Factors Affecting the Germination Ecology of Pimelea trichostachya and Its Relationship to Field Emergence
Abstract
1. Introduction
2. Results
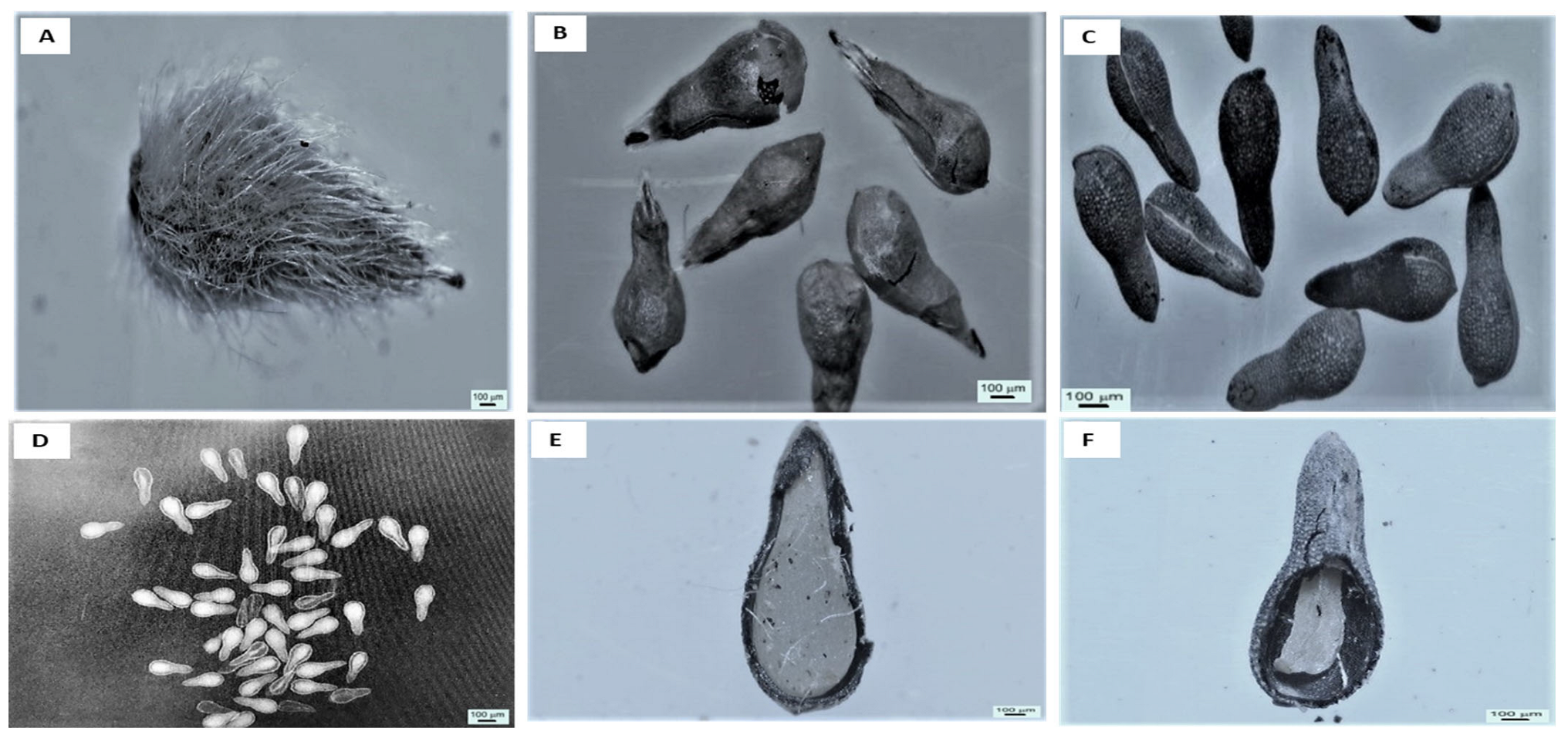
2.1. Microscopic Observations
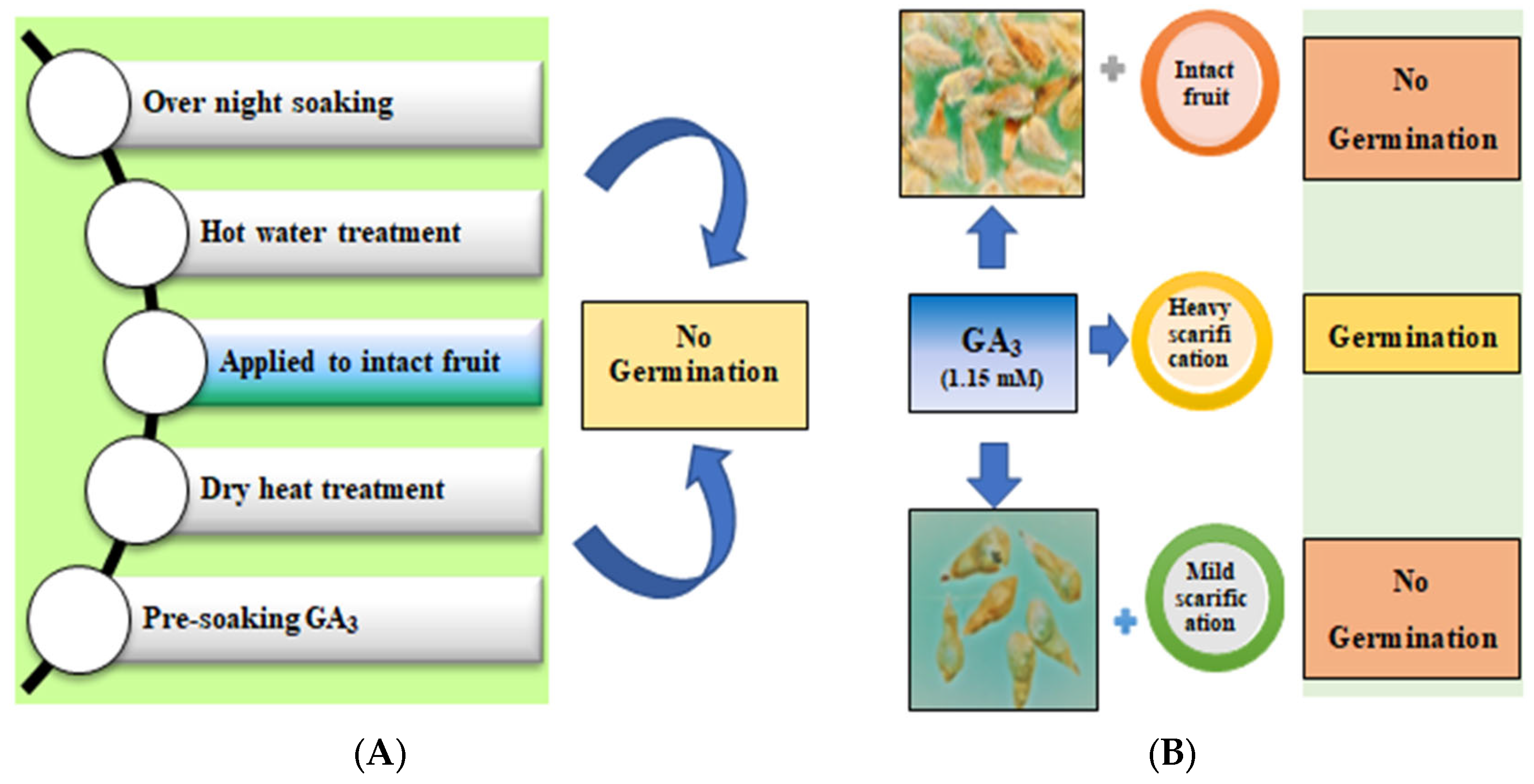
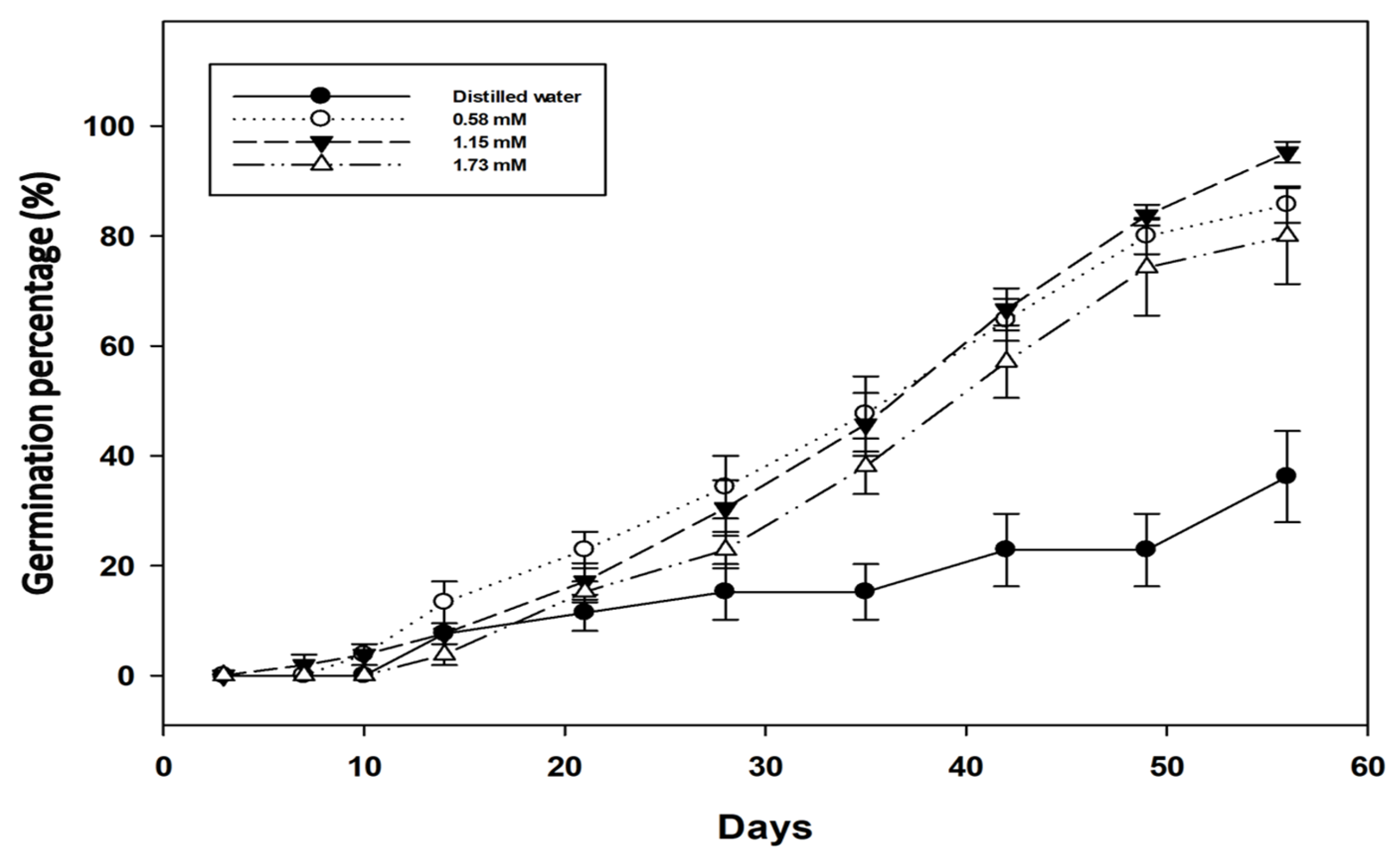
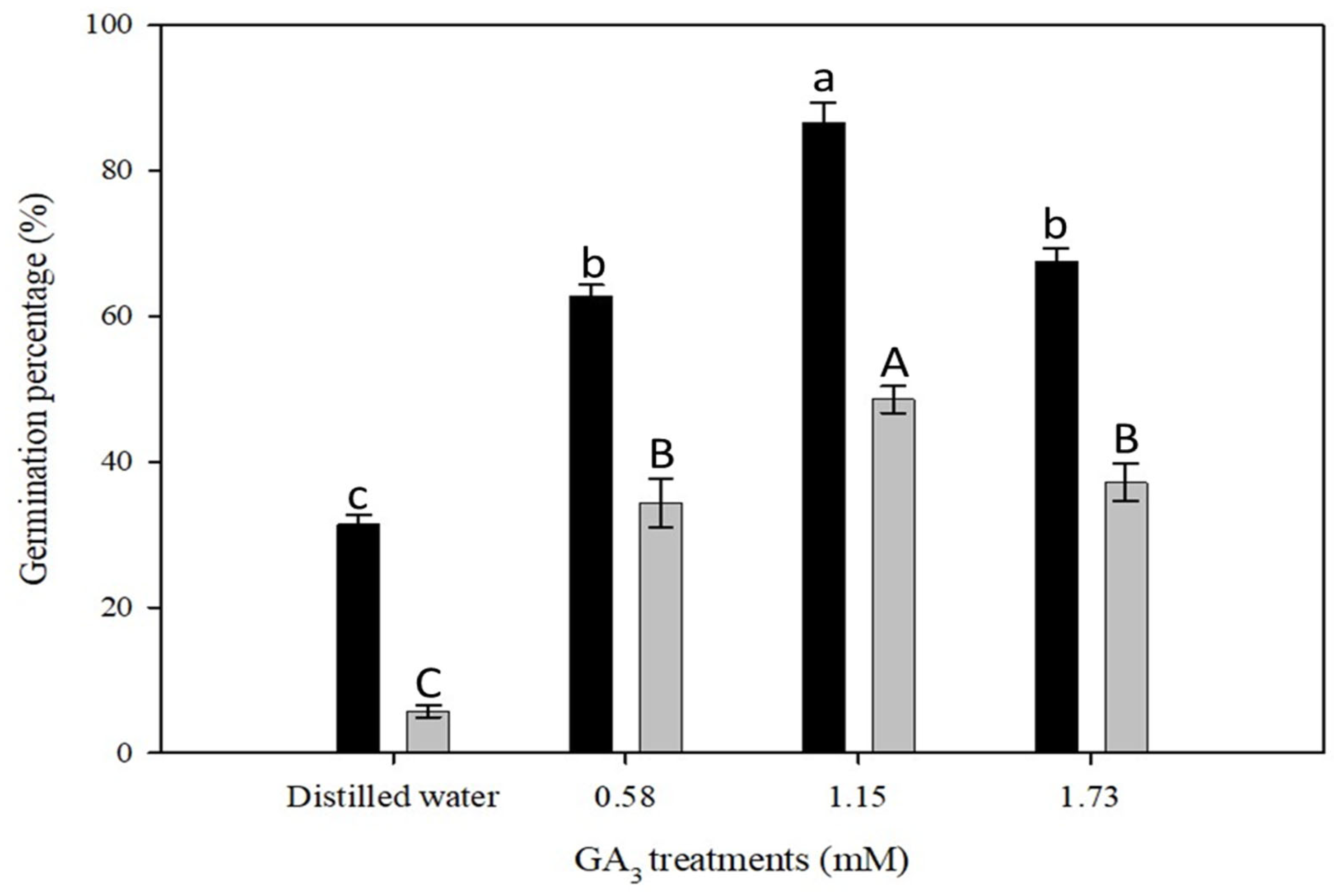
2.2. Effect of Various Dormancy Breaking Treatments
2.3. Effects of Temperature and Light on Germination
