The Highly Embryogenic Brassica napus DH4079 Line Is Recalcitrant to Agrobacterium-Mediated Genetic Transformation
Abstract
1. Introduction
2. Results and Discussion
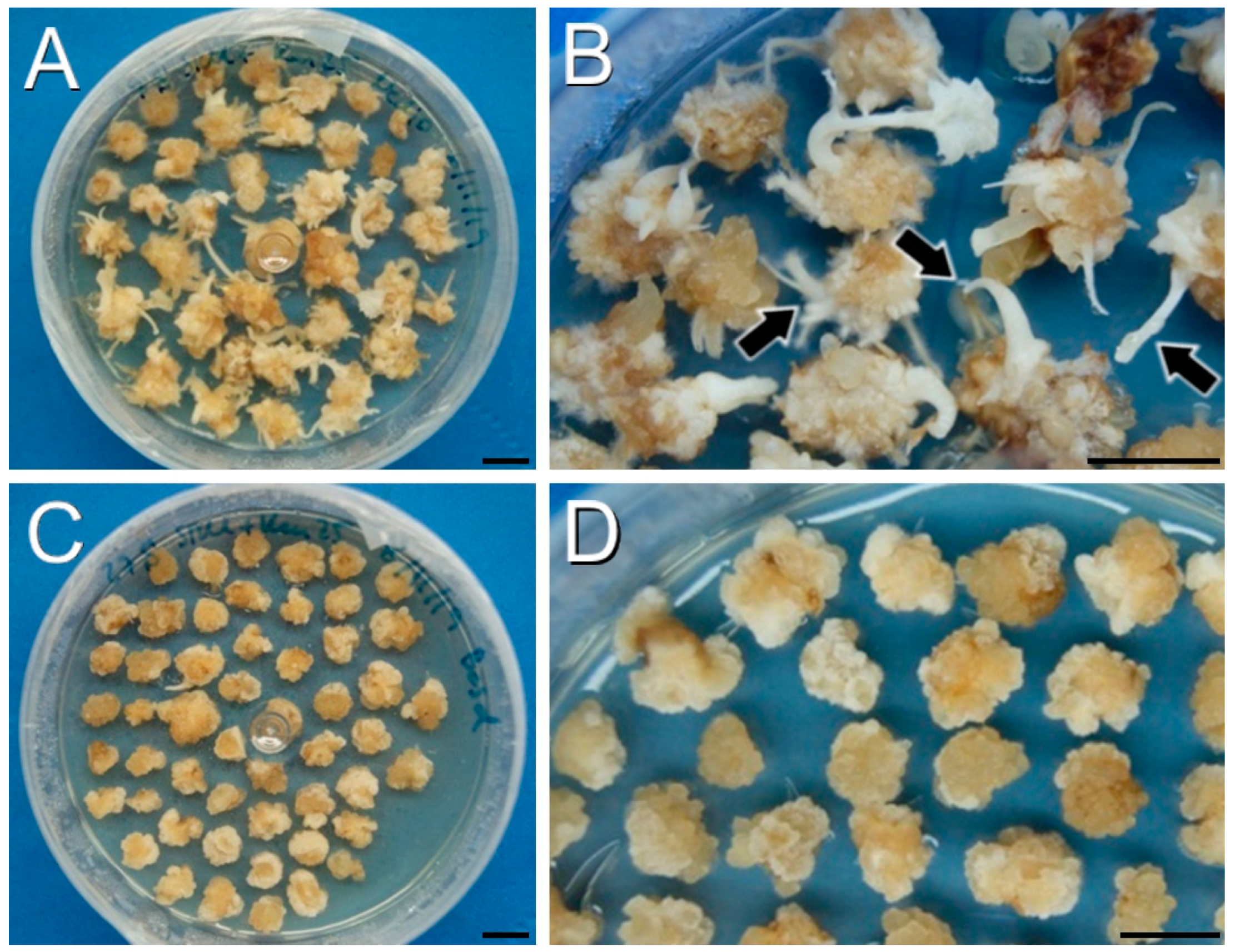
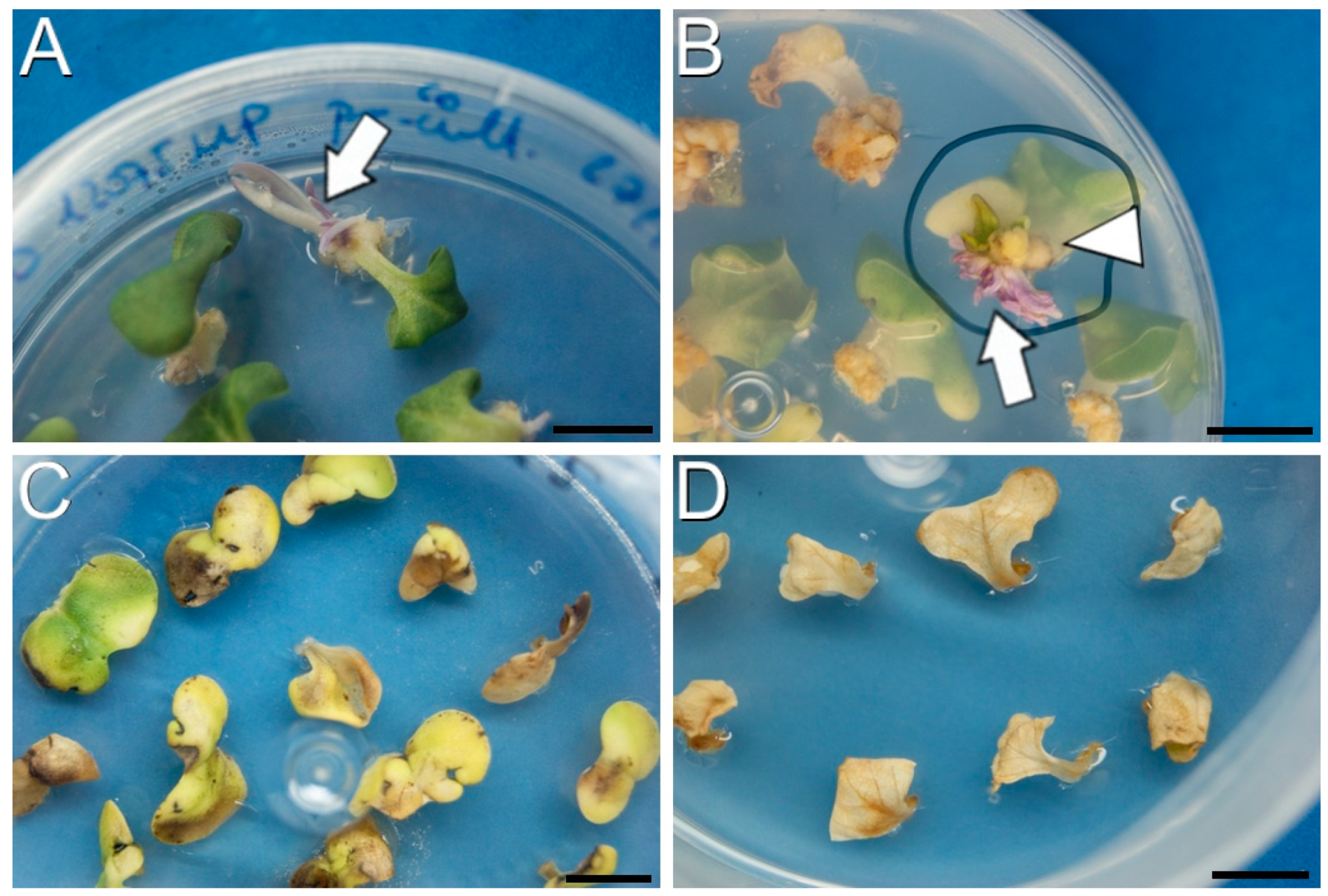
2.1. The Selective Agents Have a Critical Role in Plant Regeneration
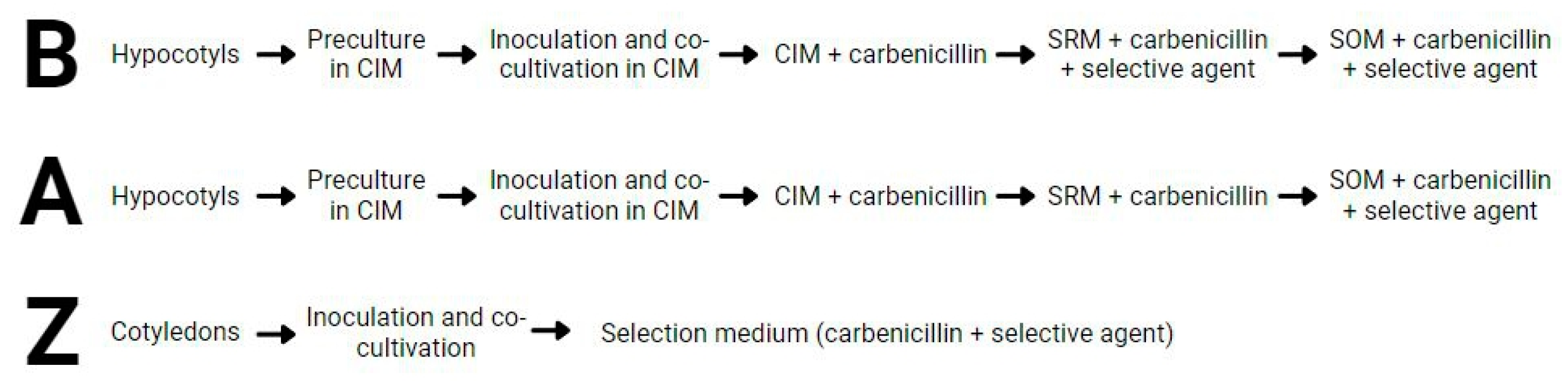
2.2. The DH12075 Line, but Not DH4079, Can Be Genetically Transformed Using Protocol A
