American Cranberry (Oxycoccus macrocarpus (Ait.) Pursh) Leaves Extract and Its Amino-Acids Preparation: The Phytochemical and Pharmacological Study
Abstract
1. Introduction
2. Results
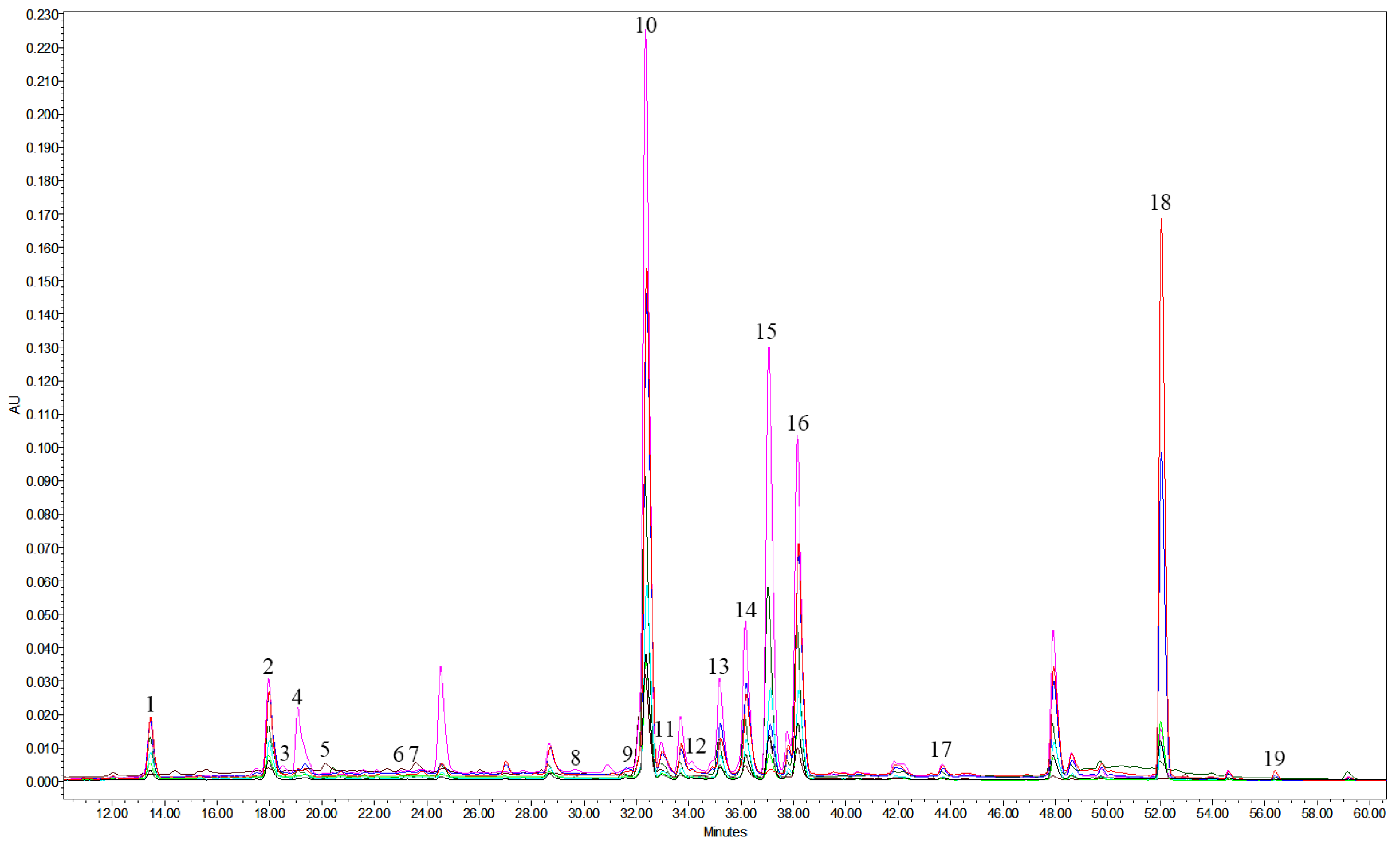
2.1. HPLC Analysis and Quantification of Major Compounds
2.2. Acute Toxicity
2.3. Hepatoprotective Activity
3. Discussion
3.1. Phytochemical Research
3.2. Hepatoprotective Activity
4. Materials and Methods
4.1. Plant Material
4.2. Extracts Preparation
4.3. Chemicals
4.4. Analysis and Quantification of Major Compounds
4.4.1. Phenolic Compounds Identification by UPLC-MS/MS
4.4.2. Phenolic Compounds Analysis by HPLC-PDA
4.5. Acute Toxicity
4.6. Hepatoprotective Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A.; Pushpangadan, P. Development of phytomedicine for liver diseases. Indian J. Pharmacol. 1999, 31, 166–175. [Google Scholar]
- Adewusi, E.A.; Afolayan, A.J. A review of natural products with hepatoprotective activity. J. Med. Plants Res. 2010, 4, 1318–1334. [Google Scholar]
- Ahsan, M.R.; Islam, K.M.; Bulbul, I.J. Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Glob. J. Pharmacol. 2009, 3, 116–122. [Google Scholar]
- Patel, K.P.; Patel, P.A.; Vunnam, R.R.; Hewlett, A.T.; Jain, R.; Jing, R.; Vunnam, S.R. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J. Clin. Virol. 2020, 128, 104386. [Google Scholar] [CrossRef]
- Huang, Y.; Miyamoto, D.; Hidaka, M.; Adachi, T.; Gu, W.L.; Eguchi, S. Regenerative medicine for the hepatobiliary system: A review. J. Hepatobiliary Pancreat. Sci. 2021, 11, 913–930. [Google Scholar] [CrossRef]
- Deshwal, N.; Sharma, A.K.; Sharma, P. Review on hepatoprotective plants. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 15–26. [Google Scholar]
- Casafont-Morencos, F.; Puente, A.; Pons-Romero, F. Infecciones bacterianas y parasitarias del hígado. Medicine 2008, 10, 563–569. [Google Scholar]
- Amengual-Guedan, M.J.; Rodríguez Sánchez, J.L. Autoinmunidad en las enfermedades del hígado (I). Immunologia 2000, 19, 90–102. [Google Scholar]
- Chattopadhyay, R.R. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J. Ethnopharmacol. 2003, 89, 217–219. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Fragoso-Antonio, S.; Valadez-Vega, C.; Solano-Solano, G.; Pérez, C.Z.; Sánchez-Gutiérrez, M.; IzquierdoVega, J.A.; Gutiérrez-Salinas, J.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Investigation on the protective effects of cranberry against the DNA damage induced by benzo[a]pyrene. Molecules 2012, 17, 4435–4451. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Cheng, M.L.; Zhang, B.F.; Mu, M.; Zhou, M.Y.; Wu, J.; Li, C.X. Effect of blueberry on hepatic and immunological functions in mice. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 164–168. [Google Scholar] [PubMed]