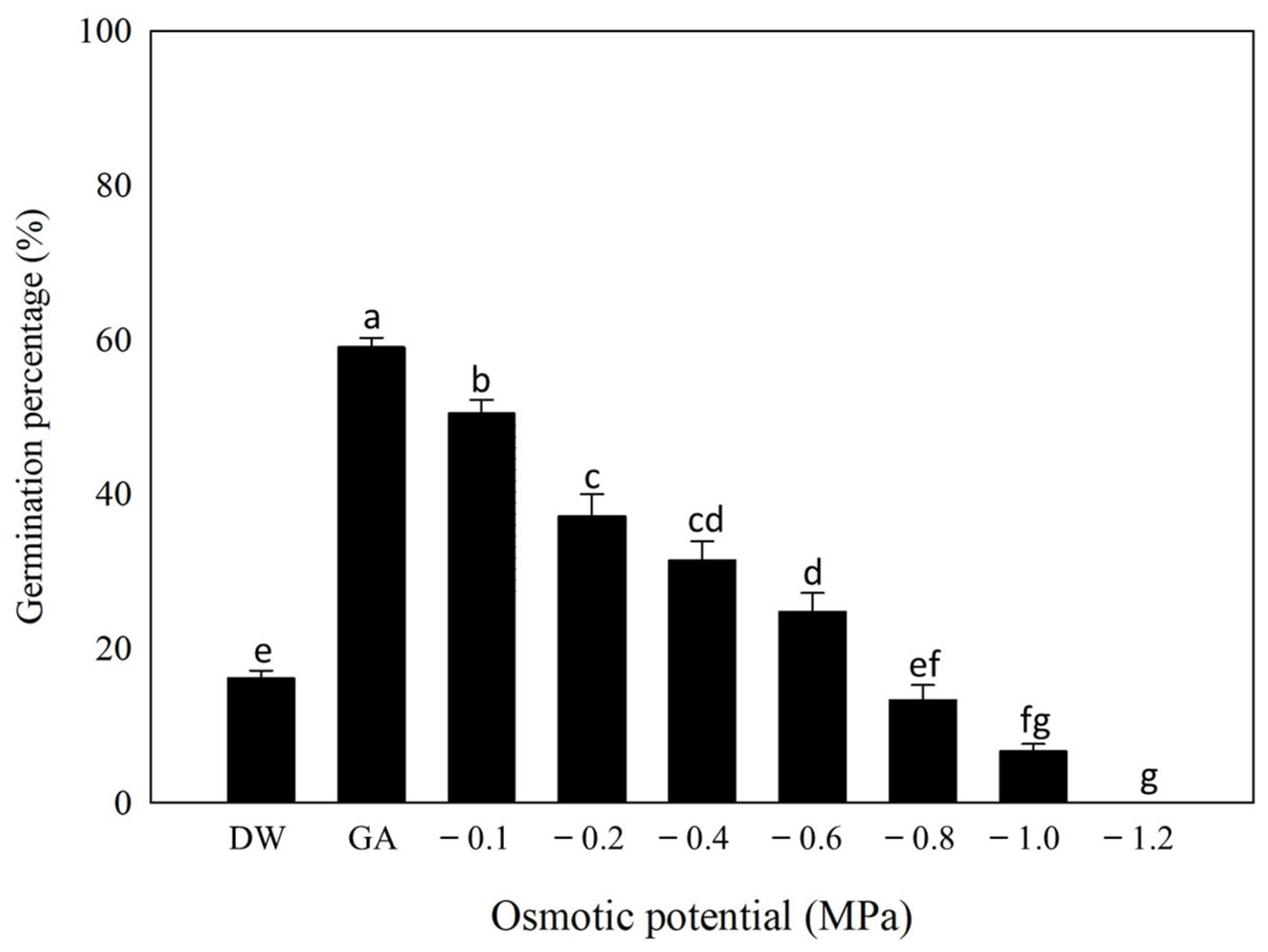
2.4. Effect of Osmotic Stress on Germination
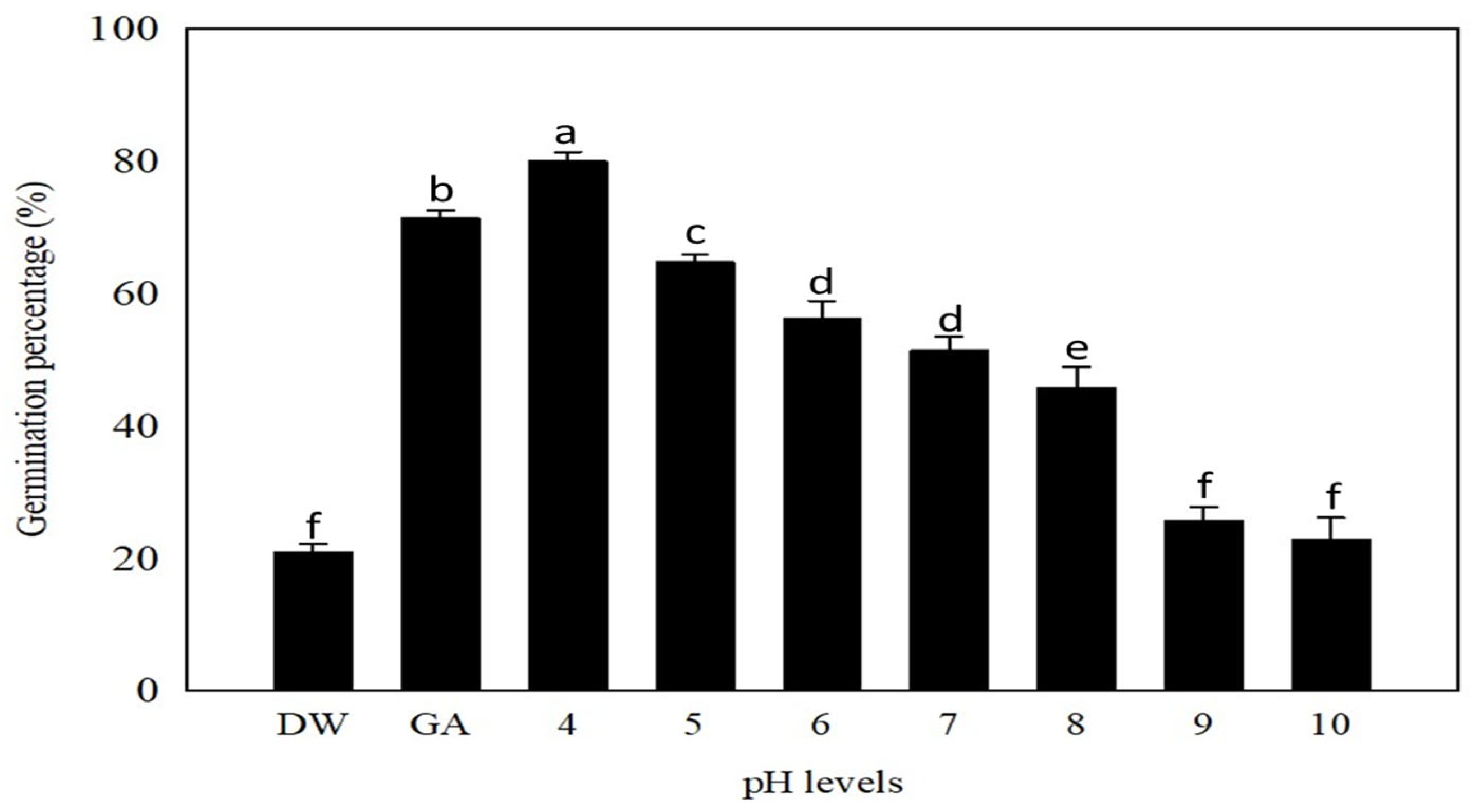
2.5. Effect of pH on Germination
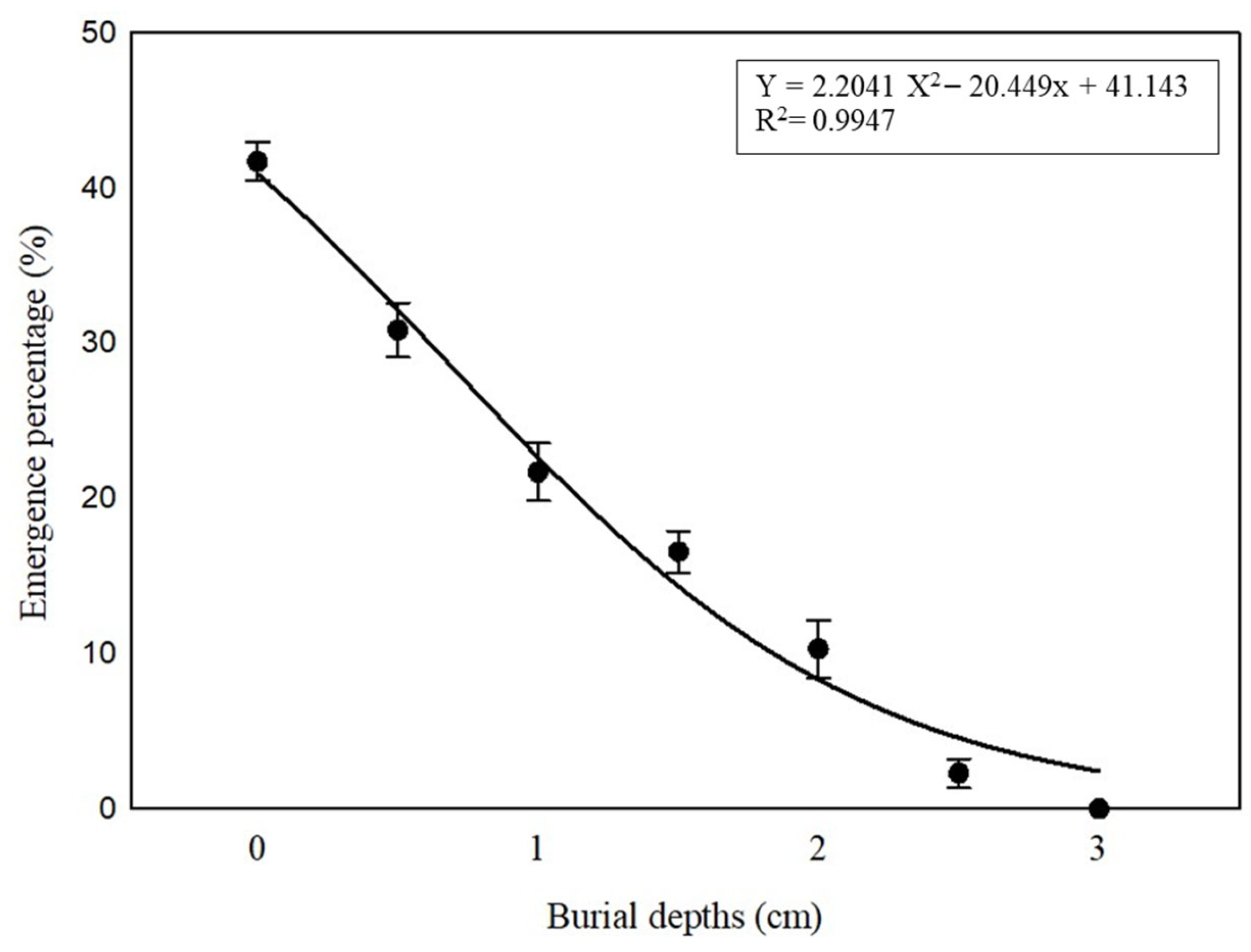
2.6. Effect of Different Burial Depths on Seedling Emergence
3. Discussion
3.1. Dormancy Mechanisms
3.2. Temperature and Light
3.3. Osmotic Stress
3.4. Substrate pH
3.5. Burial Depth
4. Materials and Methods
4.1. Seed Collection, Processing and Storage
