res. 2021, 7, 33–41. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Map of Salt-Affected Soils; FAO: Roma, Italy, 2021; Volume 3, pp. 1–3. [Google Scholar]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, F.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Sou. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Caro, M.; Cruz, V.; Cuartero, J.; Estan, M. Salinity tolerance of normal-fruited and cherry tomato cultivars. J. Plant Physiol. 1999, 3, 249–255. [Google Scholar] [CrossRef]
- Aloui, H.; Souguir, M.; Latique, S.; Hannachi, C. Germination and growth in control and primed seeds of pepper as affected by salt stress. Cercet. Agron. Mold. 2014, 3, 83–95. [Google Scholar] [CrossRef]
- Kaveh, H.; Hossein, N.; Mohammad, F.; Safieh, V.J. How Salinity Affect Germination and Emergence of Tomato Lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Rahman, M.M.; Hossain, M.; Hossain, K.F.B.; Sikder, M.T.; Shammi, M.; Rasheduzaman, M.; Hossain, M.A.; Alam, A.M.; Uddin, M.K. Effects of NaCl salinity on tomato (Lycopersicum esculentum Mill.). Plant. Exp. 2018, 3, 77–89. [Google Scholar] [CrossRef]
- Hajer, A.S.; Malibari, A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A. Responses of three tomato cultivars to sea water salinity on the seedling growth. Afr. J. Biot. 2006, 5, 855–861. [Google Scholar]
- Kumar, S. Comparative effect of NaCl and PEG on physiological and biochemical attributes during vegetative stage of tomato. Ind. J. Plant Physiol. 2021, 4, 14–23. [Google Scholar]
- Habibi, N.; Sediqui, N.; Naoki, T.; Atsushi, S.; Koshio, K. Effects of salinity on growth, physiological and biochemical responses of tomato. J. Int. Soc. Southeast Asian Agric. Sci. 2021, 27, 14–28. [Google Scholar]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Moaaz Ali, M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Abdul, R.; Babar, S.; Aman, U.; Faisal, N.; Mohsin, T.; Anket, S.; Dong, J.L. Seed priming-mediated improvement of plant morphophysiology under salt stress. In Priming and Pretreatment of Seeds and Seedlings; Springer: Berlin/Heidelberg, Germany, 2019; pp. 205–217. [Google Scholar] [CrossRef]
- Nawaz, A.; Muhammad, A.; Muhammad, M.J.; Samiya, M.K.; Huawei, C.; Jin, H. Induction of salt tolerance in tomato (Lycopersicon esculentum Mill.) seeds through sand priming. Aust. J. Crop. Sci. 2012, 6, 1199–1203. [Google Scholar]
- Theerakulpisut, P.; Lontom, W.; Kulya, J.; Bunnag, S.; Techawongstein, S. Effect of Seed Priming on Physiological Changes in Tomato Grown under Salt Stress. Acta Hort. ISHS 2011, 3, 295–300. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.-S.; Guo, H.-D.; Bai, S.-Y.; Abid, K.; Wang, X.-M.; Gao, Y.-M.; Li, J.-S. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2012, 13, 949541. [Google Scholar] [CrossRef]
- Mirabi, E.; Mahmud, H. Effect of Seed Priming on Some Characteristic of Seedling and Seed Vigor of Tomato (Lycopersicon esculentum). J. Adv. Lab. Res. Biol. 2012, 3, 237–240. [Google Scholar]
- Garcia, D.; Shuo, Z.; Samiah, A.; Yinglei, Z.; Lin, C.M.; Danfeng, H. Seed priming technology as a key strategy to increase crop plant production under adverse environmental conditions. J. Agri. Horti. Res. 2022, 5, 27–46. [Google Scholar] [CrossRef]
