Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions
Abstract
1. Introduction
2. Feeding and Nutritive Value
2.1. Metabolizable Energy
2.2. Protein and Nitrogen
| Halophytes | % Nitrogen | % Protein | References |
|---|---|---|---|
| A. saligna | 2 | 14 | [17] |
| A. brevifolia | 1.6 | 9 | [22] |
| A. halimus | 2 | 13 | |
| A. nummularia | 2 | 13 | |
| A. repanda | 3 | 21 | [23] |
| H. ammodendron | 2 | 14 | |
| H. salicornicum | 2 | 17 | [24] |
| H. strobilaceum | 1 | 7 | |
| K. foliatum | 3 | 19 | [25] |
| K. caspica | 2 | 12 | |
| L. chilense | 2 | 13 | |
| P. communis | 2 | 12 | [26] |
| S. tetranda | 1 | 7 | |
| R. soongorica | 1 | 10 | [7] |
| S. foliosa | 3 | 17 | |
| S. fruticosa | 2 | 12 | |
| T. mannifera | 1 | 7 | [18] |
| T. crinita | 1 | 10 | |
| Z. album | 1 | 6 |
2.3. Sulfur
2.4. Minerals
2.5. Organic Acids
2.6. Antioxidants
3. Halophytes and Secondary Metabolites
3.1. Phenolic Compounds
3.2. Nitrates, Saponins, and Mimosine
| Halophytes | Flavonoids (%) | Nitrates (%) | Saponins (%) | Oxalates (%) | Phenols (%) | Net Tannins (%) | Condensed Tannins (%) | References |
|---|---|---|---|---|---|---|---|---|
| A. stocksii | 0.705 | 0.659 | 0.589 | 0.841 | - | - | - | [45] |
| Acacia nilotica | 0.487 | 0.102 | 0.531 | 0.481 | 70 | 15 | 1 | |
| Toona cililate | 4 | 2.3 | 0.9 | |||||
| Avicennia marina | 0.604 | 0.567 | 4.638 | 1.621 | 16 | - | - | [15] |
| Chenopodium album | 0.281 | 0.321 | 0.447 | 0.631 | - | - | - | [46] |
| Salsola kali | - | - | - | - | 3 | - | 1.9 | [47] |
| Conocarpus erectus | 0.472 | 0.691 | 2.221 | 0.721 | - | - | - | [48] |
| Bauhinia variegate | - | - | - | - | 5 | 3.7 | 3.4 | |
| Convolvulus arvensis | 0.859 | 0.379 | 0.702 | 0.721 | - | - | - | [22] |
| Phoenix acaulis | - | - | - | - | 6 | 4.8 | 4.3 | |
| Cressa cretica | 0.572 | 0.393 | 0.665 | 0.721 | 9.5 | |||
| Anogeissus latifolia | 17.5 | 16 | 0.4 | [49] | ||||
| Enicostemma hyssopifolium | 0.991 | 0.392 | 1.351 | 1.441 | - | - | - | [39] |
| Carrisa spinarum | - | - | - | - | 6.6 | 4.5 | 4.6 | |
| Haloxylon stocksii | 0.427 | 0.345 | 2.112 | 2.341 | [50] | |||
| Ougenia oojeiuealis | - | - | - | - | 4.2 | 2.9 | 2.6 | |
| Heliotropium bacciferum | 0.455 | 0.587 | 1.825 | 0.541 | - | - | - | |
| Indigofera cordifolia | 0.563 | 0.318 | 0.536 | 0.641 | - | - | - | [16] |
| Leucaena leucocephala | - | - | - | - | 5 | 2.1 | 0.8 | |
| Indigofera oblongifolia | 0.683 | 0.458 | 7.284 | 0.781 | - | - | - | [51] |
| Ipomoea pes-caprae | 1.491 | 0.691 | 3.849 | 1.321 | - | - | - | |
| Suaeda fruticose | - | - | - | - | 32 | - | 1.5 | [9] |
| Launaea resedifolia | 0.899 | 0.557 | 1.723 | 1.621 | - | - | - | |
| Leucas urticifolia | 0.302 | 0.577 | 1.011 | 0.841 | - | - | - | |
| Prosopis cineraria | 1.113 | 0.224 | 1.324 | 0.361 | - | - | - | [16] |
| Prosopis glandulosa | 0.755 | 0.356 | 1.693 | 0.721 | - | - | - | |
| Prosopis juliflora | 0.579 | 0.282 | 2.069 | 0.481 | - | - | - | |
| Salsola imbricata | 0.147 | 0.197 | 0.732 | 1.211 | 6 | - | - | [52] |
| Salvadora oleoides | 0.164 | 0.481 | 0.726 | 1.381 | - | - | - | |
| Thespesia populnea | 0.305 | 0.256 | 8.684 | 1.511 | - | - | - | |
| Zaleya pentandra | 0.253 | 1.152 | 2.268 | 0.661 | - | - | - |
| Plants | Ruminant | Level of Inclusion (g kg−1) | Decline in CH4 Content | Effect on Other Parameters | References |
|---|---|---|---|---|---|
| Acacia mearnsii | Sheep | 41 | 9.90% | 23% reduction in tannin and 20% in monensin | [53] |
| Cattle | 9 | 31% | Digestibility reduced | ||
| H. coronarium | Cows | Sole feed | 2.35% | - | [54] |
| Lespedeza cuneata | Goats | Sole feed | 51.40% | Digestibility and protozoa numbers decreased Total volatile fatty acid unaffected | [55] |
| Quebracho tannins | Beef cattle | 10–20 | No effect | No effect on digestibility Total volatile fatty acid decreased | |
| Lotus pedunculatus | Sheep | As sole feed | No effect | No effect | [56] |
| Lespedeza striata | Goats | As sole feed | 32.9–58.4% | Digestibility and protozoal numbers decreased Total volatile fatty acid unaffected | [57] |
3.3. Alkaloids and Glucosinolates
3.4. Voluntary Feed Intake (Fiber and Salt)
3.5. Toxins
3.6. Relative Palatability
4. Biomass Production and Growth Potential of Halophytes under Saline Water or Saline Soils
5. Approaches for Feeding Value Improvement of Halophytes
6. Effect of Halophytic Fodder on Animal Performances
6.1. Animal Meat Quality and Halophytic Fodder
6.2. Wool Production
6.3. Milk Production and Quality
7. Nutritional Management and Better Use of Agro-Industrial Byproducts
7.1. Agro-Industrial By-Products Ensiling and Feed Blocks
7.2. Agro-Industrial Byproducts-Based Pellets
7.3. Role of Molasses and Other Amendments Mixture with Halophytic Fodder to Increase Animal Palatability
8. Constraints on Halophyte Consumption
8.1. Ash Content and Lignification Factor
8.2. Plant Secondary Metabolites
8.3. Alleviation of the Undesirable Secondary Compounds in Fodder