2.3. The B. napus DH4079 Line Is Also Recalcitrant to Transient A. rhizogenes Transformation
2.4. A Possible Relationship between Recalcitrance to Genetic Transformation and Doubled Haploidy?
3. Materials and Methods
3.1. Plant Material
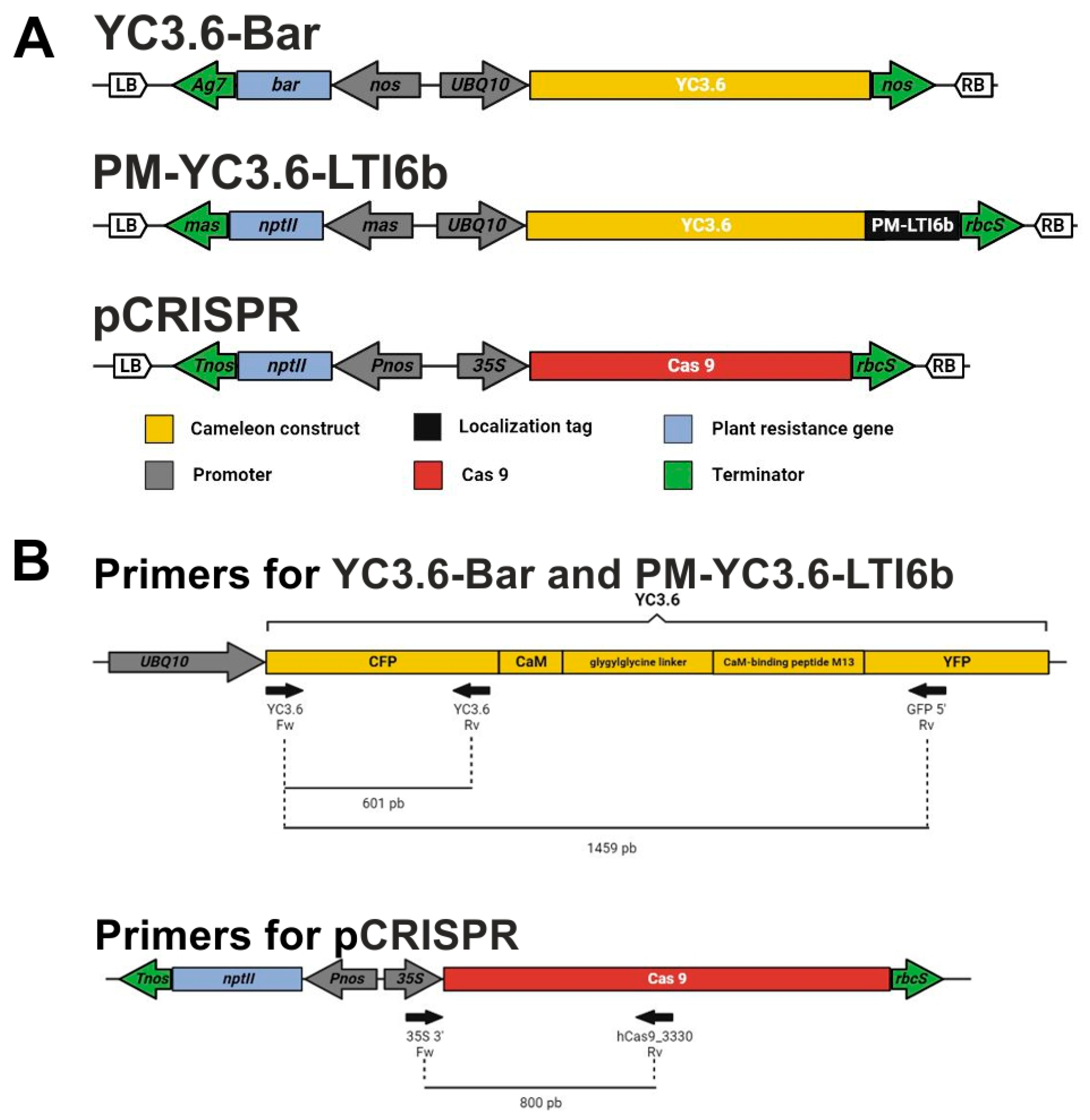
3.2. Constructs
3.3. Management and Transformation of Escherichia coli and Agrobacterium Strains
3.4. Explant Stable Transformation and Plant Regeneration
3.5. A. rhizogenes-Mediated Transient Transformation
3.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Montagu, M. It Is a Long Way to GM Agriculture. Ann. Rev. Plant. Biol. 2011, 62, 1–23. [Google Scholar] [CrossRef]
- Klümper, W.; Qaim, M. A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef]
- ISAAA. GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase (accessed on 23 May 2022).