4.2. Seed Anatomical Analysis and Fill
4.3. General Seed Germination Test Protocols
4.4. Effect of Various Dormancy Breaking Treatments
4.5. Effects of Temperature, and Light on Germination
4.6. Effect of Osmotic Stress on the Germination
4.7. Effect of pH on the Germination
4.8. Effect of Different Burial Depths on the Emergence of P. trichostachya
4.9. Experimental Design, Germination Calculations and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, G.M.; Mulham, W.E.; Milthorpe, P.L.; Leigh, J.H. Plants of Western New South Wales; Intaka Press: Sydney, NSW, Australia, 1992. [Google Scholar]
- Milson, J. Pasture Plants of North-West Queensland; Information Service QI00015; Department of Primary Industries: Brisbane, QLD, Australia, 2000. [Google Scholar]
- Kutsche, F.; Lay, B. Field Guide to the Plants of Outback South Australia; Department of Water, Land and Biodiversity Conservation, Government of South Australia: Adelaide, SA, Australia, 2003. [Google Scholar]
- Lee, J.S. George Disease in the NT. Vet. Pathol. Rep. 1990, 27, 25–26. [Google Scholar]
- AVH, The Australian Virtual Herbarium. 2022. Available online: https://avh.ala.org.au/occurrences/search?taxa=Pimelea+trichostachya (accessed on 28 February 2021).
- Chow, S.; Fletcher, M.T.; McKenzie, R.A. Analysis of daphnane orthoesters in poisonous Australian Pimelea species by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2010, 58, 7482–7487. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Silcock, R.; Ossedryver, S.; Milson, J.; Chow, S. Understanding Pimelea Poisoning of Cattle. Department of Employment, Economic Development and Innovation, Brisbane, Queensland, Australia. 2009. Available online: https://futurebeef.com.au/wp-content/uploads/2011/09/Understanding_pimelea_poisoning_of_cattle.pdf (accessed on 20 February 2021).