9. Concluding Remarks and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in Improving Plant Salinity Resistance and Use of Salinity Resistant Plants for Economic Sustainability. Crit. Rev. Environ. Sci. Technol. 2021, 52, 2150–2196. [Google Scholar] [CrossRef]
- Hasnain, M.; Abideen, Z.; Anthony Dias, D.; Naz, S.; Munir, N. Utilization of Saline Water Enhances Lipid Accumulation in Green Microalgae for the Sustainable Production of Biodiesel. BioEnergy Res. 2022, 16, 1026–1039. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous Fertilizers: Impact on Environment Sustainability, Mitigation Strategies, and Challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Kakarla, R.; Choi, J.-W.; Yun, J.-H.; Kim, B.-H.; Heo, J.; Lee, S.; Cho, D.-H.; Ramanan, R.; Kim, H.-S. Application of High-Salinity Stress for Enhancing the Lipid Productivity of Chlorella sorokiniana HS1 in a Two-Phase Process. J. Microbiol. 2018, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Chowardhara, B.; Awasthi, J.P.; Panda, S.K.; Panigrahi, K.C.S. Salinity Stress and Plant Secondary Metabolite Enhancement: In An Overview. Biotechnological Approaches to Enhance Plant Secondary Metabolites; Shahnawaz, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 49–60. [Google Scholar]
- Calone, R.; Bregaglio, S.; Sanoubar, R.; Noli, E.; Lambertini, C.; Barbanti, L. Physiological Adaptation to Water Salinity in Six Wild Halophytes Suitable for Mediterranean Agriculture. Plants 2021, 10, 309. [Google Scholar] [CrossRef]
- Tıpırdamaz, R.; Karakas, S.; Dikilitas, M. Halophytes and the Future of Agriculture. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M. A Comprehensive Phytochemical, Biological, Toxicological and Molecular Docking Evaluation of Suaeda fruticosa (L.) Forssk.: An Edible Halophyte Medicinal Plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef]
- Scopel, B.R.; de Miranda, P.H.O.; da Silva, R.S.; de Oliveira Alves, J.V.; de Veras, B.O.; Amorim, L.C.; da Rosa, M.M.; de Souza barbosa, J.I.; de Melo, M.R.C.S.; de Souza Bezerra, R. Biological Activities and Chemical Profile from Batis maritima (Bataceae), a Halophyte Species with Bioprospecting Potential. Res. Soc. Dev. 2021, 10, e391101623726. [Google Scholar] [CrossRef]
- Attia-Ismail, S.A. Halophytes as Forages. In New Perspectives in Forage Crops; Loiola Edvan, R., Rocha Bezerra, L., Eds.; IntechOpen: London, UK, 2018; pp. 69–87. [Google Scholar]
- Badri, M.; Ludidi, N. Halophytes as a Resource for Livestock in Africa: Present Status and Prospects: Present Status and Prospects. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Badri, M., Luddi, N., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 1–17. [Google Scholar]
- Elahi, M.Y.; Khusro, A.; Elghnadour, M.M.Y.; Salem, A.Z.M.; López, S. Ruminal Fermentation Kinetics of Nine Halophytic Tree Species at Different Growth Stages. Agrofor. Syst. 2019, 93, 1843–1852. [Google Scholar] [CrossRef]
- Friha, M.; Hamdi, H.; Ayeb, N.; Hajlaoui, A.; Durand, D.; Majdoub-Mathlouthi, L. Potential Use of Natural Saline Pasture for Grazing Lambs: Effect on Digestibility, Growth Performances, Carcass and Meat Quality. Small Rumin. Res. 2022, 210, 106669. [Google Scholar] [CrossRef]
- Attia-Ismail, S.A. Nutritional and Feed Value of Halophytes and Salt Tolerant Plants. In Halophytic Salt-Tolerant Feed; El Shaer, H.M., Squires, V.R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 348–357. [Google Scholar]
- Attia-Ismail, S.A. Nutrient Feasibility of Halophytic Feed Plants. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Badri, M., Luddi, N., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 1–17. [Google Scholar]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Atia, A.; Debez, A.; Rabhi, M.; Barhoumi, Z.; Haouari, C.C.; Gouia, H.; Abdelly, C.; Smaoui, A. Salt Tolerance and Potential Uses for Saline Agriculture of Halophytes from the Poaceae. In Sabkha Ecosystems; Springer: Dordrecht, The Netherlands, 2019; pp. 223–237. [Google Scholar]
- Dawei, Z.; Vu, T.S.; Huang, J.; Chi, C.Y.; Xing, Y.; Fu, D.D.; Yuan, Z.N. Effects of Calcium on Germination and Seedling Growth in Melilotus officinalis L. (Fabaceae) under Salt Stress. Pak. J. Bot 2019, 51, 1–9. [Google Scholar]
- Mausz, M.A.; Airs, R.L.; Dixon, J.L.; Widdicombe, C.E.; Tarran, G.A.; Polimene, L.; Dashfield, S.; Beale, R.; Scanlan, D.J.; Chen, Y. Microbial uptake dynamics of choline and glycine betaine in coastal seawater. Limnol. Oceanogr. 2022, 672, 1052–1064. [Google Scholar] [CrossRef]
- Abdurzakova, A.S.; Astamirova, M.A.M.; Magomadova, R.S.; Israilova, S.A.; Khanaeva, K.R.; Khasueva, B.A.; Umaeva, A.M.; Shakhgirieva, Z.I. Halophytes of Tersko-Kumsk Lowland Area, Their Protection and Rational Use. KnE Life Sci. 2019, 4, 1129–1138. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-Plant Interaction and Detoxification Strategies under Abiotic Stresses for Achieving Agricultural Resilience: A Critical Review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.A.; Hakeem, K.R. Biomass Production of Various Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Badri, M., Luddi, N., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 1–13. [Google Scholar]