- Cheshchevik, V.T.; Lapshina, E.A.; Dremza, I.K.; Zabrodskaya, S.V.; Reiter, R.J.; Prokopchik, N.I.; Zavodnik, I.B. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: Protection by melatonin and cranberry flavonoids. Toxicol. Appl. Pharmacol. 2012, 261, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Hudz, N.; Korzeniowska, K.; Wieczorek, P. Antioxidant activity of essential oils obtained from aerial part of some Lamiaceae species. Int. J. Green Pharm. 2018, 12, 200–204. [Google Scholar]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Y.; Komissarenko, A.N. Phytochemical study of the dry extract from bilberry leaves. Azerbaijan Pharm. Pharmacother. J. 2016, 16, 18–23. [Google Scholar]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush blue-berry (Vaccinium corymbosum L.) leaves extract and its modified arginine preparation for the management of metabolic syndrome—Chemical analysis and bioactivity in rat model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef]
- Chaika, N.; Mazen, M.; Koshovyi, O.; Kravchenko, G.; Goryacha, O.; Kireyev, I.; Kovalenko, S.M.; Darmograi, R. Research in phytochemical composition and hypoglycemic activity screening of the dry extracts from bearberry leaves. Sci. Pharm. Sci. 2021, 29, 42–50. [Google Scholar]
- Chaika, N.; Koshovyi, O.; Raal, A.; Kireyev, I.; Zupanets, A.; Odyntsova, V. Phytochemical profile and pharmacolog-ical activity of the dry extract from Arctostaphylos uva-ursi leaves modified with phenylalanine. Sci. Pharm. Sci. 2020, 6, 74–84. [Google Scholar]
- Koshovyi, O.M.; Vlasova, I.K.; Brukhanova, T.O.; Krasilnikova, O.A.; Kravchenko, G.B.; Zagayko, A.L.; Komisarenko, M.A. A Method of Obtaining a Therapeutic and Prophylactic Agent from the Leaves of Large-Fruited Cranberries for the Correction of Insulin-Resistant Conditions. UA Patent No. 147975, 23 June 2021. [Google Scholar]
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 3, 48–57. [Google Scholar]
- Kovalenko, V.N. Compendium 2020—Medicines; MORION: Kyiv, Ukraine, 2020; p. 2700. [Google Scholar]
- Parfenov, V.A. Use of L-lysine aescinate in central nervous system diseases. Neurol. Neuropsychiatry Psychosom. 2011, 3, 99–104. [Google Scholar] [CrossRef]
- Koshovyi, O.; Raal, A.; Kireyev, I.; Tryshchuk, N.; Ilina, T.; Romanenko, Y.; Kovalenko, S.M.; Bunyatyan, N. Phytochemical and sychotropic research of motherwort (Leonurus cardiaca L.) modified dry extracts. Plants 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, G.; Krasilnikova, O.; Raal, A.; Mazen, M.; Chaika, N.; Kireyev, I.; Grytsyk, A.; Koshovyi, O. Arctostaphylos uva-ursi L. leaves extract and its modified cysteine preparation for the management of insulin resistance: Chemical analysis and bioactivity. Nat. Prod. Bioprospect. 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186–193. [Google Scholar] [CrossRef]