- Hua, K.; Zhang, J.; Botella, J.R.; Ma, C.; Kong, F.; Liu, B.; Zhu, J.-K. Perspectives on the Application of Genome-Editing Technologies in Crop Breeding. Mol. Plant. 2019, 12, 1047–1059. [Google Scholar] [CrossRef]
- Hopp, H.E.; Spangenberg, G.; Herrera-Estrella, L. Editorial: Plant Transformation. Front. Plant Sci. 2022, 13, 876671. [Google Scholar] [CrossRef] [PubMed]
- Seguí-Simarro, J.M.; Jacquier, N.M.A.; Widiez, T. Overview of in vitro and in vivo doubled haploid technologies. In Doubled Haploid Technology, 1st ed.; Seguí-Simarro, J.M., Walker, J.M., Eds.; Methods in Molecular Biology; Springer Science + Business Media, LLC: New York, NY, USA, 2021; Volume 1, pp. 3–22. [Google Scholar]
- Jouannic, S.; Champion, A.; Seguí-Simarro, J.M.; Salimova, E.; Picaud, A.; Tregear, J.; Testillano, P.; Risueño, M.C.; Simanis, V.; Kreis, M.; et al. The protein kinases AtMAP3Kε1 and BnMAP3Kε1 are functional homologues of S. pombe cdc7p and may be involved in cell division. Plant J. 2001, 26, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Satpute, G.; Long, H.; Seguí-Simarro, J.M.; Risueño, M.C.; Testillano, P.S. Cell architecture during gametophytic and embryogenic microspore development in Brassica napus. Acta Physiol. Plant. 2005, 27, 665–674. [Google Scholar] [CrossRef]
- Weyen, J. Applications of doubled haploids in plant breeding and applied research. In Doubled Haploid Technology, 1st ed.; Seguí-Simarro, J.M., Walker, J.M., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2021; Volume 1, pp. 23–39. [Google Scholar]
- Custers, J.B.M.; Cordewener, J.H.G.; Fiers, M.A.; Maassen, B.T.H.; van Lookeren-Campagne, M.M.; Liu, C.M. Androgenesis in Brassica: A model system to study the initiation of plant embryogenesis. In Current Trends in the Embryology of Angiosperm; Bhojwani, S.S., Soh, W.Y., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 451–470. [Google Scholar]
- Ferrie, A.; Möllers, C. Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tissue Organ Cult. 2011, 104, 375–386. [Google Scholar] [CrossRef]
- Rivas-Sendra, A.; Corral-Martínez, P.; Porcel, R.; Camacho-Fernández, C.; Calabuig-Serna, A.; Seguí-Simarro, J.M. Embryogenic competence of microspores is associated with their ability to form a callosic, osmoprotective subintinal layer. J. Exp. Bot. 2019, 70, 1267–1281. [Google Scholar] [CrossRef]
- Rivas-Sendra, A.; Calabuig-Serna, A.; Seguí-Simarro, J.M. Dynamics of calcium during in vitro microspore embryogenesis and in vivo microspore development in Brassica napus and Solanum melongena. Front. Plant Sci. 2017, 8, 1177. [Google Scholar] [CrossRef]
- Parra-Vega, V.; Corral-Martínez, P.; Rivas-Sendra, A.; Seguí-Simarro, J.M. Induction of embryogenesis in Brassica napus microspores produces a callosic subintinal layer and abnormal cell walls with altered levels of callose and cellulose. Front. Plant Sci. 2015, 6, 1018. [Google Scholar] [CrossRef]
- Parra-Vega, V.; Corral-Martínez, P.; Rivas-Sendra, A.; Seguí-Simarro, J.M. Formation and excretion of autophagic plastids (plastolysomes) in Brassica napus embryogenic microspores. Front. Plant Sci. 2015, 6, 94. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Parra-Vega, V.; Seguí-Simarro, J.M. Novel features of Brassica napus embryogenic microspores revealed by high pressure freezing and freeze substitution: Evidence for massive autophagy and excretion-based cytoplasmic cleaning. J. Exp. Bot. 2013, 64, 3061–3075. [Google Scholar] [CrossRef] [PubMed]