- Centofanti, T.; Bañuelos, G. Practical Uses of Halophytic Plants as Sources of Food and Fodder. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; pp. 324–342. [Google Scholar]
- El-Shaer, H.M. Potentiality of Halophytes as Animals Fodders under Arid Conditions of Egypt. Ferchichi A.(comp.), Ferchichi a.(collab.). In Rehabilitation des Paturages et des Parcours en Milieu Mediterraneens; CIHEAM: Zaragoza, Spain, 2004; pp. 369–374. [Google Scholar]
- Yasin Ashraf, M.; Awan, A.R.; Anwar, S.; Khaliq, B.; Malik, A.; Ozturk, M. Economic Utilization of Salt-Affected Wasteland for Plant Production: A Case Study from Pakistan. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Cham, Switzerland, 2020; pp. 1–24. [Google Scholar]
- Wessels, A.G. Influence of the gut microbiome on feed intake of farm animals. Microorganisms 2022, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Altay, V.; Güvensen, A. Sustainable Use of Halophytic Taxa as Food and Fodder: An Important Genetic Resource in Southwest Asia. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Cham, Switzerland, 2019; pp. 235–257. [Google Scholar]
- Stevanovic, Z.; Stankovic, M.S.; Stankovic, J.; Janackovic, P.; Stankovic, M. Use of Halophytes as Medicinal Plants: Phytochemical Diversity and Biological Activity. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; pp. 289–343. [Google Scholar]
- Gouda, M.S.; Elsebaie, E.M. Glasswort (Salicornia spp.) as a Source of Bioactive Compounds and Its Health Benefits: A Review. Alex. J. Food. Sci. Technol 2016, 13, 1–7. [Google Scholar]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Lüttge, U. Elimination of Salt by Recretion: Salt Glands and Gland-Supported Bladders in Recretohalophytes. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; pp. 223–239. [Google Scholar]
- Li, H.; Xu, C.; Han, L.; Li, C.; Xiao, B.; Wang, H.; Yang, C. Extensive Secretion of Phenolic Acids and Fatty Acids Facilitates Rhizosphere PH Regulation in Halophyte Puccinellia tenuiflora under Alkali Stress. Physiol. Plant. 2022, 174, e13678. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with Metal Toxicity–Cues from Halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Zheng, L.; Nguyen, H.; Ni, L.; Song, S.; Sui, Y. Antioxidant Responses of Triangle Sail Mussel Hyriopsis cumingii Exposed to Toxic Microcystis aeruginosa and Thermal Stress. Sci. Total Environ. 2020, 743, 140754. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.A.A.; Al-Omar, M.S.; Al Rugaie, O.; Abdellatif, A.A.H. Comparative Phytochemical Profile and Biological Activity of Four Major Medicinal Halophytes from Qassim Flora. Plants 2021, 10, 2208. [Google Scholar] [CrossRef]
- Eissa, M.A. Effect of Compost and Biochar on Heavy Metals Phytostabilization by the Halophytic Plant Old Man Saltbush [Atriplex nummularia Lindl]. Soil Sediment Contam. Int. J. 2019, 28, 135–147. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in Biosaline Agriculture: Mechanism, Utilization, and Value Addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Ishtiyaq, S.; Kumar, H.; Varun, M.; Ogunkunle, C.O.; Paul, M.S. Role of Secondary Metabolites in Salt and Heavy Metal Stress Mitigation by Halophytic Plants. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 307–327. [Google Scholar]
- Kewan, K.Z.; Elkhouly, A.A.; Negm, A.M.; Javadi, A. Feedstock Values of Some Common Fodder Halophytes in the Egyptian Desert. In Proceedings of the 22nd International Water Technology Conference, IWTC22 Ismailia, Ismailia, Egypt, 12–13 September 2019; pp. 12–13. [Google Scholar]
- Baysal, I.; Ekizoglu, M.; Ertas, A.; Temiz, B.; Agalar, H.G.; Yabanoglu-Ciftci, S.; Temel, H.; Ucar, G.; Turkmenoglu, F.P. Identification of Phenolic Compounds by LC-MS/MS and Evaluation of Bioactive Properties of Two Edible Halophytes: Limonium effusum and L. dinuatum. Molecules 2021, 26, 4040. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as Source of Bioactive Phenolic Compounds and Their Potential Applications. Crit. Rev. Food Sci. Nutr. 2021, 63, 1078–1101. [Google Scholar] [CrossRef]
- Ebrahim, H.; Negussie, F. Effect of Secondary Compounds on Nutrients Utilization and Productivity of Ruminant Animals: A Review. J. Agric. Sci. Pr. 2020, 5, 60–73. [Google Scholar]
- Attia-Ismail, S.A. Plant Secondary Metabolites: Deleterious Effects, Remediation. In Plants, Pollutants and Remediation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 157–178. [Google Scholar]
- El Shaer, H.M. Halophytes and Salt-Tolerant Plants as Potential Forage for Ruminants in the Near East Region. Small Rumin. Res. 2010, 91, 3–12. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Domange, C.; Broudiscou, L.P.; Sauvant, D.; Berthelot, V. Rumen Function in Goats, an Example of Adaptive Capacity. J. Dairy Res. 2020, 87, 45–51. [Google Scholar] [CrossRef]