- Habibi, N.; Fakoor, M.Y.; Faqiri, M.S.; Sharaf, Z.; Hotak, M.S.; Danishyar, N.; Haris, M.M.; Osmani, S.K.; Shinohara, T.; Terada, N.; et al. Enhancing salinity tolerance in tomatoes at the reproductive stage by increasing pollen viability. Revis Bionatura 2023, 8, 25. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; Pascale, S.D. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.; Cottier, V.; Toquin, V.; Rigoli, G.; Zimmerli, L.; Metraux, J.P.; MauchMani, B. D-Aminobutyric acid-induced resistance in plants. Eur. J. Plant Pathol. 2001, 107, 29–37. [Google Scholar] [CrossRef]
- Abhinandan, K. Abiotic stress signaling in wheat–an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.; Tran, L.S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef]
- Dai, L.Y.; Zhu, H.D.; Yin, K.D.; Du, J.D.; Zhang, Y.X. Seed priming mitigates the effects of saline-alkali stress in soybean seedlings. Chil. J. Agric. Res. 2017, 77, 118–125. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2012, 94, 33–45. [Google Scholar] [CrossRef]
- Pradhan, N.; Prakash, P.; Tiwari, S.K. Osmopriming of tomato genotypes with polyethylene glycol 6000 induces tolerance to salinity stress. J. Plant Gen. 2014, 2, 111–119. [Google Scholar]
- Parra, M.; Albaete, A.; Martinez-Andujar, C.; Peerz-Alfocea, F. Increasing plant vigour and tomato fruit yield under salinity by inducing plant adaptation at the earliest seedling stage. Environ. Exp. Bot. 2007, 60, 77–85. [Google Scholar] [CrossRef]
- Debasis, M.; Rittick, M.; Bahman, K.; Smriti, S.; Pradeep, K.D.M.; Periyasamy, P. Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef]
- Wargent, J.J.; Pickup, D.A.; Paul, N.D.; Roberts, M.R. Reduction of photosynthetic sensitivity in response to abiotic stress in tomato is mediated by a new generation plant activator. J. Plant Biol. 2013, 13, 46–59. [Google Scholar] [CrossRef]
- Biswas, S.; Rasal-Monir, M.; Islam, M.; Modak, S.; Humayun, M.K. Induction of salt tolerance in tomato through seed priming. J. Plants 2019, 7, 47–56. [Google Scholar]
- Nakaune, M.; Atsushi, H.; Yin, Y.-G.; Chiaki, M.; Shinjiro, Y.; Hiroshi, E. Molecular and physiological dissection of enhanced seed germination using short-term low-concentration salt seed priming in tomato. Plant. Physiol. Biochem. 2012, 52, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Balibrea, M.E.; Parra, M.; Bolarin, M.C.; Perez-Alfocca, F. PEG induced tomato adaptation to salt stress. Aus. J. Plant Physiol. 1999, 5, 781–786. [Google Scholar]
- Raziq, A.; Wang, Y.; Mohi Ud Din, A.; Sun, J.; Shu, S.; Guo, S. A Comprehensive Evaluation of Salt Tolerance in Tomato (Var. Ailsa Craig): Responses of Physiological and Transcriptional Changes in RBOH’s and ABA Biosynthesis and Signalling Genes. Int. J. Mol. Sci. 2022, 23, 1603. [Google Scholar] [CrossRef]
- Hussein, M.M.; Nadia, E.L.; Gereadly, H.M.; EL-Desuki, M. Role of Puterscine in Resistance to Salinity of Pea Plants (Pisum sativum L.). J. Appl. Sci. Res. 2006, 2, 598–604. [Google Scholar]
- Rana, R.S. Breeding for salt resistance: Concepts and strategy. Int. J. Trop. Agric. 2018, 3, 236–254. [Google Scholar]
- Pill, W.G.; Frett, J.J.; Morneau, D.C. Germination and Seedling Emergence of Primed Tomato and Asparagus Seeds under Adverse Conditions. HortScience 1991, 26, 1160–1162. [Google Scholar] [CrossRef]
- Anwar, A.; Yu, X.; Li, Y. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef]
- Lin, Y.S.; Medlyn, B.E.; Ellsworth, D.S. Temperature responses of leaf net photosynthesis: The role of component processes. J. Tree Physiol. 2012, 32, 2019–2231. [Google Scholar] [CrossRef]
