Genome-Wide Identification and Expression Profiling of Pathogenesis-Related Protein 1 (PR-1) Genes in Durum Wheat (Triticum durum Desf.)
Abstract
1. Introduction
2. Results
2.1. Identification, Distribution, Gene Structures, and Conserved Motifs of PR-1 in Triticum durum
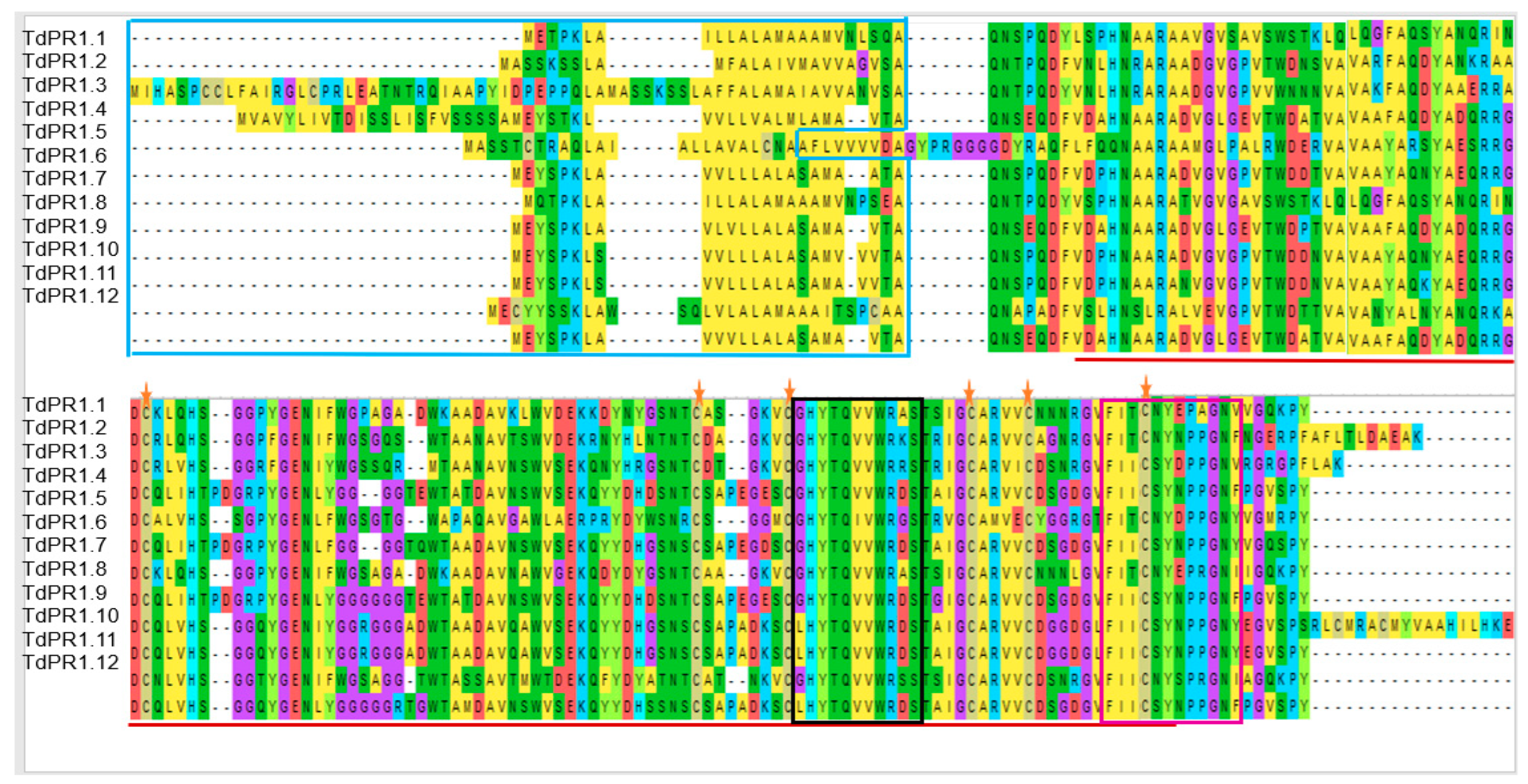
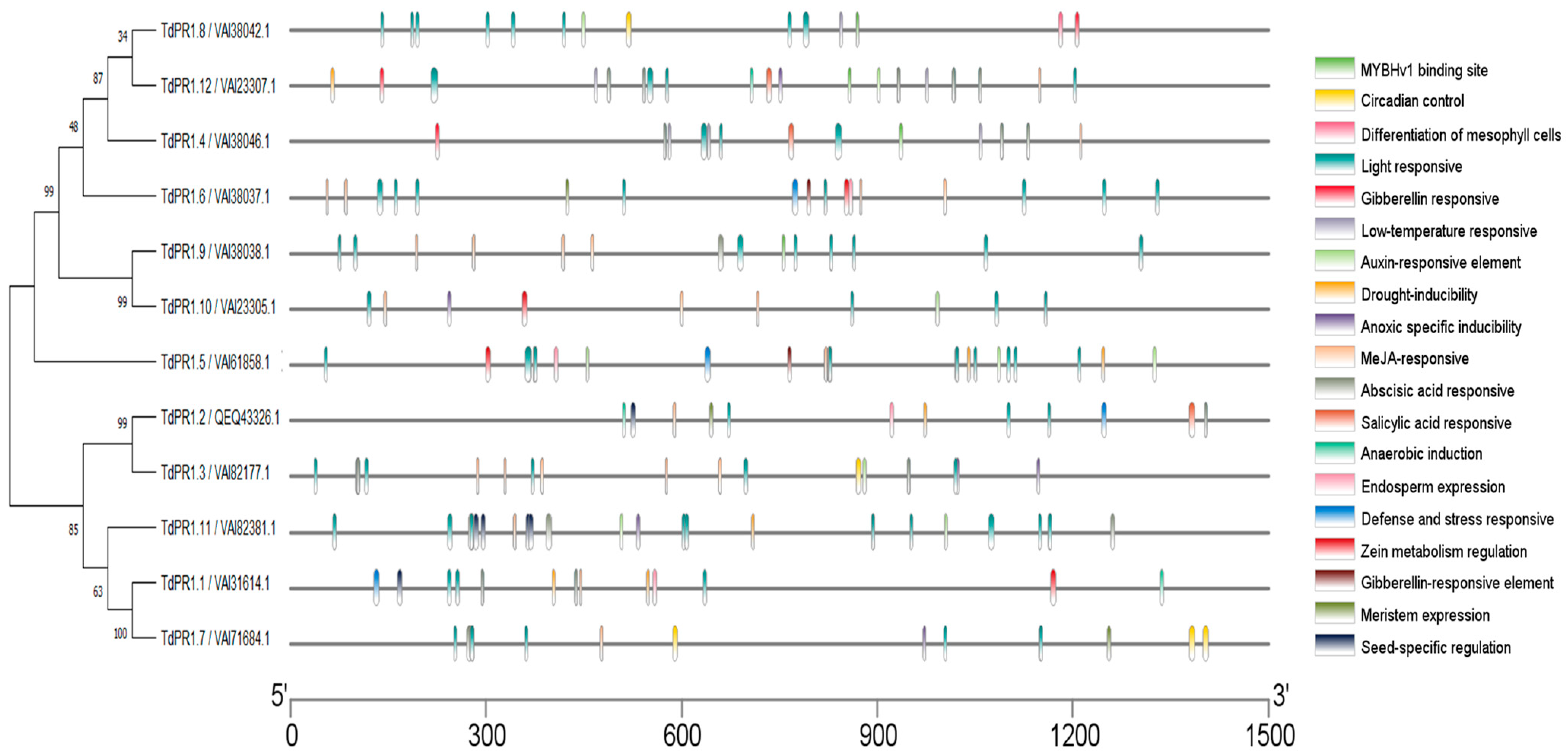
2.2. Multiple Alignment and Phylogenetic Relationship among the TdPR1 Genes
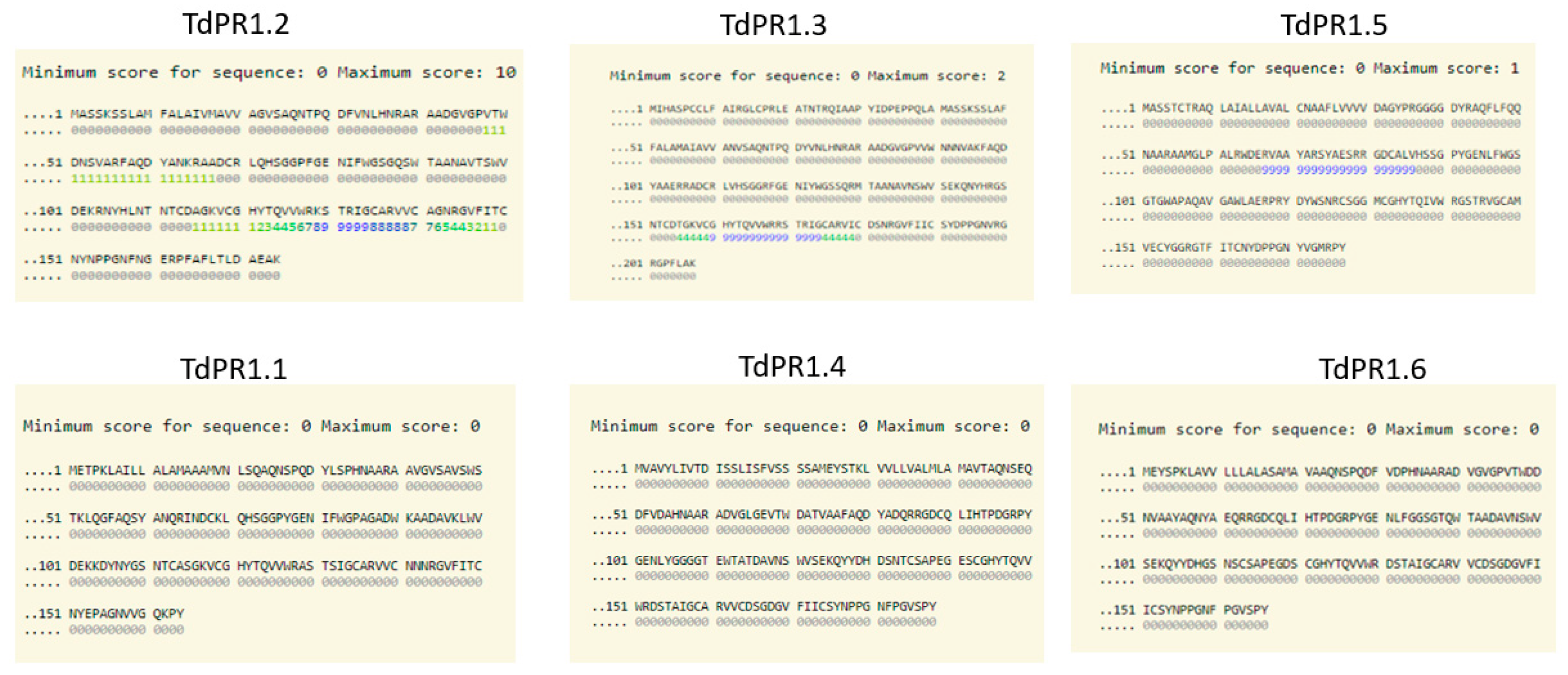
2.3. Identification of Putative Zn2+ and CaM Binding Domains
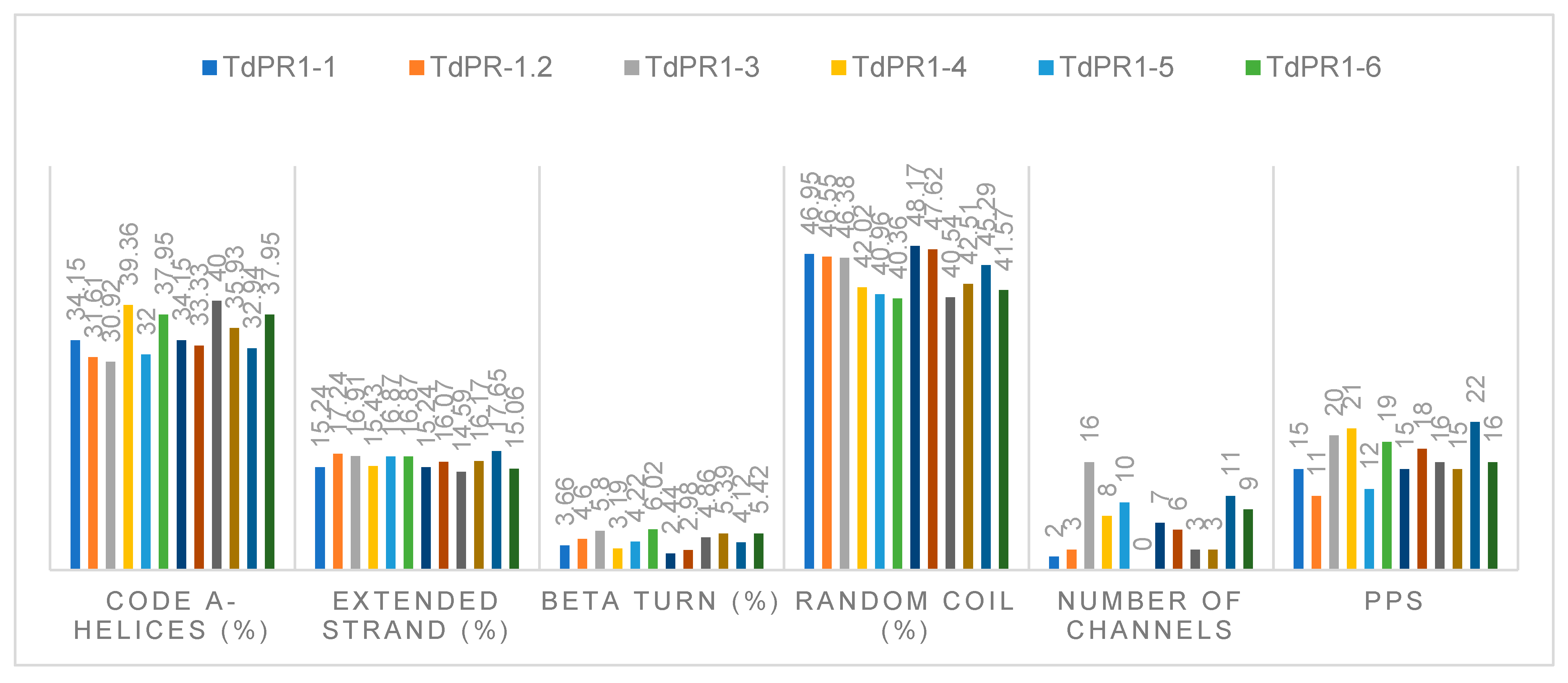
2.4. Physicochemical Properties
2.5. Prediction of Transmembrane Helices on TdPR1
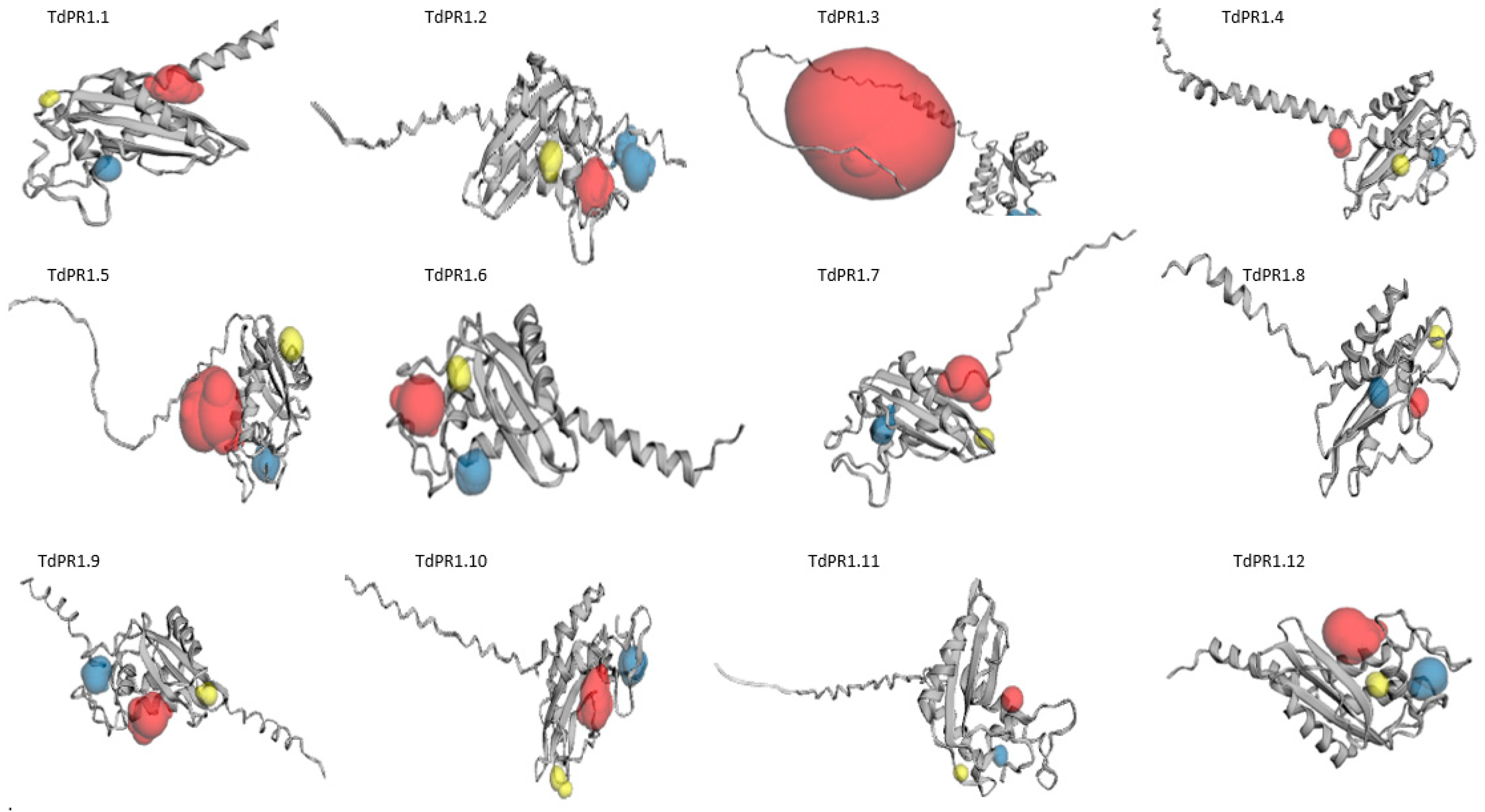
2.6. Predicted Secondary and 3D Structures of TdPR-1 Proteins
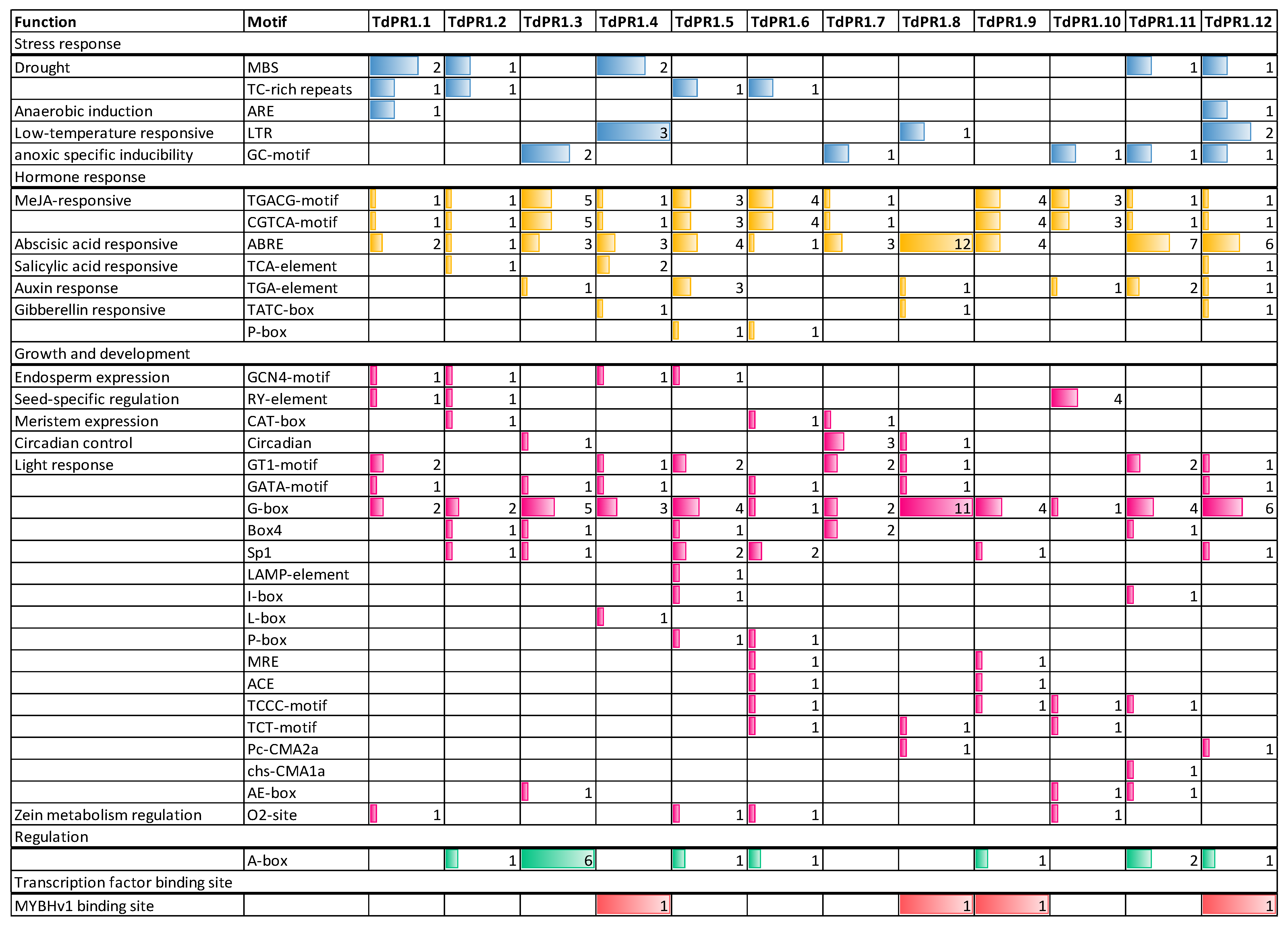
2.7. In Silico Analysis of Cis-Elements
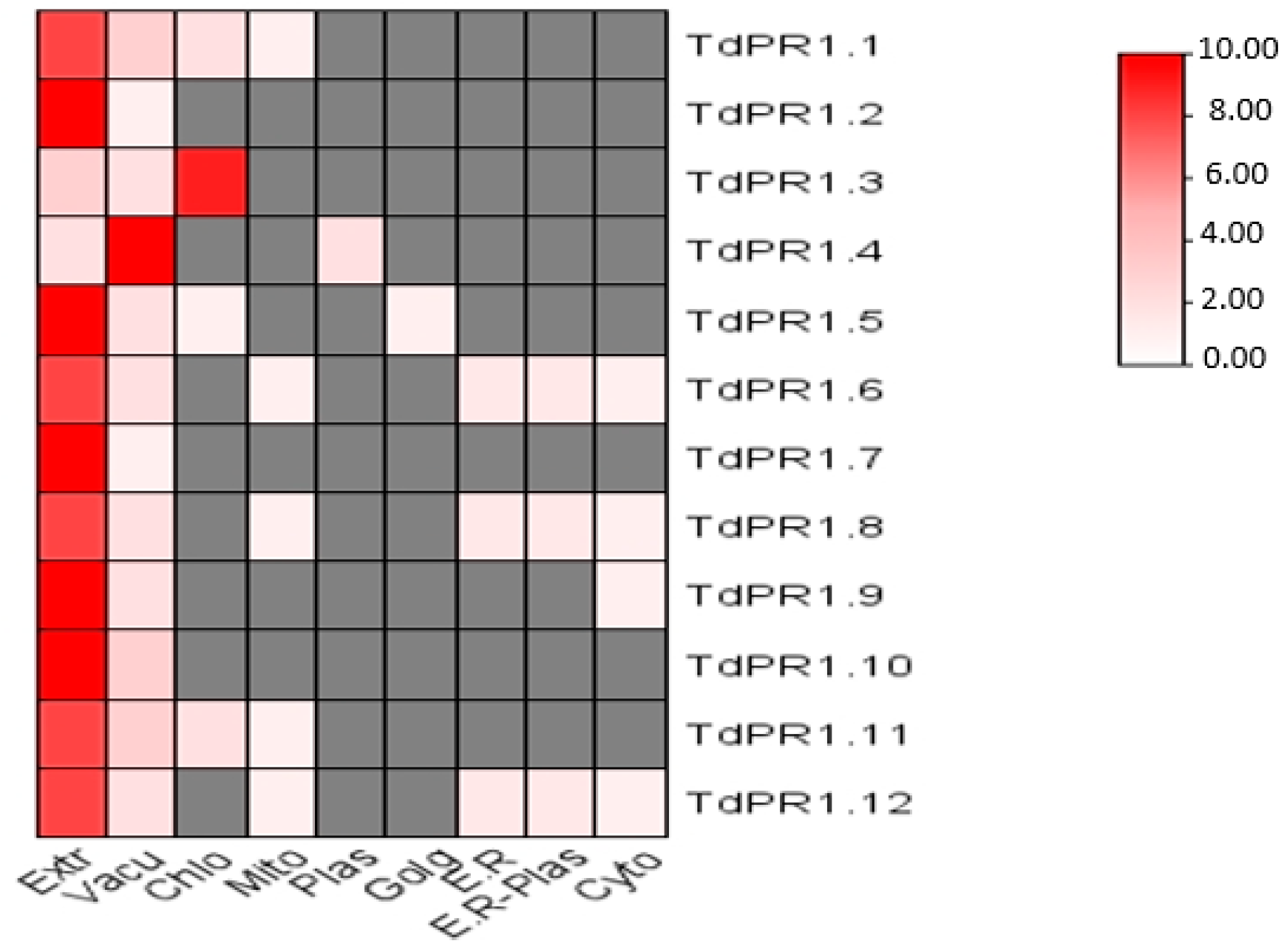
2.8. Predicting TdPR1 Pproteins Subcellular Localization
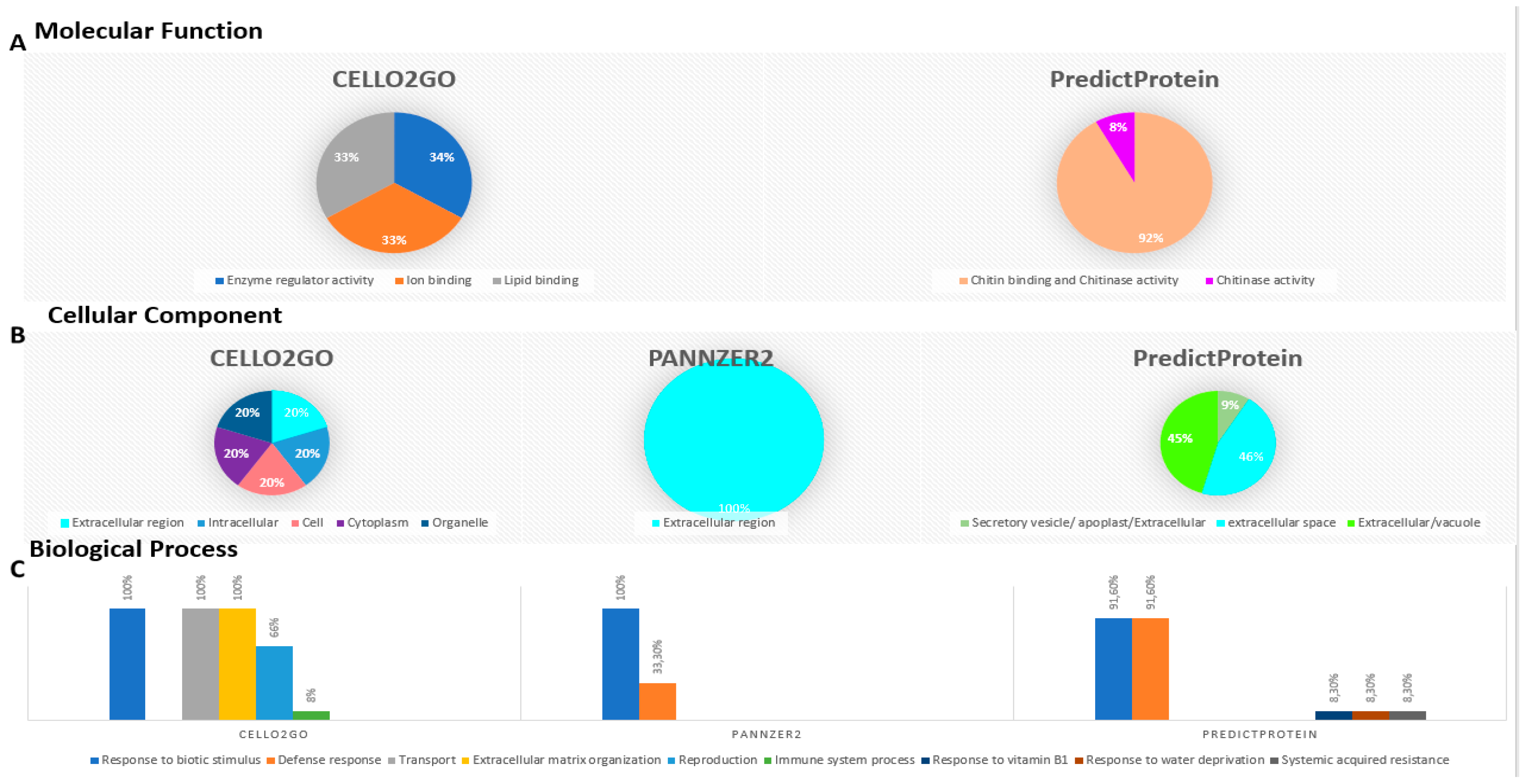
2.9. Gene Ontology (GO) Term Distribution of Triticum durum PR-1
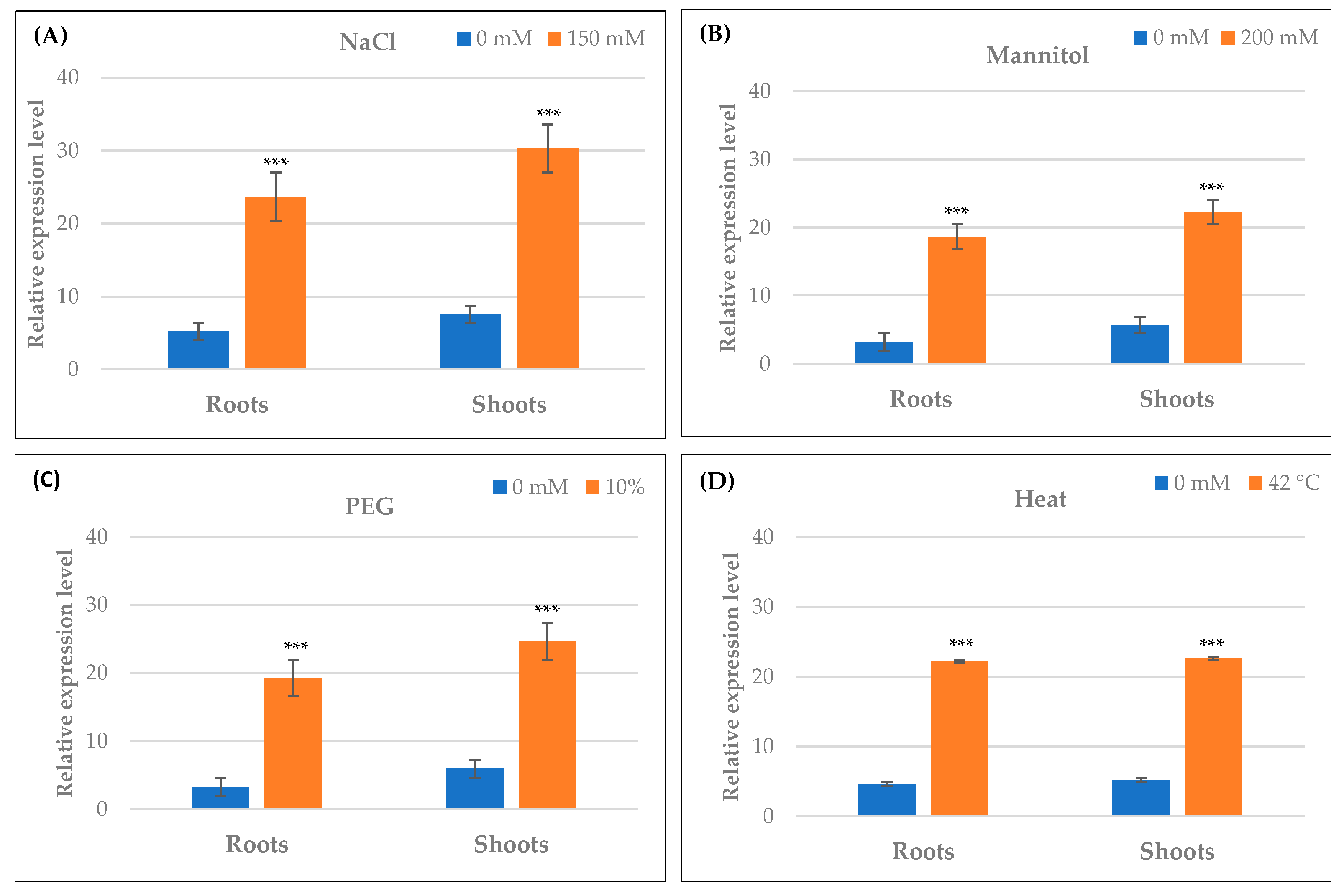
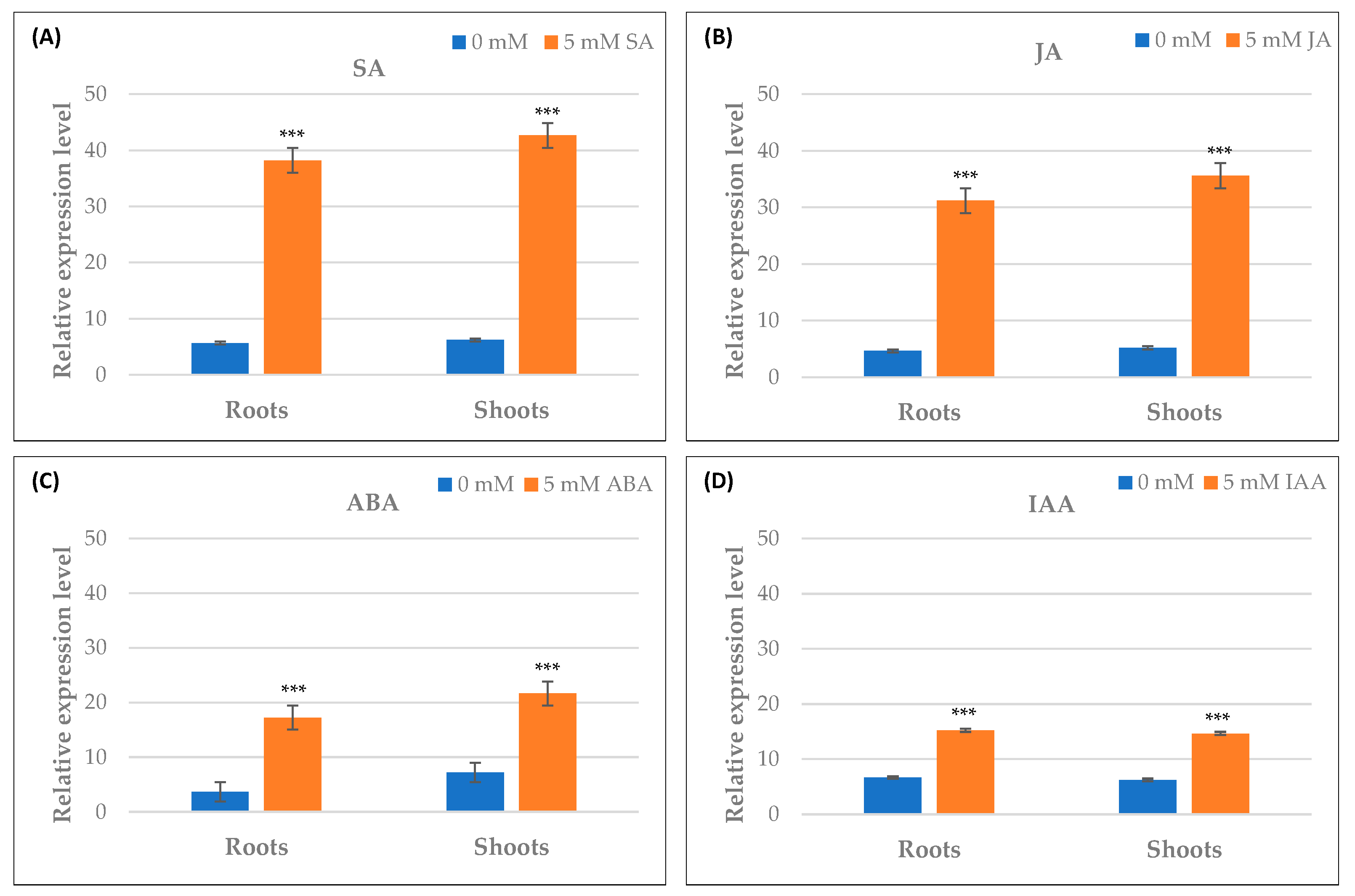
2.10. Differential Expression of TdPR1.2 Gene under Various Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Identification of PR-1 Genes from the T. durum Genome
4.2. Sequence Analysis
4.3. Topological Analysis
4.4. Secondary and Tertiary Structure Prediction
4.5. Conserved Motif, Multiple Alignment, and Phylogenetic Tree
4.6. Cis-Elements, Chromosomal Locations, and Gene Structure Analyses
4.7. Subcellular Localization and Gene Ontology Analysis
4.8. Plant Material and Stress Treatments
4.9. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, B.; Raina, A.; Khan, S. Biotic and Abiotic Stresses, Impact on Plants and Their Response. In Disease Resistance in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. Abiotic Biot. Stress Plants 2019, 1–19. [Google Scholar] [CrossRef]
- Jwa, N.-S.; Agrawal, G.K.; Rakwal, R.; Park, C.-H.; Agrawal, V.P. Molecular Cloning and Characterization of a Novel Jasmonate Inducible Pathogenesis-Related Class 10 Protein Gene, JIOsPR10, from Rice (Oryza sativa L.) Seedling Leaves. Biochem. Biophys. Res. Commun. 2001, 286, 973–983. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef]
- Punja, Z.K. Genetic Engineering of Plants to Enhance Resistance to Fungal Pathogens—A Review of Progress and Future Prospects. Can. J. Plant Pathol. 2001, 23, 216–235. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Yildiz, S.; Filiz, E. Pathogenesis Related Protein-1 (PR-1) Genes in Tomato (Solanum lycopersicum L.): Bioinformatics Analyses and Expression Profiles in Response to Drought Stress. Genomics 2020, 112, 4089–4099. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.-S.; Rakwal, R. A Novel Rice (Oryza sativa L.) Acidic PR1 Gene Highly Responsive to Cut, Phytohormones, and Protein Phosphatase Inhibitors. Biochem. Biophys. Res. Commun. 2000, 274, 157–165. [Google Scholar] [CrossRef]