- Riley, J.J. Review of Halophyte Feeding Trials with Ruminants. In Halophytic Salt-Tolerant Feed; CRC Press: Boca Raton, FL, USA, 2015; pp. 177–217. [Google Scholar]
- Nefzaoui, A.; Salem, H.B.; El Mourid, M. Innovations in Small Ruminants Feeding Systems in Arid Mediterranean Areas. In New Trends for Innovation in the Mediterranean Animal Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–116. [Google Scholar]
- Masters, D.G. Assessing the Feeding Value of Halophytes. In Halophytic and Salt Tolerant Feedstuffs: Impacts on Nutrition, Physiology and Reproduction of Livestock; CRC Press: New York, NY, USA, 2015; pp. 89–105. [Google Scholar]
- Salem, H.B.; Makkar, H.P.S.; Nefzaoui, A. Towards Better Utilisation of Non-Conventional Feed Sources by Sheep and Goats in Some African and Asian Countries. Options Méditerranéennes Série A 2004, 59, 177–187. [Google Scholar]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in Animal Nutrition: A Review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Molina-Botero, I.C.; Arroyave-Jaramillo, J.; Valencia-Salazar, S.; Rosales, R.B.; Aguilar-Pérez, C.F.; Burgos, A.A.; Arango, J.; Ku-Vera, J.C. Effects of Tannins and Saponins Contained in Foliage of Gliricidia Sepium and Pods of Enterolobium cyclocarpum on Fermentation, Methane Emissions and Rumen Microbial Population in Crossbred Heifers. Anim. Feed Sci. Technol. 2019, 251, 1–11. [Google Scholar] [CrossRef]
- Faustino, M.V.; Faustino, M.A.F.; Pinto, D.C.G.A. Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. Int. J. Mol. Sci. 2019, 20, 1067. [Google Scholar] [CrossRef] [PubMed]
- Junior, F.P.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of Tannins-Rich Extract from Acacia mearnsii or monensin as Feed Additives on Ruminal Fermentation Efficiency in Cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in Forage Plants and Their Role in Animal Husbandry and Environmental Sustainability: A Review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Muir, J.P.; Lambert, B.D.; Kothmann, M.M. Effect of Molecular Weight of Condensed Tannins from Warm-Season Perennial Legumes on Ruminal Methane Production in Vitro. Biochem. Syst. Ecol. 2013, 50, 154–162. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane Production from in Vitro Rumen Incubations with Lotus pedunculatus and Medicago sativa, and Effects of Extractable Condensed Tannin Fractions on Methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Cardoso-Gutierrez, E.; Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Castelán-Ortega, O.A.; Chay-Canul, A.J.; Foggi, G.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; González-Ronquillo, M. Effect of Tannins from Tropical Plants on Methane Production from Ruminants: A Systematic Review. Vet. Anim. Sci. 2021, 14, 100214. [Google Scholar] [CrossRef]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Kumar, A.; Chand, G. Halophytes: The Plants of Therapeutic Medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 271–287. [Google Scholar]
- Amirifar, A.; Hemati, A.; Asgari Lajayer, B.; Pandey, J.; Astatkie, T. Impact of Various Environmental Factors on the Biosynthesis of Alkaloids in Medicinal Plants. In Environmental Challenges and Solutions; Springer: Cham, Switzerland, 2022; pp. 229–248. [Google Scholar]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Khan, M.A.; Duke, N.C. Halophytes-A Resource for the Future. Wetl. Ecol. Manag. 2001, 9, 455–456. [Google Scholar] [CrossRef]
- Dynes, R.A.; Schlink, A.C. Livestock Potential of Australian Species of Acacia. Conserv. Sci. West. Aust. 2002, 4, 117–124. [Google Scholar]
- Cock, I.E. Medicinal and Aromatic Plants-Australia; EOLSS Publishers: Oxford, UK, 2011. [Google Scholar]
- Aganga, A.A.; Tshwenyane, S.O. Feeding Values and Anti-Nutritive Factors of Forage Tree Legumes. Pak. J. Nutr. 2003, 2, 170–177. [Google Scholar]
- Squires, V.R.; El Shaer, H.M. Global Distribution and Abundance of Sources of Halophytic and Salt Tolerant Feedstuffs. In Halophytic and Salt-Tolerant Feedstuffs; CRC Press: New York, NY, USA, 2015; pp. 3–20. [Google Scholar]
- Nascimento, T.V.C.; Oliveira, R.L.; Menezes, D.R.; de Lucena, A.R.F.; Queiroz, M.A.Á.; Lima, A.; Ribeiro, R.D.X.; Bezerra, L.R. Effects of Condensed Tannin-Amended Cassava Silage Blend Diets on Feeding Behavior, Digestibility, Nitrogen Balance, Milk Yield and Milk Composition in Dairy Goats. Animal 2021, 15, 100015. [Google Scholar] [CrossRef]
- Barry, T.N.; McNeill, D.M.; McNabb, W.C. Plant Secondary Compounds; Their Impact on Forage Nutritive Value and upon Animal Production. In Proceedings of the XIX International Grassland Congress São Pedro, São Paulo, Brazil, 11–21 February 2001. [Google Scholar]
- Moehlenpah, A.N.; Ribeiro, L.P.S.; Puchala, R.; Goetsch, A.L.; Beck, P.; Pezeshki, A.; Gross, M.A.; Holder, A.L.; Lalman, D.L. Water and Forage Intake, Diet Digestibility, and Blood Parameters of Beef Cows and Heifers Consuming Water with Varying Concentrations of Total Dissolved Salts. J. Anim. Sci. 2021, 99, 1–10. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards Saving Freshwater: Halophytes as Unconventional Feedstuffs in Livestock Feed: A Review. Environ. Sci. Pollut. Res. 2018, 25, 14397–14406. [Google Scholar] [CrossRef]