- Aqvila, D.; Carella, G. Polyethylene glycol 6000 priming effect on germination of aged wheat seed lots. Biol. Plant. 1984, 26, 166–173. [Google Scholar] [CrossRef]
- Cuneyt, U. Effects of Salinity on Seed Germination and Early Seedling Stage. In Abiotic Stress in Plants; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Rowland, M.K.; Patrick, A.N. Cultivation of Tomato under Dehydration and Salinity Stress: Unravelling the Physiology and Alternative Tolerance Options. In Tomato—From Cultivation to Processing Technology; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Berhanu, A.; Yohannes, G. The role of seed priming in improving seedling growth of maize (Zea mays L.) under salt stress at field conditions. Agric. Sci. 2013, 4, 666–672. [Google Scholar] [CrossRef]
- Yohannes, G.; Berhanu, A. The role of seed priming in improving seed germination and seedling growth of maize (Zea mays L.) under salt stress at laboratory conditions. Afr. J. Biotechnol. 2013, 12, 6484–6490. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Halopriming of seeds imparts tolerance to NaCl and PEG induced stress in Vigna radiata (L.) Wilczek varieties. Physiol. Mol. Biol. Plants 2014, 20, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L. SA and PEG-Induced Priming for Water Stress Tolerance in Rice Seedling. In Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2012; Volume 134. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; KhanBangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- El-Saifi, S.; Ahmed, H.; Hasan, S.; Morsi, M.; El-Shatoury, R. Seed priming influences seed germination and seedling growth of tomato under different salinity levels. J. Plant Prod. 2010, 1, 159–170. [Google Scholar] [CrossRef]
- Khan, M.; Shafi, M.; Bakht, J.; Anwar, S. Effect of salinity and seed priming on growth characters of wheat varieties. Sarhad J. Agric. 2017, 33, 435–446. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. lsoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2002, 127, 1781–1787. [Google Scholar] [CrossRef]
- Abdelgawad, F.K.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-faceted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root Respiratory Burst Oxidase Homologue-Dependent H2O2 Production Confers Salt Tolerance on a Grafted Cucumber by Controlling Na+ Exclusion and Stomatal Closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. J. Plant Physiol. 1973, 51, 173–184. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–1511. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.K.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Plant Height (cm) | Root Length (cm) | Leaves Per Plant | Leaf Length (cm) | Leaf Area (cm2) | Branches Per Plant |
|---|---|---|---|---|---|---|
| S0P0 | 13.85 ± 1.81 b–d | 12.70 ± 0.46 b | 26.67 ± 2.07 ab | 6.28 ± 0.47 ab | 5.66 ± 0.25 b–d | 14.00 ± 1.67 b |
| S0P1 | 15.25 ± 1.80 a | 14.20 ± 0.95 ab | 24.00 ± 2.97 a–d | 6.40 ± 0.55 ab | 5.57 ± 0.25 b–e | 15.50 ± 3.73 ab |
| S0P2 | 14.83 ± 1.33 ab | 16.15 ± 0.15 a | 28.17 ± 3.06 a | 6.62 ± 0.49 a | 6.36 ± 0.25 ab | 15.50 ± 3.45 a |
| S0P3 | 14.27 ± 1.56 a–c | 17.93 ± 0.15 a | 25.83 ± 3.60 a–c | 6.85 ± 0.52 a | 5.17 ± 0.25 d–g | 17.17 ± 4.49 a |
| S1P0 | 12.53 ± 1.17 b–e | 11.60 ± 0.75 bc | 21.71 ± 2.45 c–f | 4.66 ± 0.24 b–d | 6.00 ± 0.31 bc | 8.00 ± 0.89 c–f |
| S1P1 | 11.17 ± 0.99 e–h | 13.53 ± 0.45 ab | 22.84 ± 1.74 b–e | 5.16 ± 0.40 b | 5.93 ± 0.27 b–d | 9.00 ± 0.89 c–e |