- Ghorbel, M.; Zribi, I.; Haddaji, N.; Besbes, M.; Bouali, N.; Brini, F. The Wheat Pathogenesis Related Protein (TdPR1. 2) Ensures Contrasting Behaviors to E. coli Transformant Cells under Stress Conditions. Adv. Microbiol. 2021, 11, 453–468. [Google Scholar] [CrossRef]
- Alhudaib, K.; Alanazi, N.; Ghorbel, M.; El-Ganainy, S.; Brini, F. Isolation and Characterization of a Novel Pathogenesis-Related Protein-1 Gene (AvPR-1) with Induced Expression in Oat (Avena sativa L.) during Abiotic and Hormonal Stresses. Plants 2022, 11, 2284. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.; Dong, X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Van Loon, L.; Pierpoint, W.; Boller, T.; Conejero, V. Recommendations for Naming Plant Pathogenesis-Related Proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Kaur, A.; Sarao, N.; Sharma, D. Pathogenesis-Related Proteins and Their Transgenic Expression for Developing Disease-Resistant Crops: Strategies Progress and Challenges; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Gibbs, G.M.; Roelants, K.; O’bryan, M.K. The CAP Superfamily: Cysteine-Rich Secretory Proteins, Antigen 5, and Pathogenesis-Related 1 Proteins—Roles in Reproduction, Cancer, and Immune Defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.; Van Kammen, A. Polyacrylamide Disc Electrophoresis of the Soluble Leaf Proteins from Nicotiana Tabacum Var.‘Samsun’ and ‘Samsun NN’: II. Changes in Protein Constitution after Infection with Tobacco Mosaic Virus. Virology 1970, 40, 199–211. [Google Scholar] [CrossRef]
- Van Loon, L.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-Related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135162. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, N.; Zhang, Y.; Yu, Y.; Liu, S. Genome-Wide Characterization and Expression Analysis of Pathogenesis-Related 1 (PR-1) Gene Family in Tea Plant (Camellia sinensis (L.) O. Kuntze) in Response to Blister-Blight Disease Stress. Int. J. Mol. Sci. 2022, 23, 1292. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Friesen, T.L.; Faris, J.D. Molecular Characterization and Genomic Mapping of the Pathogenesis-Related Protein 1 (PR-1) Gene Family in Hexaploid Wheat (Triticum aestivum L.). Mol. Genet. Genom. 2011, 285, 485–503. [Google Scholar] [CrossRef]
- Kattupalli, D.; Srinivasan, A.; Soniya, E.V. A Genome-Wide Analysis of Pathogenesis-Related Protein-1 (PR-1) Genes from Piper Nigrum Reveals Its Critical Role during Phytophthora Capsici Infection. Genes 2021, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Silva, F.; Venancio, T.M. Pathogenesis-Related Protein 1 (PR-1) Genes in Soybean: Genome-Wide Identification, Structural Analysis and Expression Profiling under Multiple Biotic and Abiotic Stresses. Gene 2022, 809, 146013. [Google Scholar] [CrossRef]