- Anon, M. Introduction of Salt-Tolerant Forage Production Systems to Salt-Affected Lands in Sinai Peninsula in Egypt: A Pilot Demonstration Project; Final Report; DRC: Cairo Governorate, Egypt; ICBA: Dubai, United Arab Emirates, 2009. [Google Scholar]
- Masters, D.G.; Benes, S.E.; Norman, H.C. Biosaline Agriculture for Forage and Livestock Production. Agric. Ecosyst. Environ. 2007, 119, 234–248. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ahmed, M.Z.; Gul, B.; Khan, M.A. Moderate salinity stimulates growth and photosynthesis of Phragmites karka by water relations and tissue specific ion regulation. Environ. Exp. Bot. 2014, 105, 70–76. [Google Scholar] [CrossRef]
- Katoch, R. Nutritional Quality of Major Forage Grasses of Himalayan Region. In Nutritional Quality Management of Forages in the Himalayan Region; Springer: Singapore, 2022; pp. 279–308. [Google Scholar]
- Dear, B.S.; Ewing, M.A. The Search for New Pasture Plants to Achieve More Sustainable Production Systems in Southern Australia. Aust. J. Exp. Agric. 2008, 48, 387–396. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Marinucci, M.T.; Lupi, P.; Conte, G. Milk Fatty Acid Composition, Rumen Microbial Population, and Animal Performances in Response to Diets Rich in Linoleic Acid Supplemented with Chestnut or Quebracho Tannins in Dairy Ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef]
- Ballard, R.A.; Peck, D.M. Sensitivity of the Messina (Melilotus siculus)–Sinorhizobium medicae Symbiosis to Low pH. Crop Pasture Sci. 2021, 72, 754–761. [Google Scholar] [CrossRef]
- Shehata, M.F. Feeding Camels on Halophytic Plants and Their Effects on Meat Quality Characteristics and Products. In Management and Development of Agricultural and Natural Resources in Egypt’s Desert; Springer: Berlin/Heidelberg, Germany, 2021; pp. 517–532. [Google Scholar]
- Bonanno, A.; Di Miceli, G.; Di Grigoli, A.; Frenda, A.S.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of Feeding Green Forage of Sulla (Hedysarum coronarium L.) on Lamb Growth and Carcass and Meat Quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Landau, S.; Or, D.; Kababya, D.; Bruckental, I.; Nitsan, Z. Analytical Approach and Effects of Condensed Tannins in Carob Pods (Ceratonia siliqua) on Feed Intake, Digestive and Metabolic Responses of Kids. Livest. Sci. 2006, 99, 29–38. [Google Scholar] [CrossRef]
- de Matos Teixeira, A.; Junior, G.D.; Velasco, F.O.; de Faria Júnior, W.G.; Rodriguez, N.M.; Rodrigues, J.A.; McAllister, T.; Gonçalves, L.C. Intake and Digestibility of Sorghum (Sorghum bicolor, L. Moench) Silages with Different Tannin Contents in Sheep. Rev. Bras. Zootec. 2014, 43, 14–19. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of Quebracho Tannin Extract on Intake, Digestibility, Rumen Fermentation, and Methane Production in Crossbred Heifers Fed Low-Quality Tropical Grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Exploring the Benefits of Feeding Tannin Containing Diets for Enhancing the Nutritional Values of Milk and Meat of Ruminants. Indian J. Anim. Health 2014, 53, 63–76. [Google Scholar]
- Sun, H.X.; Zhong, R.Z.; Liu, H.W.; Wang, M.L.; Sun, J.Y.; Zhou, D.W. Meat Quality, Fatty Acid Composition of Tissue and Gastrointestinal Content, and Antioxidant Status of Lamb Fed Seed of a Halophyte (Suaeda glauca). Meat Sci. 2015, 100, 10–16. [Google Scholar] [CrossRef]
- Rashid, M.; Archimède, H. Response of Lactating Blackbelly Ewes to Fed Pellets of Leucaena leucocephala Leaves with Dry Banana Fruits. In Proceedings of the Caribbean Science and Innovation Meeting; HAL Université des Antilles: French West Indies, France, 2019. [Google Scholar]
- AlSuwaiegh, S.B.; Almotham, A.M.; Alyousef, Y.M.; Mansour, A.T.; Al-Sagheer, A.A. Influence of Functional Feed Supplements on the Milk Production Efficiency, Feed Utilization, Blood Metabolites, and Health of Holstein Cows during Mid-Lactation. Sustainability 2022, 14, 8444. [Google Scholar] [CrossRef]
- Beck, M.R.; Al-Marashdeh, O.; Gregorini, P. Low Levels of a Seaweed (Ecklonia radiata) Extract Alter in Vitro Fermentation Products but Not in Combination with Quebracho (Schinopsis quebracho-colorado) Tannins. Appl. Anim. Sci. 2019, 35, 476–481. [Google Scholar] [CrossRef]
- Cabiddu, A.; Molle, G.; Decandia, M.; Spada, S.; Fiori, M.; Piredda, G.; Addis, M. Responses to Condensed Tannins of Flowering Sulla (Hedysarum coronarium L.) Grazed by Dairy Sheep: Part 2: Effects on Milk Fatty Acid Profile. Livest. Sci. 2009, 123, 230–240. [Google Scholar] [CrossRef]