| S1P2 | 12.70 ± 1.31 a–e | 13.53 ± 0.55 ab | 22.98 ± 2.04 b–e | 4.77 ± 0.26 bc | 6.95 ± 0.31 a | 10.67 ± 0.82 b–d |
| S1P3 | 14.12 ± 1.52 a–d | 12.30 ± 0.72 b | 22.20 ± 2.40 c–f | 4.55 ± 0.27 b–e | 7.14 ± 0.27 a | 11.17 ± 1.17 bc |
| S2P0 | 10.98 ± 0.70 e–h | 9.10 ± 0.20 d | 18.14 ± 3.12 f–h | 3.93 ± 0.19 e–g | 4.14 ± 0.25 hi | 7.83 ± 0.75 c–f |
| S2P1 | 11.93 ± 1.00 c–f | 10.67 ± 0.57 bc | 19.89 ± 1.75 d–h | 4.14 ± 0.28 c–f | 3.71 ± 0.25 i | 8.17 ± 0.75 c–f |
| S2P2 | 12.23 ± 1.25 c–f | 11.00 ± 1.00 bc | 20.60 ± 1.35 d–g | 3.97 ± 0.17 d–g | 4.77 ± 0.25 e–h | 8.83 ± 0.75 c–e |
| S2P3 | 13.25 ± 1.14 a–d | 11.80 ± 0.20 b | 22.91 ± 0.97 b–e | 4.13 ± 0.21 c–f | 4.39 ± 0.25 g–i | 9.17 ± 0.75 c–e |
| S3P0 | 9.75 ± 0.99 gh | 8.73 ± 0.68 d-g | 17.47 ± 1.42 gh | 3.91 ± 0.15 e–g | 5.51 ± 0.31 c–f | 7.00 ± 0.89 ef |
| S3P1 | 10.57 ± 1.58 f–h | 10.37 ± 0.65 bc | 19.07 ± 0.90 e–h | 4.14 ± 0.34 c–f | 5.79 ± 0.27 b–d | 7.67 ± 0.82 c–f |
| S3P2 | 11.58 ± 0.74 d–g | 9.27 ± 0.31 cd | 19.65 ± 0.94 e–h | 3.38 ± 0.24 g | 4.15 ± 0.31 hi | 7.50 ± 0.84 def |
| S3P3 | 11.18 ± 1.28 e–h | 9.93 ± 0.60 cd | 18.06 ± 1.39 f–h | 3.66 ± 0.31 fg | 3.72 ± 0.27 i | 8.00 ± 0.89 c–f |
| S4P0 | 8.85 ± 0.84 h | 7.53 ± 0.68 e | 16.11 ± 0.96 h | 3.57 ± 0.19 gh | 5.72 ± 0.25 b–d | 5.17 ± 0.75 f |
| S4P1 | 11.22 ± 0.81 e–h | 8.10 ± 0.62 de | 18.22 ± 1.38 f–h | 3.83 ± 0.18 fg | 4.02 ± 0.25 hi | 6.33 ± 0.52 ef |
| S4P2 | 10.58 ± 0.91 f–h | 7.90 ± 0.20 de | 17.25 ± 1.15 gh | 3.64 ± 0.41 fg | 4.73 ± 0.25 f–h | 7.00 ± 0.89 ef |
| S4P3 | 10.23 ± 0.65 f–h | 8.77 ± 0.64 de | 17.97 ± 1.17 f–h | 3.81 ± 0.35 fg | 5.96 ± 0.25 b–d | 8.00 ± 0.89 c–f |
| S | ** | *** | *** | *** | *** | *** |
| P | * | ** | *** | ** | *** | *** |
| * | * | * | * | * | * |
| Treatments | Root | Shoot | Leaf | |||
|---|---|---|---|---|---|---|
| FW (g) | DW (g) | FW (g) | DW (g) | FW (g) | DW (g) | |
| S0P0 | 0.53 ± 0.08 a–d | 0.24 ± 0.04 b–d | 7.07 ± 1.32 a | 1.35 ± 0.31 a | 0.48 ± 0.01 a | 0.05 ± 0.01 a–d |
| S0P1 | 0.63 ± 0.07 ab | 0.27 ± 0.07 ab | 5.36 ± 0.99 b–d | 1.07 ± 0.26 a–c | 0.46 ± 0.01 ab | 0.04 ± 0.01 b–e |
| S0P2 | 0.57 ± 0.03 a–c | 0.21 ± 0.04 a–e | 4.58 ± 0.27 b–f | 0.74 ± 0.09 b–f | 0.43 ± 0.01 cd | 0.05 ± 0.01 a–c |
| S0P3 | 0.74 ± 0.06 a | 0.30 ± 0.04 a | 5.40 ± 1.31 b–d | 0.91 ± 0.30 b–e | 0.40 ± 0.01 ef | 0.06 ± 0.02 a |
| S1P0 | 0.51 ± 0.25 b–e | 0.27 ± 0.07 ab | 4.04 ± 0.52 b–f | 0.86 ± 0.12 b–f | 0.46 ± 0.01 a–c | 0.04 ± 0.01 b–d |
| S1P1 | 0.56 ± 0.05 a–c | 0.25 ± 0.08 a–c | 5.55 ± 1.06 a–c | 1.32 ± 0.13 a | 0.44 ± 0.01 bc | 0.05 ± 0.01 a–d |
| S1P2 | 0.26 ± 0.18 c–f | 0.12 ± 0.05 c–f | 1.57 ± 0.20 e–g | 0.75 ± 0.02 b–f | 0.41 ± 0.01 de | 0.05 ± 0.02 a–c |
| S1P3 | 0.15 ± 0.01 f | 0.15 ± 0.09 b–f | 1.41 ± 0.10 e–g | 0.99 ± 0.09 a–d | 0.38 ± 0.01 fg | 0.05 ± 0.03 ab |
| S2P0 | 0.21 ± 0.09 ef | 0.11 ± 0.03 c–f | 3.41 ± 2.23 b–g | 0.67 ± 0.06 d–g | 0.37 ± 0.01 gh | 0.03 ± 0.01 c–g |
| S2P1 | 0.30 ± 0.11 c–f | 0.15 ± 0.05 b–f | 6.26 ± 2.98 ab | 1.09 ± 0.13 ab | 0.36 ± 0.02 g–i | 0.03 ± 0.02 c–g |
| S2P2 | 0.23 ± 0.09 d–f | 0.11 ± 0.01 c–f | 3.62 ± 1.08 b–f | 0.71 ± 0.05 b–f | 0.34 ± 0.01 i–k | 0.04 ± 0.01 b–e |
| S2P3 | 0.39 ± 0.08 b–f | 0.17 ± 0.04 a–f | 4.96 ± 1.73 a–e | 0.68 ± 0.04 c–g | 0.32 ± 0.02 kl | 0.04 ± 0.01 b–d |
| S3P0 | 0.24 ± 0.02 d–f | 0.09 ± 0.03 ef | 1.34 ± 0.16 fg | 0.44 ± 0.03 g | 0.35 ± 0.02 h–j | 0.03 ± 0.01 d–g |
| S3P1 | 0.18 ± 0.09 ef | 0.10 ± 0.04 d–f | 2.28 ± 0.57 c–g | 0.79 ± 0.03 b–f | 0.32 ± 0.01 j–l | 0.03 ± 0.02 c–g |