- Li, Z.T.; Dhekney, S.A.; Gray, D.J. PR-1 Gene Family of Grapevine: A Uniquely Duplicated PR-1 Gene from a Vitis Interspecific Hybrid Confers High Level Resistance to Bacterial Disease in Transgenic Tobacco. Plant Cell Rep. 2011, 30, 1–11. [Google Scholar] [CrossRef]
- Edreva, A. Pathogenesis-Related Proteins: Research Progress in the Last 15 Years. Gen. Appl. Plant Physiol. 2005, 31, 105–124. [Google Scholar]
- Chu, N.; Zhou, J.-R.; Rott, P.C.; Li, J.; Fu, H.-Y.; Huang, M.-T.; Zhang, H.-L.; Gao, S.-J. ScPR1 Plays a Positive Role in the Regulation of Resistance to Diverse Stresses in Sugarcane (Saccharum spp.) and Arabidopsis thaliana. Ind. Crops Prod. 2022, 180, 114736. [Google Scholar] [CrossRef]
- Anuradha, C.; Arumugam, C.; Suthanthiram, B.; Raman, T.; Giribabu, P.; Uma, S. Genome-Wide Analysis of Pathogenesis-Related Protein 1 (PR-1) Gene Family from Musa spp. and Its Role in Defense Response during Stresses. Gene 2022, 821, 146334. [Google Scholar] [CrossRef] [PubMed]
- Chassot, C.; Nawrath, C.; Métraux, J. Cuticular Defects Lead to Full Immunity to a Major Plant Pathogen. Plant J. 2007, 49, 972–980. [Google Scholar] [CrossRef]
- Shin, S.H.; Pak, J.-H.; Kim, M.J.; Kim, H.J.; Oh, J.S.; Choi, H.K.; Jung, H.W.; Chung, Y.S. An Acidic Pathogenesis-Related1 Gene of Oryza Grandiglumis Is Involved in Disease Resistance Response against Bacterial Infection. Plant Pathol. J. 2014, 30, 208. [Google Scholar] [CrossRef]
- Schneiter, R.; Di Pietro, A. The CAP Protein Superfamily: Function in Sterol Export and Fungal Virulence. Biomol. Concepts 2013, 4, 519–525. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lee, C.-Y.; Cheng, K.-T.; Chang, W.-H.; Huang, R.-N.; Nam, H.G.; Chen, Y.-R. Quantitative Peptidomics Study Reveals That a Wound-Induced Peptide from PR-1 Regulates Immune Signaling in Tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef]
- Fernández, C.; Szyperski, T.; Bruyere, T.; Ramage, P.; Mösinger, E.; Wüthrich, K. NMR Solution Structure of the Pathogenesis-Related Protein P14a. J. Mol. Biol. 1997, 266, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Van Strien, E. The Families of Pathogenesis-Related Proteins, Their Activities, and Comparative Analysis of PR-1 Type Proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Ghorbel, M.; Zribi, I.; Missaoui, K.; Drira-Fakhfekh, M.; Azzouzi, B.; Brini, F. Differential Regulation of the Durum Wheat Pathogenesis-Related Protein (PR1) by Calmodulin TdCaM1. 3 Protein. Mol. Biol. Rep. 2021, 48, 347–362. [Google Scholar] [CrossRef]