- Morales, R.; Ungerfeld, E.M. Use of Tannins to Improve Fatty Acids Profile of Meat and Milk Quality in Ruminants: A Review. Chil. J. Agric. Res. 2015, 75, 239–248. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential Use of Olive By-Products in Ruminant Feeding: A Review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Vera, N.; Gutiérrez-Gómez, C.; Williams, P.; Allende, R.; Fuentealba, C.; Ávila-Stagno, J. Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In vitro Rumen Fermentation Parameters. Animals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Amelchanka, S.L.; Kreuzer, M.; Leiber, F. Utility of Buckwheat (Fagopyrum esculentum Moench) as Feed: Effects of Forage and Grain on in Vitro Ruminal Fermentation and Performance of Dairy Cows. Anim. Feed Sci. Technol. 2010, 155, 111–121. [Google Scholar] [CrossRef]
- Colombini, S.; Broderick, G.A.; Galasso, I.; Martinelli, T.; Rapetti, L.; Russo, R.; Reggiani, R. Evaluation of Camelina sativa (L.) Crantz Meal as an Alternative Protein Source in Ruminant Rations. J. Sci. Food Agric. 2014, 94, 736–743. [Google Scholar] [CrossRef]
- Denninger, T.M.; Schwarm, A.; Birkinshaw, A.; Terranova, M.; Dohme-Meier, F.; Muenger, A.; Eggerschwiler, L.; Bapst, B.; Wegmann, S.; Clauss, M. Immediate Effect of Acacia mearnsii Tannins on Methane Emissions and Milk Fatty Acid Profiles of Dairy Cows. Anim. Feed Sci. Technol. 2020, 261, 114388. [Google Scholar] [CrossRef]
- Torkashvand, N.; Soltan Dalal, M.M.; Mousivand, M.; Hashemi, M. Canola Meal and Tomato Pomace as Novel Substrates for Production of Thermostable Bacillus Subtilis T4b Xylanase with Unique Properties. Biomass Convers. Biorefin. 2020, 12, 3373–3385. [Google Scholar] [CrossRef]
- Onte, S.; Bhattacharjee, S.; Arif, M.; Dey, D. Non-Conventional Feed Resources. Agriallis 2019, 1, 1–35. [Google Scholar]
- Mishra, B.; Varjani, S.; Karthikeya Srinivasa Varma, G. Agro-Industrial by-Products in the Synthesis of Food Grade Microbial Pigments: An Eco-Friendly Alternative. In Green Bio-Processes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 245–265. [Google Scholar]
- Meral, R.; Kose, Y.E.; Ceylan, Z.; Cavidoglu, İ. The Potential Use of Agro-Industrial by-Products as Sources of Bioactive Compounds: A Nanotechnological Approach. Stud. Nat. Prod. Chem. 2022, 73, 435–466. [Google Scholar]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N. Nutritive Value of Some Agro-Industrial by-Products for Ruminants-A Review. World J. Zool 2008, 3, 40–46. [Google Scholar]
- Moore, R. Principles of Animal Nutrition; Scientific e-Resources: Singapore, 2018; ISBN 1839474475. [Google Scholar]
- Meneses, M.; Martínez-Marín, A.L.; Madrid, J.; Martínez-Teruel, A.; Hernández, F.; Megías, M.D. Ensilability, in Vitro and in Vivo Values of the Agro-Industrial by-products of Artichoke and Broccoli. Environ. Sci. Pollut. Res. 2020, 27, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.B.; Znaidi, I.-A. Partial Replacement of Concentrate with Tomato Pulp and Olive Cake-Based Feed Blocks as Supplements for Lambs Fed Wheat Straw. Anim. Feed Sci. Technol. 2008, 147, 206–222. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M. Reviewing the Benefits of Grazing/Browsing Semiarid Rangeland Feed Resources and the Transference of Bioactivity and Pro-Healthy Properties to Goat Milk and Cheese: Obesity, Insulin Resistance, Inflammation and Hepatic Steatosis Prevention. Animals 2021, 11, 2942. [Google Scholar] [CrossRef]
- Pinto Filho, J.S.; Cunha, M.V.; Souza, E.J.O.; Santos, M.V.F.; Lira, M.A.; Moura, J.G.; Rodrigues, J.; Silva, C.S. Performance, Carcass Features, and Non-Carcass Components of Sheep Grazed on Caatinga Rangeland Managed with Different Forage Allowances. Small Rumin. Res. 2019, 174, 103–109. [Google Scholar] [CrossRef]
- Rudiger, U.; El Ayed, M.; Idoudi, Z.; Frija, A.; Nefzaoui, A.; Gharsi, M. Nutrient-Dense Livestock Feed Pellets Manufactured in Tunisia Can Compete with Imported Feed Concentrates. Bus. Model Brief. 2021. [Google Scholar]
- Collado, R.; Monedero, E.; Casero-Alonso, V.M.; Rodríguez-Aragón, L.J.; Hernández, J.J. Almond Shells and Exhausted Olive Cake as Fuels for Biomass Domestic Boilers: Optimization, Performance and Pollutant Emissions. Sustainability 2022, 14, 7271. [Google Scholar] [CrossRef]
- Gihad, E.A.; Shaer, H.M.E.L. Utilization of Halophytes by Livestock on Rangelands Problems and Prospects. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Squires, V.R., Ayoub, A.T., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 1994; pp. 77–96. [Google Scholar]
- El Shaer, H.M. Potential Use of Halophytes and Salt-Tolerant Forages as Animal Feed in the Arab Region: An Overview. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 2479–2498. [Google Scholar]
- Shellito, S.M.; Ward, M.A.; Lardy, G.P.; Bauer, M.L.; Caton, J.S. Effects of Concentrated Separator By-Product (Desugared Molasses) on Intake, Ruminal Fermentation, Digestion, and Microbial Efficiency in Beef Steers Fed Grass Hay. J. Anim. Sci. 2006, 84, 1535–1543. [Google Scholar] [CrossRef]
- Preston, T.R.; Sansoucy, R.; Aarts, G. Molasses as Animal Feed: An Overview; Sugarcane as Feed, FAO Animal Production and Health Papers 72. In Proceeding of the FAO Expert Consultation, St. Domingo, Dominic Repub, 7–11 July 1986. [Google Scholar]