| S3P2 | 0.16 ± 0.05 ef | 0.08 ± 0.01 ef | 1.94 ± 0.62 d–g | 0.59 ± 0.02 e–g | 0.30 ± 0.02 lm | 0.04 ± 0.01 c–g |
| S3P3 | 0.16 ± 0.04 ef | 0.09 ± 0.03 ef | 1.41 ± 0.59 e–g | 0.77 ± 0.02 b–f | 0.27 ± 0.03 n–p | 0.04 ± 0.02 c–g |
| S4P0 | 0.10 ± 0.03 fg | 0.06 ± 0.01 f | 1.14 ± 0.43 fg | 0.49 ± 0.02 fg | 0.29 ± 0.01 mn | 0.02 ± 0.01 g |
| S4P1 | 0.12 ± 0.07 f | 0.06 ± 0.02 f | 1.27 ± 1.28 fg | 0.68 ± 0.13 c–g | 0.27 ± 0.01 m–o | 0.03 ± 0.03 fg |
| S4P2 | 0.08 ± 0.01 fg | 0.05 ± 0.01 f | 0.84 ± 0.47 g | 0.63 ± 0.02 d–g | 0.26 ± 0.01 op | 0.02 ± 0.01 g |
| S4P3 | 0.13 ± 0.02 ef | 0.06 ± 0.01 f | 0.88 ± 0.05 g | 0.53 ± 0.01 e–g | 0.24 ± 0.01 p | 0.03 ± 0.01 e–g |
| S | *** | ** | ** | ** | *** | *** |
| P | * | * | * | * | * | * |
| ** | * | ** | * | ** | * | |
| Treatments | Pn (μmol CO2 m−2 s−1) | E (μmol CO2 m−2 s−1) | gs (mol CO2 m−2 s−1) | LT (°C) |
|---|---|---|---|---|
| S0P0 | 5.89 ± 0.05 b | 0.37 ± 0.02 c | 0.32 ± 0.02 b | 25.44 ± 1.04 c–e |
| S0P1 | 5.93 ± 0.03 ab | 0.40 ± 0.02 bc | 0.36 ± 0.01 ab | 23.33 ± 0.91 ef |
| S0P2 | 5.99 ± 0.03 ab | 0.43 ± 0.02 ab | 0.38 ± 0.01 a | 22.03 ± 0.74 f |
| S0P3 | 6.03 ± 0.04 a | 0.45 ± 0.01 a | 0.37 ± 0.02 a | 21.37 ± 1.22 f |
| S1P0 | 5.24 ± 0.02 e | 0.31 ± 0.01 d | 0.21 ± 0.01 d | 27.59 ± 0.79 bc |
| S1P1 | 5.55 ± 0.02 cd | 0.37 ± 0.02 c | 0.26 ± 0.01 c | 25.89 ± 1.04 b–e |
| S1P2 | 5.51 ± 0.03 d | 0.40 ± 0.01 bc | 0.27 ± 0.01 c | 25.19 ± 1.04 c–e |
| S1P3 | 5.64 ± 0.03 c | 0.43 ± 0.02 ab | 0.27 ± 0.01 c | 25.55 ± 0.86 b–e |
| S2P0 | 4.60 ± 0.03 l | 0.15 ± 0.01 f | 0.15 ± 0.02 ef | 25.77 ± 0.78 b–e |
| S2P1 | 5.06 ± 0.04 f–h | 0.21 ± 0.03 e | 0.19 ± 0.01 de | 24.01 ± 1.04 d–f |
| S2P2 | 5.02 ± 0.05 g–i | 0.22 ± 0.01 e | 0.20 ± 0.02 d | 23.31 ± 1.04 ef |
| S2P3 | 5.15 ± 0.04 ef | 0.25 ± 0.01 e | 0.21 ± 0.02 d | 23.51 ± 1.04 ef |
| S3P0 | 4.57 ± 0.03 l | 0.15 ± 0.02 f | 0.07 ± 0.02 h | 31.23 ± 0.40 a |
| S3P1 | 5.01 ± 0.05 g–i | 0.21 ± 0.02 e | 0.14 ± 0.01 fg | 28.28 ± 0.40 b |
| S3P2 | 5.98 ± 0.05 hi | 0.23 ± 0.01 e | 0.15 ± 0.01 ef | 27.25 ± 0.66 bc |
| S3P3 | 5.11 ± 0.05 fg | 0.23 ± 0.02 e | 0.15 ± 0.02 ef | 26.45 ± 1.35 b–d |
| S4P0 | 4.39 ± 0.02 m | 0.09 ± 0.01 g | 0.02 ± 0.01 i | 32.27 ± 0.80 a |
| S4P1 | 4.85 ± 0.02 jk | 0.13 ± 0.02 fg | 0.11 ± 0.02 gh | 27.49 ± 0.81 bc |
| S4P2 | 4.82 ± 0.02 k | 0.15 ± 0.01 f | 0.12 ± 0.01 fg | 26.83 ± 0.15 bc |
| S4P3 | 4.95 ± 0.02 ij | 0.14 ± 0.02 f | 0.12 ± 0.02 fg | 27.63 ± 0.67 bc |
| S | *** | *** | *** | *** |
| P | ** | * | ** | ** |
| *** | ** | ** | *** |
| Treatments | Fruit Size (mm) | Fruit Weight (g) | Fruits Per Plant | Yield Per Plant (g) |
|---|---|---|---|---|
| S0P0 | 19.6 ± 1.1 b–d | 9.04 ± 2.00 a | 18.17 ± 4.17 a–c | 63.45 ± 13.68 a–c |
| S0P1 | 19.4 ± 0.9 cd | 8.84 ± 2.05 a | 21.00 ± 3.58 ab | 67.01 ± 11.90 ab |
| S0P2 | 19.0 ± 1.8 cd | 8.44 ± 2.33 ab | 21.00 ± 3.63 ab | 73.87 ± 13.93 a |
| S0P3 | 20.7 ± 0.9 a | 8.07 ± 1.74 a–c | 22.33 ± 5.96 a | 74.61 ± 20.58 a |
| S1P0 | 17.5 ± 0.8 d–h | 6.12 ± 1.82 a–d | 18.67 ± 1.21 a–c | 41.96 ± 13.71 c–f |
| S1P1 | 18.8 ± 0.8 b–e | 6.01 ± 1.88 a–d | 20.17 ± 1.83 ab | 42.78 ± 25.72 c–f |
| S1P2 | 18.3 ± 0.8 c–f | 5.75 ± 1.83 b–e | 21.33 ± 1.63 a | 44.50 ± 14.24 b–e |
| S1P3 | 22.2 ± 1.0 a | 5.05 ± 1.41 c–f | 22.00 ± 1.41 a | 47.34 ± 24.07 b–d |
| S2P0 | 15.3 ± 0.8 h–j | 4.91 ± 2.33 d–f | 16.50 ± 1.05 b–e | 33.74 ± 0.84 d–g |
| S2P1 | 16.5 ± 0.8 e–h | 4.87 ± 2.42 d–f | 17.67 ± 1.51 a–d | 28.65 ± 2.67 d–g |
| S2P2 | 16.0 ± 0.6 f–i | 4.47 ± 0.84 d–g | 18.17 ± 1.60 a–c | 35.00 ± 1.93 d-e |