- Seo, J.S.; Diloknawarit, P.; Park, B.S.; Chua, N. ELF18-INDUCED LONG NONCODING RNA 1 Evicts Fibrillarin from Mediator Subunit to Enhance PATHOGENESIS-RELATED GENE 1 (PR1) Expression. New Phytol. 2019, 221, 2067–2079. [Google Scholar] [CrossRef]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-Related Protein 1b1 (PR1b1) Is a Major Tomato Fruit Protein Responsive to Chilling Temperature and Upregulated in High Polyamine Transgenic Genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef] [PubMed]
- Kiba, A.; Nishihara, M.; Nakatsuka, T.; Yamamura, S. Pathogenesis-Related Protein 1 Homologue Is an Antifungal Protein in Wasabia Japonica Leaves and Confers Resistance to Botrytis Cinerea in Transgenic Tobacco. Plant Biotechnol. 2007, 24, 247–253. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, E.N.; Kim, K.D.; Hwang, B.K.; Islam, R.; Shin, J.S. Overexpression of a Pepper Basic Pathogenesis-Related Protein 1 Gene in Tobacco Plants Enhances Resistance to Heavy Metal and Pathogen Stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, X.; Wang, R.; Li, A.; Zhao, G.; Zhao, J.; Jing, R. Identification of Wheat Stress-Responding Genes and TaPR-1-1 Function by Screening a CDNA Yeast Library Prepared Following Abiotic Stress. Sci. Rep. 2019, 9, 141. [Google Scholar] [CrossRef]
- Hussain, R.M.F.; Sheikh, A.H.; Haider, I.; Quareshy, M.; Linthorst, H.J.M. Arabidopsis WRKY50 and TGA Transcription Factors Synergistically Activate Expression of PR1. Front. Plant Sci. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- van Verk, M.; Neeleman, L.; Bol, J.; Linthorst, H. Tobacco Transcription Factor NtWRKY12 Interacts with TGA2.2 in Vitro and in Vivo. Front. Plant Sci. 2011, 2, 32. [Google Scholar] [CrossRef]
- Sall, A.T.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; van Ginkel, M.; Bassi, F.M. Durum Wheat (Triticum durum Desf.): Origin, Cultivation and Potential Expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Feldman, M. Origin of Cultivated Wheat. In The World Wheat Book: A History of Wheat Breeding; Intercept Ltd.: Bambous, Mauritius, 2001; pp. 3–53. [Google Scholar]
- Ben Krima, S.; Slim, A.; Gelisse, S.; Kouki, H.; Nadaud, I.; Sourdille, P.; Yahyoui, A.; Ben M’Barek, S.; Suffert, F.; Marcel, T. Life Story of Tunisian Durum Wheat Landraces Revealed by Their Genetic and Phenotypic Diversity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Robbana, C.; Kehel, Z.; Ammar, K.; Guzmán, C.; Naceur, M.B.; Amri, A. Unlocking the Patterns of the Tunisian Durum Wheat Landraces Genetic Structure Based on Phenotypic Characterization in Relation to Farmer’s Vernacular Name. Agronomy 2021, 11, 634. [Google Scholar] [CrossRef]
- Huhn, M.R.; Elias, E.M.; Ghavami, F.; Kianian, S.F.; Chao, S.; Zhong, S.; Alamri, M.S.; Yahyaoui, A.; Mergoum, M. Tetraploid Tunisian Wheat Germplasm as a New Source of Fusarium Head Blight Resistance. Crop Sci. 2012, 52, 136–145. [Google Scholar] [CrossRef]