- Singh, A.P.; Wilson, T.; Kalk, A.J.; Cheong, J.; Vorsa, N. Isolation of specific cranberry flavonoids for biological activity assessment. Food Chem. 2009, 116, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- Stobnicka, A.; Gniewosz, M. Antimicrobial protection of minced pork meat with the use of swamp cranberry (Vaccinium oxycoccos L.) fruit and pomace extracts. J. Food Sci. Technol. 2018, 55, 62–71. [Google Scholar] [CrossRef]
- Marzullo, L.; Ochkur, O.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Orlandini, S.; Del Bubba, M. Quality by Design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine; Naukova Dumka: Kyiv, Ukraine, 1999. [Google Scholar]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019.
- State Pharmacopoeia of Ukraine, 2nd ed.; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015. (In Ukrainian)
- Vlasova, I.K.; Koshovyi, O.M. Standardization of dry extracts from large cranberry leaves. J. Org. Pharm. Chem. 2022, 20, 40–45. [Google Scholar] [CrossRef]
- Koshevoi, O.N. Amino-acid and monosaccharide compositions of Salvia officinalis leaves. Chem. Nat. Comp. 2011, 47, 492–493. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic fractions from Vaccinium vitis-idaea L. and their antioxidant and anticancer activities assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, O.V. Preclinical Studies of Drugs; Avitsenna: Kyiv, Ukraine, 2001. (In Ukrainian) [Google Scholar]
- Kovalenko, V.M. Preclinical research of medicinal products in Ukraine. Pharmacol. Med. Toxicol. 2009, 5, 56–61. [Google Scholar]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and pharmacological research in Agrimonia eupatoria L. herb extract with anti-Inflammatory and hepatoprotective properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and pharmacological screening of a Monarda fistulosa L. dry extract based on a hydrodistilled residue by-product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- The Law of Ukraine “On the Protection of Animals from Cruel Treatment” dated 12/15/2009. Available online: https://zakon.rada.gov.ua/laws/show/3447-15#Text (accessed on 8 September 2021).
- The Order of the Ministry of Health of Ukraine No. 944 dated 14.12.2009 “On approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products”. Available online: https://zakon.rada.gov.ua/laws/show/z0972-01#Text (accessed on 1 February 2010).
- Council Directive of 18 December 1986 on the Lows, Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances (87/18/EEC)/The Rules Governing Medicinal Products in the European Community; Commission of the European Communities: Brussels, Belgium, 1991; Volume 1, pp. 145–146.