| S2P3 | 17.7 ± 0.5 d–f | 3.15 ± 0.59 d–h | 18.17 ± 0.75 a–c | 34.95 ± 2.42 d–g |
| S3P0 | 12.2 ± 0.5 kl | 2.82 ± 0.43 e–h | 11.17 ± 0.75 fg | 18.73 ± 2.25 fg |
| S3P1 | 14.0 ± 0.9 i–k | 2.70 ± 0.51 f–h | 13.00 ± 0.63 d–g | 21.95 ± 3.64 e–g |
| S3P2 | 12.8 ± 0.8 kl | 2.45 ± 0.55 f–h | 12.67 ± 0.82 e–g | 20.09 ± 1.21 fg |
| S3P3 | 15.7 ± 0.8 gh | 2.15 ± 0.05 f–h | 14.17 ± 1.47 c–g | 24.29 ± 1.91 d–g |
| S4P0 | 11.2 ± 1.0 l | 2.03 ± 0.23 f–h | 9.83 ± 1.17 g | 16.78 ± 1.03 g |
| S4P1 | 12.2 ± 0.8 kl | 1.62 ± 0.07 gh | 12.50 ± 0.55 e–g | 17.00 ± 1.79 g |
| S4P2 | 13.3 ± 0.8 jk | 1.20 ± 0.13 h | 14.00 ± 0.89 c–g | 17.72 ± 4.14 g |
| S4P3 | 11.7 ± 0.8 kl | 1.18 ± 0.10 h | 15.33 ± 1.03 c–f | 19.77 ± 1.53 fg |
| S | ** | ** | *** | *** |
| P | * | Ns | *** | ** |
| * | * | * | * |
| Treatments | Fruit Color (* a Value) | Fruit Brix (%) | Glucose | Fructose | Sucrose | Citric Acid | Malic Acid | Vitamin C |
|---|---|---|---|---|---|---|---|---|
| S0P0 | 46.54 ± 2.01 bc | 4.20 ± 0.46 i | 51.19 ± 3.56 ab | 72.67 ± 2.50 de | 16.05 ± 1.59 ab | 5.47 ± 0.14 d–f | 3.03 ± 0.31 b–d | 23.83 ± 1.89 f–h |
| S0P1 | 55.54 ± 2.01 b | 4.65 ± 0.25 hi | 46.41 ± 2.72 b–d | 76.57 ± 3.48 cd | 16.61 ± 1.52 a | 5.04 ± 0.13 ef | 2.48 ± 0.42 d | 22.27 ± 1.53 gh |
| S0P2 | 46.75 ± 1.73 bc | 4.83 ± 0.68 g–i | 46.38 ± 2.77 b–d | 77.82 ± 2.80 b–d | 14.26 ± 0.72 b–d | 4.75 ± 0.24 f | 2.37 ± 0.48 d | 21.27 ± 1.42 h |
| S0P3 | 41.55 ± 1.57 c | 4.73 ± 0.21 g–i | 47.34 ± 3.22 bc | 79.41 ± 1.88 a–d | 16.87 ± 1.56 a | 4.71 ± 0.17 f | 2.65 ± 0.21 cd | 21.93 ± 0.65 h |
| S1P0 | 36.52 ± 1.86 d | 6.93 ± 1.00 f–i | 51.74 ± 3.39 ab | 82.08 ± 3.07 a–c | 13.64 ± 1.12 b–e | 6.37 ± 0.44 c–e | 3.88 ± 0.27 a–c | 26.80 ± 2.16 d–g |
| S1P1 | 68.53 ± 1.15 ab | 8.10 ± 1.00 d–f | 57.45 ± 2.14 a | 84.80 ± 2.27 ab | 14.32 ± 1.05 a–d | 5.36 ± 0.35 ef | 2.70 ± 0.41 cd | 30.80 ± 0.20 a–d |
| S1P2 | 58.53 ± 1.15 b | 8.23 ± 0.42 c–f | 56.45 ± 1.84 a | 85.70 ± 3.42 a | 15.75 ± 1.31 ab | 5.05 ± 0.35 ef | 2.65 ± 0.29 cd | 31.40 ± 2.80 a–d |
| S1P3 | 51.30 ± 1.76 bc | 7.40 ± 0.44 d–g | 57.48 ± 2.54 a | 79.66 ± 3.35 a–d | 15.20 ± 2.11 a–c | 4.69 ± 0.49 f | 3.41 ± 0.46 b–d | 27.33 ± 1.85 c–f |
| S2P0 | 42.17 ± 1.01 c | 7.43 ± 0.71 d–g | 41.31 ± 2.62 c–f | 77.64 ± 1.91 b–d | 12.62 ± 0.67 b–e | 6.72 ± 0.54 b–d | 4.06 ± 0.82 ab | 31.00 ± 1.40 a–d |
| S2P1 | 40.32 ± 1.10 c | 8.00 ± 0.95 d–f | 46.99 ± 1.09 bc | 79.95 ± 2.02 a–d | 13.86 ± 0.94 b–e | 5.26 ± 0.33 ef | 3.31 ± 0.35 b–d | 25.00 ± 0.20 e–h |
| S2P2 | 71.26 ± 1.06 a | 7.23 ± 0.50 e–h | 44.67 ± 0.96 b–e | 79.69 ± 1.85 a–d | 13.69 ± 1.06 a–d | 5.64 ± 0.37 d–f | 3.43 ± 0.5 b–d | 28.93 ± 1.47 b–e |
| S2P3 | 61.26 ± 1.06 b | 10.13 ± 0.42 a–c | 44.88 ± 2.38 b–e | 81.64 ± 1.92 a–c | 13.39 ± 1.16 a–d | 5.34 ± 0.34 ef | 3.60 ± 0.52 a–d | 30.33 ± 1.03 b–d |
| S3P0 | 38.67 ± 3.59 d | 9.17 ± 1.45 c–f | 34.16 ± 2.05 fg | 63.52 ± 2.08 f | 11.97 ± 0.23 c–f | 7.89 ± 0.22 ab | 4.07 ± 0.52 ab | 30.53 ± 0.83 b–d |
| S3P1 | 51.16 ± 0.87 bc | 9.70 ± 2.21 a–d | 38.53 ± 1.11 ef | 67.71 ± 2.40 ef | 12.80 ± 1.58 b–e | 5.82 ± 0.37 d–f | 3.30 ± 0.42 b–d | 31.47 ± 0.64 a–c |
| S3P2 | 45.32 ± 1.69 bc | 8.47 ± 0.99 c–f | 39.62 ± 1.54 d–f | 62.32 ± 1.65 f | 11.87 ± 1.31 c–f | 5.52 ± 0.46 d–f | 3.52 ± 0.34 a–d | 30.87 ± 1.40 a–d |
| S3P3 | 74.57 ± 1.14 a | 7.70 ± 0.72 d–f | 40.64 ± 3.56 c–f | 62.16 ± 2.09 f | 12.65 ± 0.61 b–e | 5.44 ± 0.21 d–f | 3.27 ± 0.20 b–d | 28.20 ± 1.59 c–f |