- Corral-Martínez, P.; Camacho-Fernández, C.; Mir, R.; Seguí-Simarro, J.M. Doubled haploid production in high- and low-response genotypes of rapeseed (Brassica napus) through isolated microspore culture. In Doubled Haploid Technology, 1st ed.; Seguí-Simarro, J.M., Walker, J.M., Eds.; Methods in Molecular Biology; Springer Science + Business Media, LLC: New York, NY, USA, 2021; Volume 2, pp. 129–144. [Google Scholar]
- Dai, C.; Li, Y.; Li, L.; Du, Z.; Lin, S.; Tian, X.; Li, S.; Yang, B.; Yao, W.; Wang, J. An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol. Breed. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Bhalla, P.L.; Singh, M.B. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat. Protoc. 2008, 3, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.M.; Walker, J.M.; Sharma, K.K. High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 1989, 8, 238–242. [Google Scholar] [CrossRef]
- Sparrow, P.A.C.; Dale, P.J.; Irwin, J.A. Brassica oleracea. In Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 417–426. [Google Scholar]
- Fry, J.; Barnason, A.; Horsch, R.B. Transformation of Brassica napus with Agrobacterium tumefaciens based vectors. Plant Cell Rep. 1987, 6, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sonntag, K.; Rudloff, E.; Han, J. Production of fertile transgenic Brassica napus by Agrobacterium-mediated transformation of protoplasts. Plant Breed. 2005, 124, 1–4. [Google Scholar] [CrossRef]
- Maheshwari, P.; Selvaraj, G.; Kovalchuk, I. Optimization of Brassica napus (canola) explant regeneration for genetic transformation. New Biotechnol. 2011, 29, 144–155. [Google Scholar] [CrossRef]
- Gocal, G.F. Gene editing in Brassica napus for basic research and trait development. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 731–748. [Google Scholar] [CrossRef]
- Sheng, X.; Yu, H.; Wang, J.; Shen, Y.; Gu, H. Establishment of a stable, effective and universal genetic transformation technique in the diverse species of Brassica oleracea. Front. Plant Sci. 2022, 13, 1021669. [Google Scholar] [CrossRef]
- Wu, L.; El-Mezawy, A.; Shah, S. A seed coat outer integument-specific promoter for Brassica napus. Plant Cell Rep. 2011, 30, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beaith, M.; Chalifoux, M.; Ying, J.; Uchacz, T.; Sarvas, C.; Griffiths, R.; Kuzma, M.; Wan, J.; Huang, Y. Shoot-specific down-regulation of protein farnesyltransferase (α-subunit) for yield protection against drought in canola. Mol. Plant. 2009, 2, 191–200. [Google Scholar] [CrossRef]
- Colby, S.M.; Meredith, C.P. Kanamycin sensitivity of cultured tissues of Vitis. Plant Cell Rep. 1990, 9, 237–240. [Google Scholar] [CrossRef]
- Hardegger, M.; Sturm, A. Transformation and regeneration of carrot (Daucus carota L.). Mol. Breed. 1998, 4, 119–127. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Liu, F.; Liu, Z.-H.; Wang, H.-M.; Yao, C.-B. Effects of kanamycin on tissue culture and somatic embryogenesis in cotton. Plant Growth Regul. 2001, 33, 137–149. [Google Scholar] [CrossRef]
- Khuong, T.T.H.; Crété, P.; Robaglia, C.; Caffarri, S. Optimisation of tomato Micro-tom regeneration and selection on glufosinate/Basta and dependency of gene silencing on transgene copy number. Plant Cell Rep. 2013, 32, 1441–1454. [Google Scholar] [CrossRef]