- Gordon, R.J.; Hungerford, N.L.; Laycock, B.; Fletcher, M.T. A review on Pimelea poisoning of livestock. Toxicon 2020, 186, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bean, A.R. A taxonomic revision of Pimelea section Epallage (Endl.) Benth. (Thymelaeaceae) in Queensland. Austrobaileya 2017, 10, 1–46. [Google Scholar]
- Silcock, R.G. Effective storage of highly germinable seeds of poisonous Pimelea species for future research. Seed Sci. Technol. 2017, 45, 428–443. [Google Scholar] [CrossRef]
- Silcock, R.G.; Mann, M.B. Germinating the seeds of three species of Pimelea sect. Epallage (Thymelaeaceae). Aust. J. Bot. 2014, 62, 74–83. [Google Scholar] [CrossRef]
- Dadswell, L.L.; Graham, T.G.; Newman, R.D.; D’Occhio, M.; Burton, D.; Schefe, C. Pimelea Poisoning in Beef Cattle: Plant Ecology, Epidemiology, Therapeutic Control and Immunogen Feasibility Studies. Final Report on DAQ.072 to Meat Research Corporation; Part 3: Technical report; Department of Primary Industries Project Report QO94021; Department of Primary Industries: Brisbane, QLD, Australia, 1994. [Google Scholar]
- Milberg, P.; Andersson, L.; Thompson, K.J.S. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S.W. Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus L.) relates to their invasiveness. Weed Sci. 2018, 66, 62–70. [Google Scholar] [CrossRef]
- Pereira, B.F.; Simão, E.; Terra Filho, J.B.; Cardoso, J.C.; Cardoso, V.J. Note on the germination of Vochysia tucanorum seeds treated with growth regulators. Braz. J. Bot. 2011, 34, 231–235. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S. Dormancy regulating chemicals alleviate innate seed dormancy and promote germination of desert annuals. J. Plant Growth Regul. 2017, 36, 300–311. [Google Scholar] [CrossRef]
- Zheng, M.Q.; Zheng, Y.R.; Jiang, L.H. Effects of one-time water supply and sand burial on seed germination and seedling emergence of four populary psammophyte in Mu Us sandy land. Acta Ecol. Sin. 2006, 26, 2474–2484. [Google Scholar]
- Du, X.; Guo, Q.; Gao, X.; Ma, K. Seed rain, soil seedbank, seed loss and regeneration of Catanopsis fargesii Franch. (Fagaceae) in a subtropical evergreen broad-leaved forest. For. Ecol. Manag. 2007, 238, 212–219. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.; Preston, C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006, 54, 854–860. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Q.; Li, X.; Ma, J.; Ling, X. Seed mass and shape, germination and plant abundance in a desertified grassland in northeastern Inner Mongolia, China. J. Arid. Environ. 2007, 69, 198–211. [Google Scholar] [CrossRef]
- De Caritat, P.; Cooper, M.; Wilford, J. The pH of Australian soils: Field results from a national survey. Soil Res. 2011, 49, 173–182. [Google Scholar] [CrossRef]