- Ben M’Barek, S.; Laribi, M.; Kouki, H.; Castillo, D.; Araar, C.; Nefzaoui, M.; Ammar, K.; Saint-Pierre, C.; Yahyaoui, A.H. Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria Tritici in Tunisia. Genes 2022, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, M.M.; Yahyaoui, A.; Rezgui, S.; Elias, E.M.; Daaloul, A. Inheritances of Fusarium Head Blight Resistance in a Cross Involving Local and Exotic Durum Wheat Cultivars. Crop Sci. 2011, 51, 2517–2524. [Google Scholar] [CrossRef]
- Abdedayem, W.; Patpour, M.; Laribi, M.; Justesen, A.F.; Kouki, H.; Fakhfakh, M.; Hovmøller, M.S.; Yahyaoui, A.H.; Hamza, S.; Ben M’Barek, S. Wheat Stem Rust Detection and Race Characterization in Tunisia. Plants 2023, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Miazzi, M.M.; Babay, E.; De Vita, P.; Montemurro, C.; Chaabane, R.; Taranto, F.; Mangini, G. Comparative Genetic Analysis of Durum Wheat Landraces and Cultivars Widespread in Tunisia. Front. Plant Sci. 2022, 13, 939609. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and Identifying Crop Landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Rachon, L.; Dziamba, S.; Obuchowski, W.; Kolodziejczyk, P. The usefulness of durum wheat (Triticum durum) and common wheat (Triticum aestivum ssp. vulgare) cultivars for pasta production. Ann. Univ. Mariae Curie-Sklodowska Sect. E Agric. 2002, 57, 77–86. [Google Scholar]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Ikai, A. Thermostability and Aliphatic Index of Globular Proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [CrossRef]
- Cutillas, P.R. Chapter Fourteen-Targeted In-Depth Quantification of Signaling Using Label-Free Mass Spectrometry. In Methods in Enzymology; Shukla, A.K., Ed.; Proteomics in Biology, Part A; Academic Press: Cambridge, MA, USA, 2017; Volume 585, pp. 245–268. [Google Scholar] [CrossRef]
- Liu, R.; Lu, J.; Xing, J.; Xue, L.; Wu, Y.; Zhang, L. Characterization and Functional Analyses of Wheat TaPR1 Genes in Response to Stripe Rust Fungal Infection. Sci. Rep. 2023, 13, 3362. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Li, X.-Y.; Wei, X.-J.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Expression and Functional Analysis of a Pathogenesis-Related Protein 1 Gene, TcLr19PR1, Involved in Wheat Resistance Against Leaf Rust Fungus. Plant Mol. Biol. Rep. 2015, 33, 797–805. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhang, W.; Zhang, J.; Wang, H.; Liu, D. Expression Profiles of Pathogenesis-Related Gene, TaLr35PR1, as It Relate to Lr35-Mediated Adult Plant Leaf Rust Resistance. Plant Mol. Biol. Rep. 2016, 34, 1127–1135. [Google Scholar] [CrossRef]