- Ganasan, K.; Huyop, F. The sensitivity of plant tissue culture and plant cell of Citrullus lanatus cv. Round dragon against BASTA. Int. J. Agric. Res. 2010, 5, 11–18. [Google Scholar] [CrossRef]
- Ricci, A.; Sabbadini, S.; Prieto, H.; Padilla, I.M.; Dardick, C.; Li, Z.; Scorza, R.; Limera, C.; Mezzetti, B.; Perez-Jimenez, M. Genetic transformation in peach (Prunus persica L.): Challenges and ways forward. Plants 2020, 9, 971. [Google Scholar] [CrossRef]
- Camacho-Fernández, C.; Seguí-Simarro, J.M.; Mir, R.; Boutilier, K.; Corral-Martínez, P. Cell Wall Composition and Structure Define the Developmental Fate of Embryogenic Microspores in Brassica napus. Front. Plant Sci. 2021, 12, 737139. [Google Scholar] [CrossRef]
- Kazan, K.; Curtis, M.D.; Goulter, K.C.; Manners, J.M. Agrobacterium tumefaciens-mediated transformation of double haploid canola (Brassica napus) lines. Funct. Plant Biol. 1997, 24, 97–102. [Google Scholar] [CrossRef]
- Dubas, E.; Moravčíková, J.; Libantová, J.; Matušíková, I.; Benková, E.; Żur, I.; Krzewska, M. The influence of heat stress on auxin distribution in transgenic B. napus microspores and microspore-derived embryos. Protoplasma 2014, 251, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Corral-Martinez, P.; Mousavi, A.; Salmanian, A.H.; Moieni, A.; Seguí-Simarro, J.M. An efficient method for transformation of pre-androgenic, isolated Brassica napus microspores involving microprojectile bombardment and Agrobacterium-mediated transformation. Acta Physiol. Plant. 2009, 31, 1313–1317. [Google Scholar] [CrossRef]
- Moore, L.; Warren, G.; Strobel, G. Involvement of a plasmid in the hairy root disease of plants caused by Agrobacterium rhizogenes. Plasmid 1979, 2, 617–626. [Google Scholar] [CrossRef]
- Christey, M.C.; Sinclair, B.K. Regeneration of transgenic kale (Brassica oleracea var. acephala), rape (B. napus) and turnip (B. campestris var. rapifera) plants via Agrobacterium rhizogenes mediated transformation. Plant Sci. 1992, 87, 161–169. [Google Scholar] [CrossRef]
- Jedličková, V.; Mácová, K.; Štefková, M.; Butula, J.; Staveníková, J.; Sedláček, M.; Robert, H.S. Hairy root transformation system as a tool for CRISPR/Cas9-directed genome editing in oilseed rape (Brassica napus). Front. Plant Sci. 2022, 13, 919290. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zeng, F.; Wang, J.; Ye, X.; Zhu, S.; Yuan, L.; Hou, J.; Wang, C. Transgenic Wucai (Brassica campestris L.) produced via Agrobacterium-mediated anther transformation in planta. Plant Cell Rep. 2019, 38, 577–586. [Google Scholar] [CrossRef]
- Park, B.-J.; Liu, Z.; Kanno, A.; Kameya, T. Genetic improvement of Chinese cabbage for salt and drought tolerance by constitutive expression of a B. napus LEA gene. Plant Sci. 2005, 169, 553–558. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Zhao, Y.; Zong, P.; Zhan, Z.; Piao, Z. Establishment of a simple and efficient Agrobacterium-mediated genetic transformation system to Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Hortic. Plant J. 2021, 7, 117–128. [Google Scholar] [CrossRef]
- Chen, L.-F.O.; Hwang, J.-Y.; Charng, Y.-Y.; Sun, C.-W.; Yang, S.-F. Transformation of broccoli (Brassica oleracea var. italica) with isopentenyltransferase gene via Agrobacterium tumefaciens for post-harvest yellowing retardation. Mol. Breed. 2001, 7, 243–257. [Google Scholar] [CrossRef]