- Herrera, C.M. Topsoil properties and seedling recruitment in Lavandula latifolia Medik.: Stage-dependence and spatial decoupling of influential parameters. Oikos 2002, 97, 260–270. [Google Scholar] [CrossRef]
- Dang, Y.P.; Moody, P.W.; Bell, M.J.; Seymour, N.P.; Dalal, R.C.; Freebairn, D.M.; Walker, S.R. Strategic tillage in no-till farming systems in Australia’s northern grains-growing regions: II. Implications for agronomy, soil and environment. Soil Tillage Res. 2015, 152, 115–123. [Google Scholar] [CrossRef]
- Navie, S.C.; Panetta, F.D.; McFadyen, R.E.; Adkins, S.W. Germinable soil seedbanks of central Queensland rangelands invaded by the exotic weed Parthenium hysterophorus L. Weed Biol. Manag. 2004, 4, 154–167. [Google Scholar] [CrossRef]
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 2000, 48, 212–216. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, G.Q.; Han, Y.N.; Liu, M.M. Effects of salt, alkali and salt–alkali mixed stresses on seed germination of the halophyte Salsola ferganica (Chenopodiaceae). Acta Ecol. Sin. 2013, 33, 354–360. [Google Scholar] [CrossRef]
- Merritt, D.; Rokich, D. Seed biology and ecology. In Australian Seeds: A Guide to Collection, Identification and Biology; Sweedman, L., Merritt, D., Eds.; CSIRO Publishing: Collingwood, VIC, Australia, 2006; pp. 19–24. [Google Scholar]
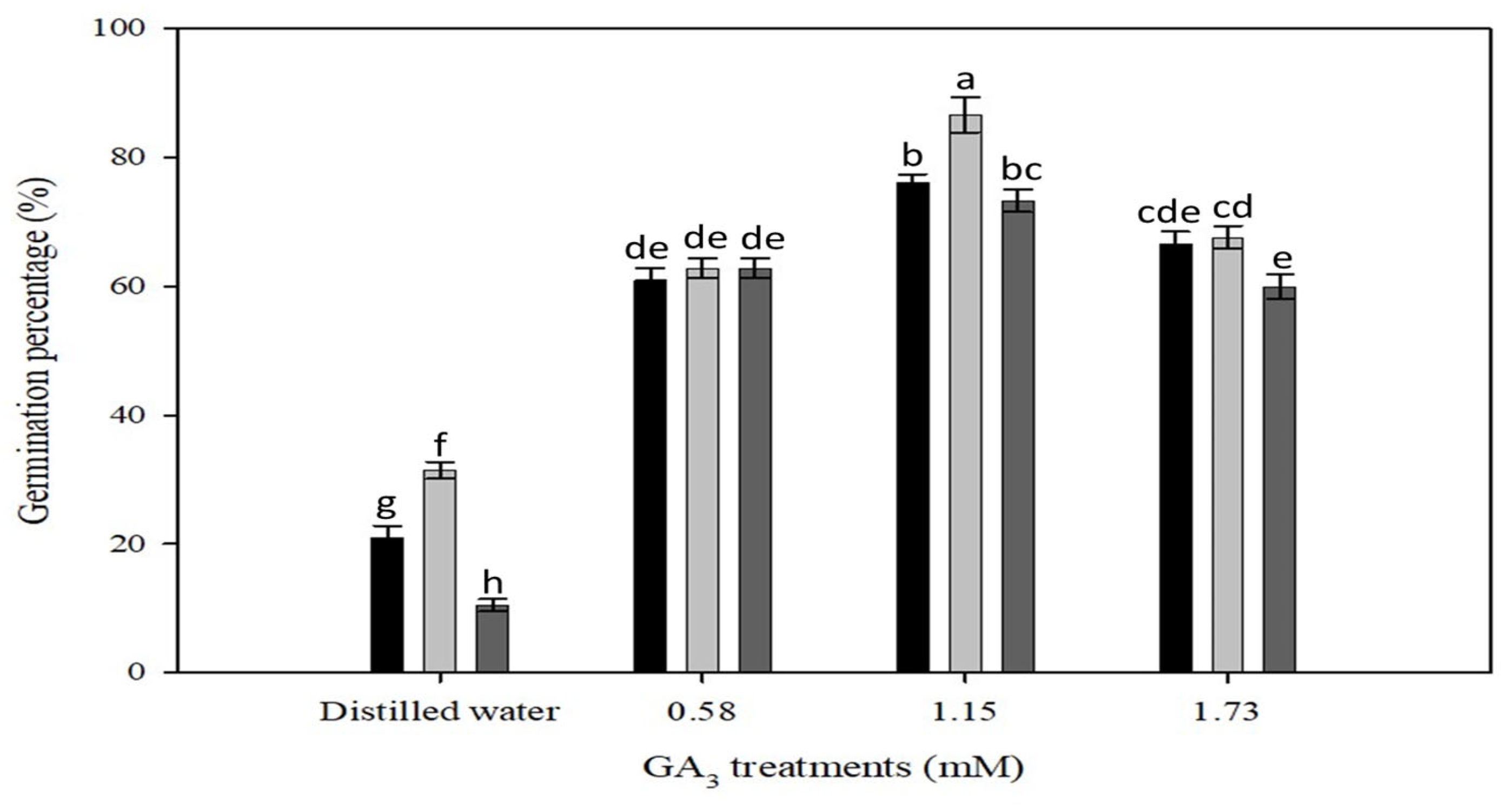

| Treatments * | Germination (%) in GA3 (mM) | |||
|---|---|---|---|---|
| 0.00 | 0.58 | 1.15 | 1.73 | |
| Intact seed | 0.0 | 0.0 | 0.0 | 0.0 |
| Mild scarification | 0.0 | 0.0 | 0.0 | 0.0 |
| Heavy scarification | 0.0 | 62 ± 1 b | 86 ± 3 a | 67 ± 1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, R.; Campbell, S.; Fletcher, M.T.; Kalaipandian, S.; Adkins, S. Factors Affecting the Germination Ecology of Pimelea trichostachya and Its Relationship to Field Emergence. Plants 2023, 12, 2112. https://doi.org/10.3390/plants12112112
Saleem R, Campbell S, Fletcher MT, Kalaipandian S, Adkins S. Factors Affecting the Germination Ecology of Pimelea trichostachya and Its Relationship to Field Emergence. Plants. 2023; 12(11):2112. https://doi.org/10.3390/plants12112112
Chicago/Turabian StyleSaleem, Rashid, Shane Campbell, Mary T. Fletcher, Sundaravelpandian Kalaipandian, and Steve Adkins. 2023. "Factors Affecting the Germination Ecology of Pimelea trichostachya and Its Relationship to Field Emergence" Plants 12, no. 11: 2112. https://doi.org/10.3390/plants12112112
APA StyleSaleem, R., Campbell, S., Fletcher, M. T., Kalaipandian, S., & Adkins, S. (2023). Factors Affecting the Germination Ecology of Pimelea trichostachya and Its Relationship to Field Emergence. Plants, 12(11), 2112. https://doi.org/10.3390/plants12112112