- Bi, W.; Zhao, S.; Zhao, J.; Su, J.; Yu, X.; Liu, D.; Kang, Z.; Wang, X.; Wang, X. Rust Effector PNPi Interacting with Wheat TaPR1a Attenuates Plant Defense Response. Phytopathol. Res. 2020, 2, 34. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, S.; Wu, W.; Yang, Y.; Cui, Z.; Wang, H.; Liu, D. TaTLP1 Interacts with TaPR1 to Contribute to Wheat Defense Responses to Leaf Rust Fungus. PLoS Genet. 2020, 16, e1008713. [Google Scholar] [CrossRef]
- Yang, F.; Melo-Braga, M.N.; Larsen, M.R.; Jørgensen, H.J.L.; Palmisano, G. Battle through Signaling between Wheat and the Fungal Pathogen Septoria Tritici Revealed by Proteomics and Phosphoproteomics. Mol. Cell. Proteom. 2013, 12, 2497–2508. [Google Scholar] [CrossRef]
- Sadeghi, M.; Reza, H.-H.; Ghasem, A.; Mohsen, M. Quantitative Gene Expression of Candidate Genes for Septoria Tritici Blotch (STB) Resistance in Wheat Infected by Mycosphaerella Graminicola. Crop Biotechnol. 2018, 7, 75–85. [Google Scholar]
- Farsad, L.K.; Mohsen, M.; Mohammad Ali, E. Quantitative Expression Analysis of Candidate Genes for Septoria Tritici Blotch Resistance in Wheat (Triticum aestivum L.). Prog. Biol. Sci. 2013, 3, 72–78. [Google Scholar]
- Liu, Q.; Xue, Q. Computational Identification of NovelPR-1-Type Genes Inoryza sativa. J. Genet. 2006, 85, 193–198. [Google Scholar] [CrossRef]
- Chen, J.; Gao, T.; Wan, S.; Zhang, Y.; Yang, J.; Yu, Y.; Wang, W. Genome-Wide Identification, Classification and Expression Analysis of the HSP Gene Superfamily in Tea Plant (Camellia sinensis). Int. J. Mol. Sci. 2018, 19, 2633. [Google Scholar] [CrossRef]
- Shikamoto, Y.; Suto, K.; Yamazaki, Y.; Morita, T.; Mizuno, H. Crystal Structure of a CRISP Family Ca2+-Channel Blocker Derived from Snake Venom. J. Mol. Biol. 2005, 350, 735–743. [Google Scholar] [CrossRef]
- Lincoln, J.E.; Sanchez, J.P.; Zumstein, K.; Gilchrist, D.G. Plant and Animal PR1 Family Members Inhibit Programmed Cell Death and Suppress Bacterial Pathogens in Plant Tissues. Mol. Plant Pathol. 2018, 19, 2111–2123. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Y.; Wei, W.; Lin, Y.; Yin, W.; Luo, C. Development of Novel Methods for Functional Evaluation of the Signal Peptide of Secreted Protein. Physiol. Mol. Plant Pathol. 2019, 106, 182–186. [Google Scholar] [CrossRef]
- Khunjan, U.; Ekchaweng, K.; Panrat, T.; Tian, M.; Churngchow, N. Molecular Cloning of HbPR-1 Gene from Rubber Tree, Expression of HbPR-1 Gene in Nicotiana Benthamiana and Its Inhibition of Phytophthora Palmivora. PLoS ONE 2016, 11, e0157591. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Poovaiah, B.W. Hydrogen Peroxide Homeostasis: Activation of Plant Catalase by Calcium/Calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef]
- Ghorbel, M.; Feki, K.; Tounsi, S.; Haddaji, N.; Hanin, M.; Brini, F. The Activity of the Durum Wheat (Triticum durum L.) Catalase 1 (TdCAT1) Is Modulated by Calmodulin. Antioxidants 2022, 11, 1483. [Google Scholar] [CrossRef]