| S4P0 | 44.57 ± 1.14 c | 8.97 ± 0.57 b–f | 26.76 ± 2.09 h | 49.57 ± 2.05 g | 8.59 ± 0.46 f | 8.62 ± 0.39 a | 4.73 ± 0.26 a | 31.93 ± 1.47 a–c |
| S4P1 | 58.22 ± 2.15 b | 10.97 ± 0.38 a–c | 28.64 ± 1.52 gh | 52.16 ± 1.86 g | 11.06 ± 0.91 d–f | 7.36 ± 0.69 bc | 4.11 ± 0.21 ab | 30.73 ± 1.50 a–d |
| S4P2 | 55.27 ± 0.60 b | 11.53 ± 0.57 ab | 28.39 ± 0.71 gh | 49.24 ± 3.75 g | 11.02 ± 1.01 d–f | 7.55 ± 0.62 bc | 3.49 ± 0.33 a–d | 35.33 ± 0.42 a |
| S4P3 | 50.28 ± 1.86 bc | 11.77 ± 1.19 a | 29.37 ± 1.98 gh | 47.97 ± 2.05 g | 10.53 ± 0.64 ef | 7.46 ± 0.94 bc | 3.59 ± 0.35 a–d | 33.00 ± 2.23 ab |
| S | ** | *** | *** | *** | *** | *** | *** | *** |
| P | * | * | ** | * | * | *** | *** | * |
| * | ** | * | * | * | * | * | *** |
| Treatments | MDA | Proline | H2O2 | Electrolyte Leakage (%) |
|---|---|---|---|---|
| S0P0 | 4.45 ± 0.47 kl | 3.64 ± 0.16 f–h | 6.21 ± 0.96 k–m | 26.08 ± 2.31 f–h |
| S0P1 | 3.93 ± 0.17 l | 3.37 ± 0.18 h | 5.26 ± 0.24 lm | 24.15 ± 1.93 h |
| S0P2 | 3.72 ± 0.36 l | 3.39 ± 0.17 h | 4.74 ± 0.37 m | 28.18 ± 2.65 d–f |
| S0P3 | 3.54 ± 0.44 l | 3.23 ± 0.12 h | 4.74 ± 0.53 m | 24.82 ± 2.39 gh |
| S1P0 | 7.74 ± 0.64 g–i | 4.23 ± 0.29 fg | 12.23 ± 2.49 hi | 36.06 ± 2.48 c–e |
| S1P1 | 6.76 ± 0.19 h–j | 3.65 ± 0.07 f–h | 9.14 ± 0.82 i–k | 27.27 ± 1.79 e–g |
| S1P2 | 6.03 ± 0.06 i–k | 3.56 ± 0.06 gh | 8.69 ± 0.54 jk | 30.27 ± 2.10 d–f |
| S1P3 | 5.76 ± 0.40 jk | 3.51 ± 0.08 gh | 8.31 ± 0.43 j–l | 27.71 ± 1.09 e–g |
| S2P0 | 9.84 ± 0.83 ef | 5.46 ± 0.41 b–d | 18.31 ± 1.20 g | 44.31 ± 1.35 a–c |
| S2P1 | 8.04 ± 0.75 gh | 4.34 ± 0.29 ef | 13.30 ± 0.84 h | 32.40 ± 1.73 d–f |
| S2P2 | 8.47 ± 0.48 f–h | 3.89 ± 0.17 f–h | 11.67 ± 0.57 h–j | 34.83 ± 1.99 c–e |
| S2P3 | 8.60 ± 0.45 fg | 3.69 ± 0.24 f–h | 12.35 ± 0.57 hi | 35.81 ± 1.24 c–e |
| S3P0 | 15.09 ± 0.22 c | 5.85 ± 0.25 bc | 27.10 ± 1.04 cd | 48.91 ± 3.77 ab |
| S3P1 | 11.83 ± 0.64 d | 5.66 ± 0.15 b–d | 24.51 ± 1.17 de | 33.97 ± 0.79 c–e |
| S3P2 | 11.52 ± 0.53 de | 5.45 ± 0.09 b–d | 23.60 ± 0.59 ef | 32.22 ± 0.94 d–f |
| S3P3 | 11.82 ± 0.64 d | 5.08 ± 0.06 de | 20.77 ± 1.16 fg | 33.61 ± 1.06 d–f |
| S4P0 | 22.29 ± 0.82 a | 7.07 ± 0.72 a | 35.93 ± 1.59 a | 50.36 ± 1.38 a |
| S4P1 | 19.40 ± 0.99 b | 5.90 ± 0.12 b | 32.90 ± 1.51 ab | 36.23 ± 1.12 c–e |
| S4P2 | 18.33 ± 0.71 b | 5.36 ± 0.21 b–d | 30.36 ± 1.40 bc | 38.53 ± 0.94 b–d |
| S4P3 | 17.75 ± 0.46 b | 5.12 ± 0.13 cd | 28.80 ± 1.13 c | 37.04 ± 0.86 c–e |
| S | *** | *** | *** | *** |
| P | *** | *** | *** | ** |
| ** | *** | ** | *** |
| Treatments | P | Mg | Fe | Zn | |||
|---|---|---|---|---|---|---|---|
| S0P0 | 0.23 ± 0.006 i | 31.52 ± 1.01 bc | 8.46 ± 0.03 c | 1.55 ± 0.015 m | 5.73 ± 0.012 c | 0.25 ± 0.011 ab | 0.019 ± 0.002 h |
| S0P1 | 0.19 ± 0.01 j | 34.56 ± 0.77 ab | 8.63 ± 0.04 a | 1.44 ± 0.010 n | 5.83 ± 0.014 b | 0.26 ± 0.015 ab | 0.008 ± 0.002 ij |
| S0P2 | 0.16 ± 0.005 j | 36.26 ± 1.28 a | 8.56 ± 0.06 ab | 1.38 ± 0.016 o | 5.88 ± 0.012 a | 0.28 ± 0.013 ab | 0.006 ± 0.001 jk |
| S0P3 | 0.17 ± 0.008 j | 34.51 ± 1.30 ab | 8.52 ± 0.03 bc | 1.34 ± 0.018 o | 5.88 ± 0.009 a | 0.28 ± 0.011 a | 0.004 ± 0.001 k |
| S1P0 | 0.32 ± 0.008 f | 27.55 ± 0.78 de | 7.26 ± 0.03 e | 1.94 ± 0.019 j | 5.01 ± 0.012 e | 0.20 ± 0.009 cd | 0.039 ± 0.003 d |
| S1P1 | 0.27 ± 0.002 gh | 31.22 ± 1.15 bc | 7.99 ± 0.03 d | 1.84 ± 0.013 k | 5.09 ± 0.015 d | 0.24 ± 0.010 bc | 0.025 ± 0.002 g |
| S1P2 | 0.26 ± 0.004 g–i | 30.61 ± 0.85 cd | 7.97 ± 0.03 d | 1.81 ± 0.021 kl | 5.12 ± 0.013 d | 0.26 ± 0.011 ab | 0.020 ± 0.001 h |