- Alsersy, H.; Salem, A.Z.M.; Borhami, B.E.; Olivares, J.; Gado, H.M.; Mariezcurrena, M.D.; Yacuot, M.H.; Kholif, A.E.; El-dawy, M.; Hernandez, S.R. Effect of M Editerranean Saltbush (Atriplex halimus) Ensilaging with Two Developed Enzyme Cocktails on Feed Intake, Nutrient Digestibility and Ruminal Fermentation in Sheep. Anim. Sci. J. 2015, 86, 51–58. [Google Scholar] [CrossRef]
- Dagar, J.C.; Sharma, P.C.; Chaudhari, S.K.; Jat, H.S.; Ahamad, S. Climate Change Vis-a-Vis Saline Agriculture: Impact and Adaptation Strategies. In Innovative Saline Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 5–53. [Google Scholar]
- Attia-Ismail, S.A. Factors Limiting and Methods of Improving Nutritive and Feeding Values of Halophytes in Arid, Semi-Arid and Coastal Areas. In Proceedings of the International Conference on Biosaline Agriculture & High Salinity Tolerance, Mugla, Turkey, 9–14 January 2005; pp. 9–14. [Google Scholar]
- Polizel, D.M.; Marques, S.S.; Westphalen, M.F.; Gouvea, V.N.; de Castro Ferraz Júnior, M.V.; Miszura, A.A.; Barroso, J.P.R.; Limede, A.C.; Ferreira, E.M.; Pires, A.V. Narasin Inclusion for Feedlot Lambs Fed a Diet Containing High Amounts of Ground Flint Corn. Sci. Agric. 2020, 78. [Google Scholar] [CrossRef]
- Selim, E.; Fisun, K.O.C.; Özdüven, L.; Eseceli, H.; Evren, C.; Karadağ, H. In Situ and In Vitro Nutritive Value Assessment of Styrax officinalis L. as an Alternative Forage Source for Goat Feeding. J. Agric. Sci. 2022, 28, 181–188. [Google Scholar]
- Petrie, J.R.; Zhou, X.-R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W. Development of a Brassica napus (Canola) Crop Containing Fish Oil-like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, G.H.; Pirasteh-Anosheh, H. Comparison of the Accumulation of Elements, Ash Content and Biomass of Some Halophytes Species under Irrigating with Sea Water. Desert Manag. 2020, 7, 63–74. [Google Scholar]
- EL-Saadany, S.A.; Omar, H.H. Effect of Feeding Barki Ewesby Halophytic Plants on the Gross Chemical Composition, Elements Content and Anti-Oxidants Compounds. J. Food Dairy Sci. 2018, 9, 403–409. [Google Scholar] [CrossRef]
- Abd El Tawab, A.M.; Khattab, M.S.A. Utilization of Polyethylene Glycol and Tannase Enzyme to Reduce the Negative Effect of Tannins on Digestibility, Milk Production and Animal Performance. Asian J. Anim. Vet. Adv. 2018, 13, 201–209. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]

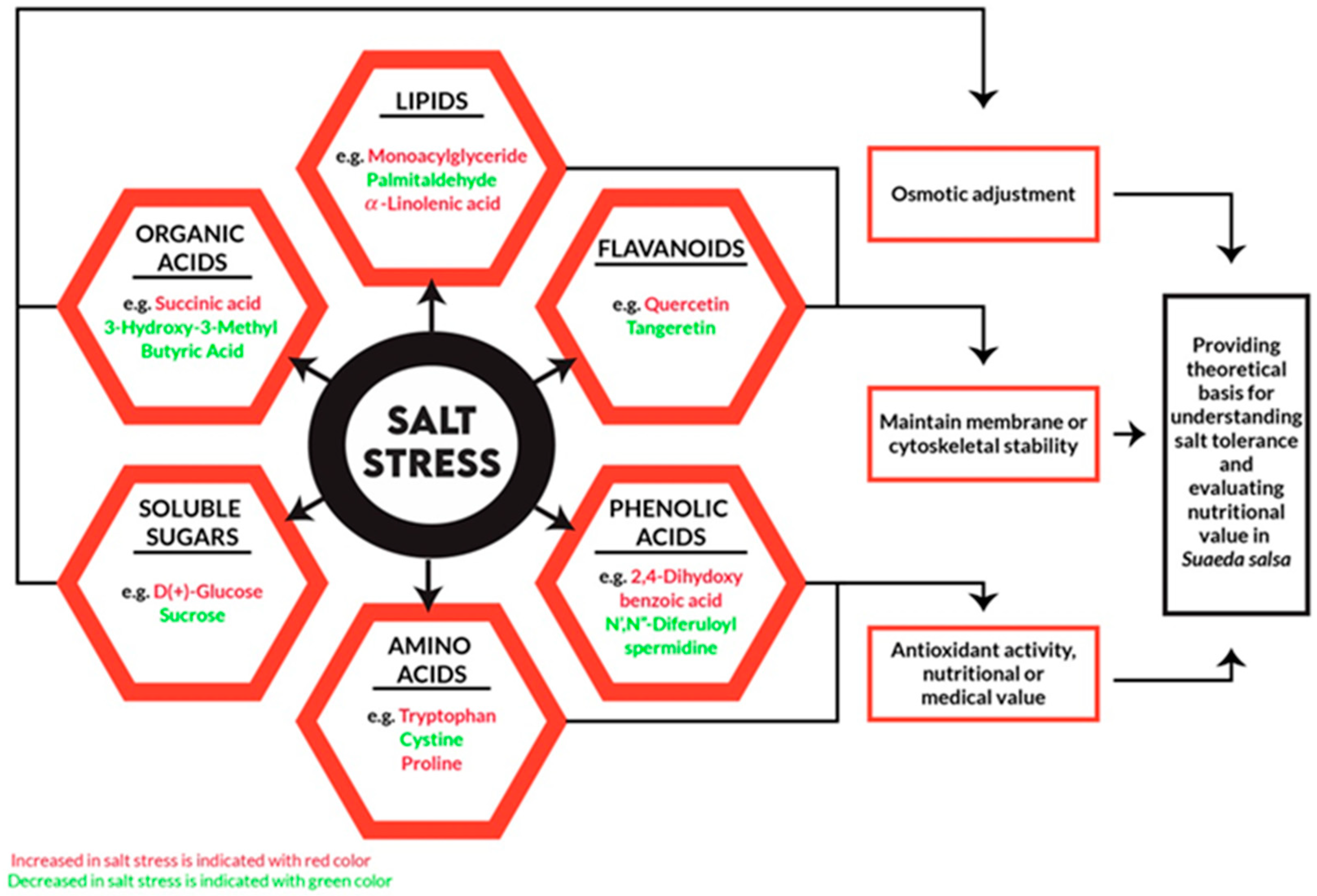
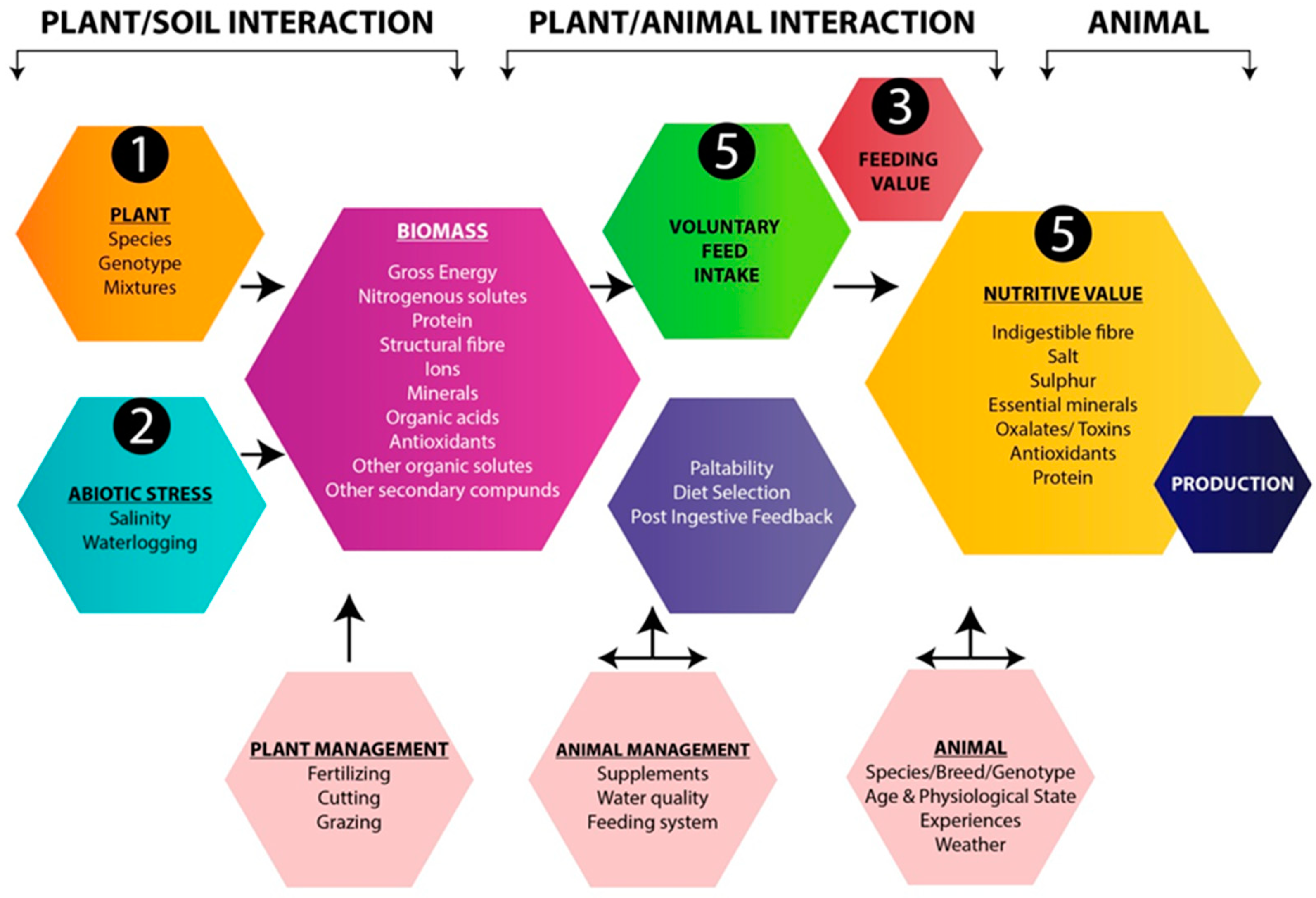
| Ruminant | Thiocyanate Glucosinolate (mol g−1 Diet) | Effect on Ruminants |
|---|---|---|
| Calves | 2.5 | No effect one thyroid function and liver |
| Steers | 14 | No effect on growth and feed renovation |
| Cows | 12 | Prompted iodine deficit |
| 25 | Feed intake and milk production decrease | |
| ≥24 | ||
| 32 | Thyroid disruption and fertility reduction | |
| Sheep | 2.3 | Weight reduction |
| 16 | Growth Reduction | |
| 18 | Thyroid weight increment | |
| 34 | Growth reduction | |
| <4 | No effect | |
| ≥4 | Prompted iodine deficit and affected thyroid weight and histology | |
| 1.7 | Reduction in estradiol causing reproductive disorders |
| Halophytes | Plant Part | Toxins | References |
|---|---|---|---|
| A. aneura | Phyllode | Oxalate | [43] |
| Tannin | |||
| A. burrowii | Flowers | Hydrogen cyanide | |
| A. cambagei | Phyllode | Hydrogen cyanide | |
| Timber, Bark | Oxalate | ||
| A. decora | Browse | Abortive agent | [62] |
| A. deanei | Browse | Hydrogen cyanide | |
| A. cana | Browse | Selenium | |
| A. doratoxylon | Browse | Cyanogenic glycoside | [63] |
| A. longifolia | Browse | Hydrogen cyanide | |
| A. georgina | Seeds, Pods | Fluoroacetate | [64] |
| Browse | Hyrolytic enzyme only |
| Halophytes | Goats | Camels | Sheep | References |
|---|---|---|---|---|
| Acacia albida | PP | HP | PP | [15] |
| A. elbaica | PP | HP | PP | |
| A. mellifera | FP | HP | PP | |
| A. reddiana | PP | HP | FP | [27] |
| Acacia tortilis | FP | HP | NP | |
| Arocnemom glaucum | -- | HP | NP | [65] |
| Astragalus eremophilus | -- | HP | NP | |
| Avicennia marina | HP | HP | NP | |
| Blepharic ciliaris | -- | HP | HP | [16] |
| Cadaba farinose | PP | HP | HP | |
| Cadaba oblonifia | HP | PP | HP | [27] |
| Calligonum comosum | HP | HP | NP | [15] |
| Convolvulus hvstrix | PP | HP | HP | [48] |
| Halopeplis prefaliala | HP | HP | HP | |
| Heliotropium leuteum | HP | HP | HP | [27] |
| Indigofera spinosa | PP | HP | NP | |
| Leptadenia pyrotechnica | HP | FP | PP | |
| Lycium shawii | HP | HP | PP | [50] |
| Maerua crassifolia | HP | HP | HP | |
| Ochradenus baccatus | HP | HP | HP | [66] |
| Panicum turgidum | HP | HP | HP | |
| Pergularia tomentosa | HP | HP | FP | |
| Plantago ciliate | FP | HP | HP | [67] |
| Salsola baryosma | FP | HP | FP | [68] |
| Leptadenia pyrotechnica | FP | PP | -- | |
| Suaeda monaica | HP | HP | PP | [69] |
| Taverniera aegyptiaca | FP | FP | HP | [16] |
| Trichodesma ehrenbergu | HP | HP | -- | |
| Zygophyllum coccineum | PP | HP | HP |