- Ghorbel, M.; Zaidi, I.; Robe, E.; Ranty, B.; Mazars, C.; Galaud, J.-P.; Hanin, M. The Activity of the Wheat MAP Kinase Phosphatase 1 Is Regulated by Manganese and by Calmodulin. Biochimie 2015, 108, 13–19. [Google Scholar] [CrossRef]
- Ghorbel, M.; Zaidi, I.; Ebel, C.; Hanin, M. Differential regulation of the durum wheat MAPK phosphatase 1 by calmodulin, bivalent cations and possibly mitogen activated protein kinase 3. Plant Physiol. Biochem. 2019, 135, 242–252. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Galaud, J.-P. Plant Calmodulins and Calmodulin-Related Proteins: Multifaceted Relays to Decode Calcium Signals. Plant Signal. Behav. 2006, 1, 96–104. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent Advances in Calcium/Calmodulin-Mediated Signaling with an Emphasis on Plant-Microbe Interactions. Plant Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef]
- Shi, F. Cloning and Function Study of Pathogenesis-Related Protein Genes ZmPR-1 and ZmPR-4. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2019. [Google Scholar]
- LiXia, H.; Chao, G.; YongMei, C.; FangGui, Z.; Xin, L. Gene cloning and expression analysis of pathogenesis-related protein 1 in Vitis vinifera. Plant Physiol. Commun. 2012, 48, 57–62. [Google Scholar]
- Kothari, K.S.; Dansana, P.K.; Giri, J.; Tyagi, A.K. Rice Stress Associated Protein 1 (OsSAP1) Interacts with Aminotransferase (OsAMTR1) and Pathogenesis-Related 1a Protein (OsSCP) and Regulates Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1057. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kuang, J.; Wang, F.; Chen, L.; Hong, K.; Xiao, Y.; Xie, H.; Lu, W.; Chen, J. Molecular Characterization of PR and WRKY Genes during SA- and MeJA-Induced Resistance against Colletotrichum Musae in Banana Fruit. Postharvest Biol. Technol. 2013, 79, 62–68. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-Related Genes of PR1, PR2, PR4, and PR5 Families Are Involved in the Response to Fusarium Infection in Garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 Years of the SMART Protein Domain Annotation Resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s Conserved Domain Database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Yang, J.; Roy, A.; Zhang, Y. BioLiP: A Semi-Manually Curated Database for Biologically Relevant Ligand–Protein Interactions. Nucleic Acids Res. 2013, 41, D1096–D1103. [Google Scholar] [CrossRef]