- Naeem, I.; Munir, I.; Durrett, T.P.; Iqbal, A.; Aulakh, K.S.; Ahmad, M.A.; Khan, H.; Khan, I.A.; Hussain, F.; Shuaib, M. Feasible regeneration and agro bacterium-mediated transformation of Brassica juncea with Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) gene. Saudi J. Biol. Sci. 2020, 27, 1324–1332. [Google Scholar] [CrossRef]
- Bhalla, P.L.; Smith, N. Agrobacterium tumefaciens-mediated transformation of cauliflower, Brassica oleracea var. botrytis. Mol. Breed. 1998, 4, 531–541. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Viswakarma, N.; Bhat, S.; Kirti, P.; Singh, B.; Chopra, V. Agrobacterium-mediated transformation of cauliflower: Optimization of protocol and development of Bt-transgenic cauliflower. J. Biosci. 2002, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Rafat, A.; Abd Aziz, M.; Abd Rashid, A.; Abdullah, S.N.A.; Kamaladini, H.; Sirchi, M.T.; Javadi, M. Optimization of Agrobacterium tumefaciens-mediated transformation and shoot regeneration after co-cultivation of cabbage (Brassica oleracea subsp. capitata) cv. KY Cross with AtHSP101 gene. Sci. Hort. 2010, 124, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Singh, M.B.; Swoboda, I.; Bhalla, P.L. Agrobacterium-mediated transformation and generation of male sterile lines of Australian canola. Aust. J. Agric. Res. 2005, 56, 353–361. [Google Scholar] [CrossRef]
- Malik, M.R.; Wang, F.; Dirpaul, J.; Zhou, N.; Hammerlindl, J.; Keller, W.; Abrams, S.R.; Ferrie, A.M.R.; Krochko, J.E. Isolation of an embryogenic line from non-embryogenic Brassica napus cv. Westar through microspore embryogenesis. J. Exp. Bot. 2008, 59, 2857–2873. [Google Scholar] [CrossRef]
- Corral-Martinez, P.; Siemons, C.; Horstman, A.; Angenent, G.C.; de Ruijter, N.; Boutilier, K. Live Imaging of embryogenic structures in Brassica napus microspore embryo cultures highlights the developmental plasticity of induced totipotent cells. Plant Reprod. 2020, 33, 143–158. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M.; Nuez, F. Embryogenesis induction, callogenesis, and plant regeneration by in vitro culture of tomato isolated microspores and whole anthers. J. Exp. Bot. 2007, 58, 1119–1132. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M. Androgenesis in solanaceae. In In Vitro Embryogenesis; Germanà, M.A., Lambardi, M., Eds.; Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2016; Volume 1359, pp. 209–244. [Google Scholar]
- Seguí-Simarro, J.M.; Nuez, F. Meiotic metaphase I to telophase II is the most responsive stage of microspore development for induction of androgenesis in tomato (Solanum lycopersicum). Acta Physiol. Plant. 2005, 27, 675–685. [Google Scholar] [CrossRef]
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; Den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012, 69, 181–192. [Google Scholar] [CrossRef]
- Vazquez-Vilar, M.; Bernabé-Orts, J.M.; Fernandez-Del-Carmen, A.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. A modular toolbox for gRNA-Cas9 genome engineering in plants based on the GoldenBraid standard. Plant Methods 2016, 12, 10. [Google Scholar] [CrossRef]


| Genotype | Protocol | Plasmid | Resistance | Explants | Plants/Explant | Percentage of Positive Plants |
|---|---|---|---|---|---|---|
| 4079 | B | YC3.6-Bar | BASTA | 296 | 0.00 | 0% |
| 4079 | B | PM-YC3.6-LTI6b | Kanamycin | 266 | 0.00 | 0% |
| 12075 | B | YC3.6-Bar | BASTA | 285 | 0.00 | 0% |
| 12075 | B | PM-YC3.6-LTI6b | Kanamycin | 403 | 0.00 | 0% |
| 4079 | Z | YC3.6-Bar | BASTA | 138 | 0.00 | 0% |