| Tannin Source | Dosage (DM CT) | Period (Days) | Effects | References |
|---|---|---|---|---|
| Hedysarum coronarium | 1.8% | 63 | Increase linoleic acid | [79] |
| Ceratonia siliqua | 2.7% | 45 | Increase rumenic acid and linoleic acid, Reduce n-3 FA | [80] |
| Sorghum bicolor | 1.7–3.5% | 103–123 | No effect in muscle FA Composition | [81] |
| S. quebracho | 4.0% | 60 | Increase t10-18:1, total trans-18:1 and PUFA Reduce SFA | [82] |
| Acacia mearnsii | 14.1% | 260–283 | Reduce rumenic acid | [83] |
| Juniperus pinchotii | 3.1–4.4% | 86 | Increase SFA, rumenic acid and Δ-9 Desaturase index | |
| Terminalia chebula | 0.6–1.8% | 90 | Increase rumenic acid, MUFA and linoleic acid |
| Treatments | Control | T1 | T2 | T3 |
|---|---|---|---|---|
| Slaughter weight (kg) | 28 | 29 | 31 | 30 |
| Carcass weight (kg) | 10 | 11.5 | 11 | 12 |
| Dressing (%) | 38.80 | 40.29 | 38.02 | 38.89 |
| pH (24 h) | 5.75 | 5.60 | 5.55 | 5.65 |
| C10:0 | 0.12 | 0.16 | 0.17 | 0.11 |
| C12:0 | 0.22 | 0.27 | 0.43 | 0.20 |
| C14:0 | 3.06 | 3.68 | 4.75 | 2.83 |
| C16:0 | 21.59 | 24.96 | 25.46 | 23.13 |
| C16:1 | 1.08 | 1.37 | 1.23 | 1.00 |
| C18:0 | 19.95 | 21.00 | 20.73 | 24.90 |
| C18:1 cis-9 | 33.62 | 34.22 | 30.47 | 30.48 |
| C18:1 trans-11 | 1.16 | 1.63 | 1.69 | 2.24 |
| C18:2 | 0.29 | 0.40 | 0.30 | 0.44 |
| C18:2 n−6 | 8.78 | 6.25 | 7.95 | 8.34 |
| C18:3 | 0.03 | 0.03 | 0.04 | 0.04 |
| C18:3 | 0.43 | 0.49 | 0.51 | 0.52 |
| C20:0 | 0.15 | 0.13 | 0.19 | 0.17 |
| C20:1 | 0.04 | 0.04 | 0.06 | 0.06 |
| C20:3 | 0.28 | 0.17 | 0.18 | 0.19 |
| C20:4 | 4.56 | 2.40 | 2.93 | 2.50 |
| C22:6 n−3 | 0.14 | 0.10 | 0.10 | 0.10 |
| Other fatty acid | 4.47 | 2.66 | 2.69 | 2.71 |
| PUFA | 14.42 | 9.88 | 12.11 | 12.17 |
| SFA | 46.86 | 50.19 | 51.67 | 51.34 |
| P:S | 0.30 | 0.19 | 0.20 | 0.23 |
| n-6 | 13.66 | 8.86 | 11.10 | 11.07 |
| n-3 | 0.55 | 0.61 | 0.62 | 0.63 |
| n-6/n-3 ratio | 14.6 | 18.5 | 17.5 | 1.72 |
| Tannin Source | Dosage (DM CT) | Duration (Days) | Effects | References |
|---|---|---|---|---|
| Schinopsis quebracho-colorado | 70% | 27 | Fatty acids profile of milk remains the same | [87] |
| Hedysarum coronarium | 2.7% | 56 | Increases linoleic acid and milk fat content, as well as a reduction in vaccenic acid, rumenic acid, and milk urea content | [88] |
| Lens culinaris | 74% | 50 | Reduction in linoleic acid, oleic and stearic acids, and milk fat content | [89] |
| Olea europaea | 94% | 50 | Reduction in linoleic, oleic, and stearic acids, as well as energy-corrected milk yield | [90] |
| S. balansae | 3% | 21 | Reduce milk urea content Increase linoleic acid | [91] |
| F. esculentum | 82% | 26 | Increase linoleic acid Reduce vaccenic acid and t10-18:1 | [92] |
| C. sativa | 50% | 30 | Fatty acids profile of milk remains the same | [93] |
| S. quebracho | 2% | 30 | [69] | |
| A. mearnsii | 400 g day−1CT | 25 | [94] |
| Basal Diet | Supplements | Growth Rate (g Day −1) | Cost Reduction | References |
|---|---|---|---|---|
| Stubble browsing | Conc. (250 g day−1) | 96 | −81% | [49] |
| Conc. (150 g day−1), wheat bran (10%), olive cake (40%), poultry litter (25%), bentonite (20%), salt (5%) | 137 | −80% | ||
| Wheat straw | Conc. (500 g day−1) | 64 | −81% | [102] |
| Conc. (125 g day−1) + Wheat bran (25%), wheat flour (15%), olive cake (30%), rapeseed meal (10%), urea (4%), quicklime (8%), salt (5%), minerals (1%) | 67 | −12% | ||
| Straw (310 g day−1) | Conc. (800 g day−1) | 121 | −11% | [47] |
| Conc. (300 g day−1) + Wheat bran (28%), barley (10%), molasses (44%), sesames hull (5%), white cement (5%), minerals (3%), urea (5%) | 110 | −11% | ||
| Fresh Acacia leaves | Wheat bran (28%), olive cake (38%), wheat flour (11%), quicklime (12%), salt (5%), minerals (1%), urea (5%) | 15 | −11% | [103] |
| Wheat bran (23%), olive cake (31.2%), wheat flour (9%), quicklime (9.9%), salt (4.1%), minerals (0.8%), urea (4.1%), PEG (18%) | 62 | −11% | ||
| Rangeland browsing | Conc. (300 g day−1) | 26 | −11% | [104] |
| Wheat bran (28%), olive cake (38%), wheat flour (11%), quicklime (12%), salt (5%), minerals (1%), urea (5%) | 40 | −11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnain, M.; Abideen, Z.; Ali, F.; Hasanuzzaman, M.; El-Keblawy, A. Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions. Plants 2023, 12, 2150. https://doi.org/10.3390/plants12112150
Hasnain M, Abideen Z, Ali F, Hasanuzzaman M, El-Keblawy A. Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions. Plants. 2023; 12(11):2150. https://doi.org/10.3390/plants12112150
Chicago/Turabian StyleHasnain, Maria, Zainul Abideen, Faraz Ali, Mirza Hasanuzzaman, and Ali El-Keblawy. 2023. "Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions" Plants 12, no. 11: 2150. https://doi.org/10.3390/plants12112150
APA StyleHasnain, M., Abideen, Z., Ali, F., Hasanuzzaman, M., & El-Keblawy, A. (2023). Potential of Halophytes as Sustainable Fodder Production by Using Saline Resources: A Review of Current Knowledge and Future Directions. Plants, 12(11), 2150. https://doi.org/10.3390/plants12112150