| Phenolic Compounds | Content in the Extract, µg/g * | |||||||
|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | |
| Cyanidin-3-O-galactoside | ND | NQ | ND | ND | NQ | NQ | 54 ± 9 | 105 ± 6 |
| Cyanidin-3-O-arabinoside | ND | NQ | ND | ND | NQ | NQ | 129 ± 11 | 210 ± 4 |
| Hyperoside | 16,125 ± 307 | 6848 ± 67 | 908 ± 6 | 8044 ± 104 | 10,705 ± 109 | 6407 ± 241 | 5279 ± 20 | 5604 ± 13 |
| Isoquercitrin | 1167 ± 35 | 399 ± 4 | 171 ± 5 | 480 ± 15 | 667 ± 2 | 397 ± 11 | 339 ± 4 | 404 ± 9 |
| Reynoutrin | 924 ± 64 | 1586 ± 15 | 188 ± 5 | 2007 ± 9 | 2542 ± 21 | 1499 ± 61 | 1084 ± 3 | 0760 ± 14 |
| Quercetin-3-O-arabinopyranoside | 5821 ± 58 | 2414 ± 23 | 243 ± 11 | 2903 ± 56 | 3911 ± 46 | 2306 ± 54 | 1801 ± 5 | 1548 ± 15 |
| Avicularin | 12,860 ± 407 | 3084 ± 27 | 595 ± 8 | 5930 ± 119 | 6446 ± 54 | 1712 ± 69 | 707 ± 14 | 67 ± 3 |
| Quercitrin | 12,333 ± 361 | 4703 ± 39 | 477 ± 15 | 5569 ± 113 | 7541 ± 87 | 4655 ± 102 | 3777 ± 6 | 3858 ± 36 |
| Quercetin | 952 ± 0.067 | 2823 ± 46 | 103 ± 8 | 717 ± 10 | 2612 ± 67 | 4153 ± 107 | 4096 ± 11 | 6992 ± 42 |
| Kaempferol-3-O-rhamnoside | 351 ± 13 | 110 ± 2 | 4 ± 1 | 175 ± 2 | 196 ± 5 | 111 ± 9 | 117 ± 2 | 119 ± 1 |
| Kaempferol | 50 ± 2 | 54 ± 1 | 9 ± 1 | 14 ± 1 | 52 ± 2 | 68 ± 3 | 49 ± 1 | 82 ± 1 |
| Chlorogenic acid | 2356 ± 35 | 1117 ± 15 | 133 ± 3 | 685 ± 12 | 1599 ±46 | 1222 ± 26 | 657 ± 10 | 672 ± 2 |
| Neochlorogenic acid | 2497 ± 108 | 720 ± 17 | 47 ± 1 | 687 ± 6 | 1733 ± 64 | 1093 ± 17 | 687 ± 10 | 735 ± 21 |
| 4-O-caffeoylquinic acid | 1100 ± 45 | 834 ± 19 | 175 ± 1 | 649 ± 31 | 913 ± 8 | 863 ± 25 | 208 ± 2 | 235 ± 5 |
| p-Coumaric acid | 136 ± 10 | 70 ± 1 | 11 ± 1 | 17 ± 1 | 93 ± 6 | 71 ± 3 | 30 ± 2 | 16 ± 1 |
| (+)-Catechin | 911 ± 42 | 415 ± 7 | 509 ± 2 | 666 ± 16 | 758 ± 6 | 484 ± 82 | 391 ± 23 | 383 ± 4 |
| (–)-Epicatechin | 247 ± 3 | 1407 ± 55 | 190 ± 17 | 2147 ± 97 | 2166 ± 59 | 1540 ± 60 | 886 ± 12 | 444 ± 9 |
| Procyanidin A1 | 1313 ± 26 | 1007 ± 69 | 139 ± 7 | 026 ± 67 | 1398 ± 93 | 1209 ± 36 | 447 ± 16 | 297 ± 19 |
| Procyanidin A2 | 2053 ± 32 | 1689 ± 25 | 69 ± 3 | 2823 ± 6 | 4047 ± 74 | 2708 ± 49 | 1303 ± 16 | 902 ± 7 |
| Total amount (mg/g) | 64,196 | 29,280 | 3971 | 34,539 | 47,379 | 30,498 | 22,041 | 23,433 |
| Group # | Group of Experimental Animals | m animal, | m liver | LWC |
|---|---|---|---|---|
| 1 | Intact animals | 174.2 ± 11.7 | 6.1 ± 0.4 | 3.5 ± 0.1 |
| 2 | Control pathology (CCl4) | 235.0 ± 15.9 | 12.0 ± 0.6 | 5.1 ± 0.5 |
| 3 | E1 | 210.0 ± 9.4 | 8.9 ± 0.7 | 4.2 ± 0.2 |
| 4 | E2 | 233.3 ± 8.6 | 8.9 ± 0.6 | 3.8 ± 0.2 |
| 5 | E3 | 218.3 ± 12.3 | 8.1 ± 0.7 | 3.7 ± 0.2 |
| 6 | E4 | 221.7 ± 12.3 | 9.3 ± 0.9 | 4.2 ± 0.3 |
| 7 | E5 | 191.7 ± 7.9 | 7.9 ± 0.6 | 4.1 ± 0.2 |
| 8 | E6 | 210.0 ± 9.6 | 8.4 ± 0.5 | 4.0 ± 0.2 |