| 4079 | Z | PM-YC3.6-LTI6b | Kanamycin | 143 | 0.02 | 0% |
| 12075 | Z | YC3.6-Bar | BASTA | 98 | 0.00 | 0% |
| 12075 | Z | PM-YC3.6-LTI6b | Kanamycin | 138 | 0.46 | 0% |
| 4079 | A | YC3.6-Bar | BASTA | 358 | 2.48 | 0% |
| 4079 | A | PM-YC3.6-LTI6b | Kanamycin | 361 | 0.64 | 0% |
| 4079 | A | CRISPR | Kanamycin | 222 | 0.12 | 0% |
| 12075 | A | CRISPR | Kanamycin | 153 | 0.19 | 3.4% |
| Protocol | Plants/Explant |
|---|---|
| B | 0.00 ± 0.00 b |
| Z | 0.12 ± 0.06 ab |
| A | 1.10 ± 0.12 a |
| Psi | TBI | TBII | SOC | Liquid YM | Solid YM | MGL | |
|---|---|---|---|---|---|---|---|
| Potassium acetate (mM) | 30 | ||||||
| RbCl2 (mM) | 100 | 10 | |||||
| CaCl2·2H2O (mM) | 10 | 75 | |||||
| MnCl2·4H2O (mM) | 50 | ||||||
| MOPS (mM) | 10 | ||||||
| MgCl2 (mM) | 52.63 | 10 | |||||
| NaCl (mM) | 10 | 1.7 | 1.7 | 85.5 | |||
| KCl (mM) | 2.5 | ||||||
| MgSO4 (mM) | 10 | ||||||
| MgSO4·7H2O (mM) | 0.8 | 0.8 | 0.4 | ||||
| Glucose (mM) | 20 | ||||||
| K2HPO4·3H2O (mM) | 2.2 | 2.2 | 1.4 | ||||
| Tryptone (%) | 2 | 2 | 0.5 | ||||
| Yeast extract (%) | 0.5 | 0.5 | 0.04 | 0.04 | 0.25 | ||
| Mannitol (%) | 1 | 1 | 0.5 | ||||
| Glycerol (% v/v) | 15 | 15 | |||||
| L-glutamic acid (g/L) | 1 | ||||||
| Biotin (mg/L) | 1 | ||||||
| Bacteriological agar (%) | 1 | ||||||
| Rifampicin (mg/L) | 50 | ||||||
| pH | 7.6 | 5.8 | 6.5 | 7 | 7 | 7 | 7 |
| PM-YC3.6-LTI6b | YC3.6-Bar | CRISPRp | |
|---|---|---|---|
| Kanamycin | 50 mg/L | 50 mg/L | |
| Spectinomycin | 100 mg/L | ||
| Streptomycin | 100 mg/L |
| GM | CIM | SRM | SOM | RRM | CCM | SM | RM | |
|---|---|---|---|---|---|---|---|---|
| MS + V (g/L) | 4.6 | 4.6 | 4.6 | 4.6 | 2.3 | 4.6 | 4.6 | 2.3 |
| MES (g/L) | 2.5 | 2.5 | 2.5 | 2.5 | ||||
| Myo-inositol (g/L) | 1 | 1 | 1 | 1 | ||||
| AgNO3 (mg/L) | 5 | 5 | ||||||
| 2,4-D (mg/L) | 1 | |||||||
| BAP (mg/L) | 5 | 0.05 | 2 | 2 | ||||
| Sucrose (g/L) | 20 | 30 | 30 | 30 | 10 | 30 | 30 | 10 |
| Plant agar (g/L) | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Carbenicillin (mg/L) | 500 | 500 | 100 | 500 | 500 | |||
| pH | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 |
| Oligonucleotide | Sequence (5′ to 3′) |
|---|---|
| 35S 3′ Fw | GATGACGCACAATCCCACTATCC |
| hCas9_3330 Rv | GCAGAATGGCGTCTGACAGG |
| YC3.6 Fw | TAAACGGCCACAGGTTCAGC |
| YC3.6 Rv | CGATCACATGGTCCTGCTGGA |
| GFP5′ Rv | GCGACGTAAACGGCCACAAGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabuig-Serna, A.; Mir, R.; Porcel, R.; Seguí-Simarro, J.M. The Highly Embryogenic Brassica napus DH4079 Line Is Recalcitrant to Agrobacterium-Mediated Genetic Transformation. Plants 2023, 12, 2008. https://doi.org/10.3390/plants12102008
Calabuig-Serna A, Mir R, Porcel R, Seguí-Simarro JM. The Highly Embryogenic Brassica napus DH4079 Line Is Recalcitrant to Agrobacterium-Mediated Genetic Transformation. Plants. 2023; 12(10):2008. https://doi.org/10.3390/plants12102008
Chicago/Turabian StyleCalabuig-Serna, Antonio, Ricardo Mir, Rosa Porcel, and Jose M. Seguí-Simarro. 2023. "The Highly Embryogenic Brassica napus DH4079 Line Is Recalcitrant to Agrobacterium-Mediated Genetic Transformation" Plants 12, no. 10: 2008. https://doi.org/10.3390/plants12102008
APA StyleCalabuig-Serna, A., Mir, R., Porcel, R., & Seguí-Simarro, J. M. (2023). The Highly Embryogenic Brassica napus DH4079 Line Is Recalcitrant to Agrobacterium-Mediated Genetic Transformation. Plants, 12(10), 2008. https://doi.org/10.3390/plants12102008