| S1P3 | 0.24 ± 0.003 hi | 32.54 ± 1.08 bc | 7.99 ± 0.03 d | 1.79 ± 0.014 l | 5.13 ± 0.015 d | 0.27 ± 0.013 ab | 0.010 ± 0.001 i |
| S2P0 | 0.37 ± 0.075 e | 21.53 ± 0.92 hi | 6.38 ± 0.03 h | 2.13 ± 0.017 g | 4.71 ± 0.013 g | 0.12 ± 0.012 gh | 0.048 ± 0.003 c |
| S2P1 | 0.32 ± 0.064 f | 26.34 ± 0.96 ef | 6.53 ± 0.02 g | 2.05 ± 0.015 h | 4.80 ± 0.014 f | 0.18 ± 0.011 d–f | 0.031 ± 0.003 f |
| S2P2 | 0.32 ± 0.008 f | 25.61 ± 1.14 e-g | 6.61 ± 0.02 fg | 2.01 ± 0.015 hi | 4.81 ± 0.015 f | 0.18 ± 0.011 de | 0.025 ± 0.022 g |
| S2P3 | 0.28 ± 0.010 g | 27.55 ± 1.22 de | 6.62 ± 0.03 f | 1.99 ± 0.017 ij | 4.84 ± 0.008 f | 0.20 ± 0.009 cd | 0.018 ± 0.001 h |
| S3P0 | 0.46 ± 0.006 b | 19.57 ± 1.13 ij | 5.35 ± 0.02 j | 2.61 ± 0.016 d | 4.15 ± 0.009 i | 0.08 ± 0.008 i | 0.052 ± 0.004 b |
| S3P1 | 0.40 ± 0.005 de | 22.57 ± 1.34 g–i | 5.85 ± 0.02 i | 2.53 ± 0.013 e | 4.24 ± 0.009 h | 0.13 ± 0.012 gh | 0.035 ± 0.004 e |
| S3P2 | 0.40 ± 0.008 de | 24.65 ± 0.88 e–h | 5.82 ± 0.03 i | 2.48 ± 0.012 ef | 4.26 ± 0.011 h | 0.15 ± 0.014 e–g | 0.031 ± 0.003 f |
| S3P3 | 0.37 ± 0.006 e | 26.69 ± 1.12 ef | 5.80 ± 0.02 i | 2.47 ± 0.011 f | 4.27 ± 0.010 h | 0.19 ± 0.013 de | 0.025 ± 0.003 g |
| S4P0 | 0.52 ± 0.004 a | 16.62 ± 0.85 j | 4.18 ± 0.03 l | 3.23 ± 0.014 a | 3.72 ± 0.015 l | 0.06 ± 0.011 i | 0.062 ± 0.005 a |
| S4P1 | 0.46 ± 0.007 b | 19.58 ± 1.08 ij | 4.36 ± 0.02 k | 3.11 ± 0.018 b | 3.83 ± 0.012 k | 0.10 ± 0.015 hi | 0.041 ± 0.004 d |
| S4P2 | 0.45 ± 0.008 bc | 22.43 ± 1.28 g–i | 4.40 ± 0.03 k | 3.06 ± 0.017 bc | 3.85 ± 0.013 jk | 0.13 ± 0.011 gh | 0.031 ± 0.004 f |
| S4P3 | 0.42 ± 0.009 cd | 23.62 ± 1.20 f–h | 4.39 ± 0.02 k | 3.05 ± 0.021 c | 3.88 ± 0.013 j | 0.14 ± 0.013 fg | 0.027 ± 0.003 g |
| S | *** | *** | *** | *** | *** | *** | *** |
| P | *** | *** | *** | *** | *** | *** | *** |
| * | ** | *** | * | * | * | *** |
| Abbreviations | Salinity | Priming |
|---|---|---|
| S0P0 | 0 mM | 0 MPa |
| S0P1 | 0 mM | −0.4 MPa |
| S0P2 | 0 mM | −0.8 MPa |
| S0P3 | 0 mM | −1.2 MPa |
| S1P0 | 50 mM | 0 MPa |
| S1P1 | 50 mM | −0.4 MPa |
| S1P2 | 50 mM | −0.8 MPa |
| S1P3 | 50 mM | −1.2 MPa |
| S2P0 | 100 mM | 0 MPa |
| S2P1 | 100 mM | −0.4 MPa |
| S2P2 | 100 mM | −0.8 MPa |
| S2P3 | 100 mM | −1.2 MPa |
| S3P0 | 150 mM | 0 MPa |
| S3P1 | 150 mM | −0.4 MPa |
| S3P2 | 150 mM | −0.8 MPa |
| S3P3 | 150 mM | −1.2 MPa |
| S4P0 | 200 mM | 0 MPa |
| S4P1 | 200 mM | −0.4 MPa |
| S4P2 | 200 mM | −0.8 MPa |
| S4P3 | 200 mM | −1.2 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, N.; Aryan, S.; Amin, M.W.; Sanada, A.; Terada, N.; Koshio, K. Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants. Plants 2023, 12, 2187. https://doi.org/10.3390/plants12112187
Habibi N, Aryan S, Amin MW, Sanada A, Terada N, Koshio K. Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants. Plants. 2023; 12(11):2187. https://doi.org/10.3390/plants12112187
Chicago/Turabian StyleHabibi, Nasratullah, Shafiqullah Aryan, Mohammad Wasif Amin, Atsushi Sanada, Naoki Terada, and Kaihei Koshio. 2023. "Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants" Plants 12, no. 11: 2187. https://doi.org/10.3390/plants12112187
APA StyleHabibi, N., Aryan, S., Amin, M. W., Sanada, A., Terada, N., & Koshio, K. (2023). Potential Benefits of Seed Priming under Salt Stress Conditions on Physiological, and Biochemical Attributes of Micro-Tom Tomato Plants. Plants, 12(11), 2187. https://doi.org/10.3390/plants12112187