| 9 | E7 | 225.0 ± 11.0 | 9.2 ± 1.1 | 4.1 ± 0.3 |
| 10 | E8 | 226.7 ± 12.7 | 9.2 ± 0.7 | 4.1 ± 0.1 |
| 11 | Silibor | 216.7 ± 6.4 | 8.6 ± 0.5 | 3.9 ±0.1 |
| # | Group of Animals | Biochemical Indicators | |||
|---|---|---|---|---|---|
| Blood Serum | Liver Homogenate | ||||
| AlAt, μmol/h.mL | AsAt, μmol/h.mL | TBA-Reactants nmol/mL | TBA-Reactants nmol/mL | ||
| 1 | Intact animals | 0.76 ± 0.05 | 0.96 ± 0.06 | 3.23 ± 0.12 | 2.45 ± 0.09 |
| 2 | Control pathology (CCl4) | 2.56 ± 0.11 * | 2.32 ± 0.11 * | 5.96 ± 0.28 * | 6.15 ± 0.26 * |
| 3 | E1 | 1.28 ± 0.04 */** | 1.36 ± 0.06 */**/# | 4.21 ± 0.20 */**/# | 3.64 ± 0.13 */** |
| 4 | E2 | 0.96 ± 0.04 */**/# | 1.09 ± 0.05 */**/# | 3.52 ± 0.16 */**/# | 2.90 ± 0.14 */**/# |
| 5 | E3 | 0.85 ± 0.06 */**/# | 1.04 ± 0.052 */**/# | 3.38 ± 0.12 */**/# | 2.76 ± 0.15 */**/# |
| 6 | E4 | 1.31 ± 0.05 */** | 1.34 ± 0.05 */**/# | 4.15 ± 0.21 */**/# | 3.78 ± 0.17 */**/# |
| 7 | E5 | 1.26 ± 0.06 */** | 1.30 ± 0.05 */**/# | 4.01 ± 0.22 */** | 3.80 ± 0.17 */**/# |
| 8 | E6 | 1.06 ± 0.06 */**/# | 1.16 ± 0.06 */**/# | 3.71 ± 0.16 */**/# | 3.24 ± 0.17 */**/# |
| 9 | E7 | 1.21 ± 0.05 */** | 1.28 ± 0.07 */** | 3.97 ± 0.26 */** | 3.65 ± 0.17 */** |
| 10 | E8 | 1.10 ± 0.04 */**/# | 1.12 ± 0.05 */**/# | 3.80 ± 0.17 */**/# | 3.10 ± 0.17 */**/# |
| 11 | Silibor | 1.27 ± 0.06 */** | 1.24 ± 0.05 */** | 3.90 ± 0.16 */** | 3.57 ± 0.16 */** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshovyi, O.; Vlasova, I.; Jakštas, V.; Vilkickytė, G.; Žvikas, V.; Hrytsyk, R.; Grytsyk, L.; Raal, A. American Cranberry (Oxycoccus macrocarpus (Ait.) Pursh) Leaves Extract and Its Amino-Acids Preparation: The Phytochemical and Pharmacological Study. Plants 2023, 12, 2010. https://doi.org/10.3390/plants12102010
Koshovyi O, Vlasova I, Jakštas V, Vilkickytė G, Žvikas V, Hrytsyk R, Grytsyk L, Raal A. American Cranberry (Oxycoccus macrocarpus (Ait.) Pursh) Leaves Extract and Its Amino-Acids Preparation: The Phytochemical and Pharmacological Study. Plants. 2023; 12(10):2010. https://doi.org/10.3390/plants12102010
Chicago/Turabian StyleKoshovyi, Oleh, Inna Vlasova, Valdas Jakštas, Gabrielė Vilkickytė, Vaidotas Žvikas, Roman Hrytsyk, Lyubov Grytsyk, and Ain Raal. 2023. "American Cranberry (Oxycoccus macrocarpus (Ait.) Pursh) Leaves Extract and Its Amino-Acids Preparation: The Phytochemical and Pharmacological Study" Plants 12, no. 10: 2010. https://doi.org/10.3390/plants12102010
APA StyleKoshovyi, O., Vlasova, I., Jakštas, V., Vilkickytė, G., Žvikas, V., Hrytsyk, R., Grytsyk, L., & Raal, A. (2023). American Cranberry (Oxycoccus macrocarpus (Ait.) Pursh) Leaves Extract and Its Amino-Acids Preparation: The Phytochemical and Pharmacological Study. Plants, 12(10), 2010. https://doi.org/10.3390/plants12102010