- Yang, J.; Roy, A.; Zhang, Y. Protein–Ligand Binding Site Recognition Using Complementary Binding-Specific Substructure Comparison and Sequence Profile Alignment. Bioinformatics 2013, 29, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin Target Database. J. Struct. Func. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Hirokawa, T.; Boon-Chieng, S.; Mitaku, S. SOSUI: Classification and Secondary Structure Prediction System for Membrane Proteins. Bioinformatics 1998, 14, 378–379. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Dosztányi, Z.; Simon, I. TMDET: Web Server for Detecting Transmembrane Regions of Proteins by Using Their 3D Coordinates. Bioinformatics 2005, 21, 1276–1277. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. SOPMA: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and Structure-Based Prediction of Eukaryotic Protein Phosphorylation Sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of Post-Translational Glycosylation and Phosphorylation of Proteins from the Amino Acid Sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, Y.; Lee, M.; Laskowski, R.A.; Ryu, S.E.; Sugihara, K.; Kim, D.-S. BetaCavityWeb: A Webserver for Molecular Voids and Channels. Nucleic Acids Res. 2015, 43, W413–W418. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed Atlas of Surface Topography of Proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE Signature Matches and ProRule-Associated Functional and Structural Residues in Proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Schneider, T.D.; Stephens, R.M. Sequence Logos: A New Way to Display Consensus Sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Jiangtao, C.; Yingzhen, K.; Qian, W.; Yuhe, S.; Daping, G.; Jing, L.; Guanshan, L. MapGene2Chrom, a Tool to Draw Gene Physical Map Based on Perl and SVG Languages. Yi Chuan 2015, 37, 91–97. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A User-Friendly Online Tool for Drawing Genetic Maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35 (Suppl. S2), W585–W587. [Google Scholar] [CrossRef]
- Törönen, P.; Medlar, A.; Holm, L. PANNZER2: A Rapid Functional Annotation Web Server. Nucleic Acids Res. 2018, 46, W84–W88. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-S.; Cheng, C.-W.; Su, W.-C.; Chang, K.-C.; Huang, S.-W.; Hwang, J.-K.; Lu, C.-H. CELLO2GO: A Web Server for Protein SubCELlular LOcalization Prediction with Functional Gene Ontology Annotation. PLoS ONE 2014, 9, e99368. [Google Scholar] [CrossRef]
- Yachdav, G.; Kloppmann, E.; Kajan, L.; Hecht, M.; Goldberg, T.; Hamp, T.; Hönigschmid, P.; Schafferhans, A.; Roos, M.; Bernhofer, M.; et al. PredictProtein—An Open Resource for Online Prediction of Protein Structural and Functional Features. Nucleic Acids Res. 2014, 42, W337–W343. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
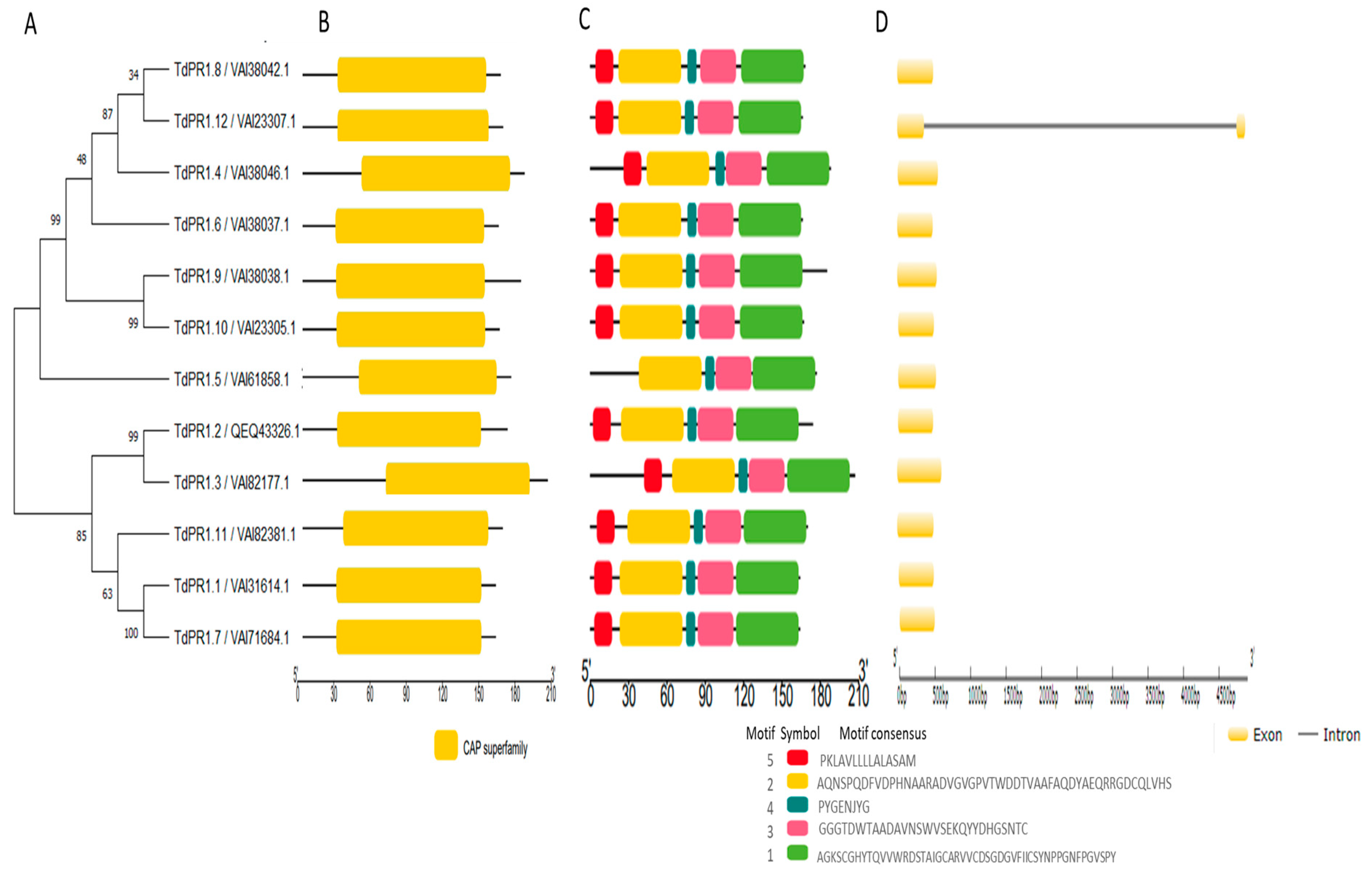



| Gene Name | Locus/Protein Id | Gene Identifier/ ORF Names/Locus Tag | Chr | Strand | EMBL ID | Start | End | N° Exon |
|---|---|---|---|---|---|---|---|---|
| TdPR1.1 | VAI31614.1 | TRITD5Bv1G112400 | Chr5B | − | LT934120.1 | 330,646,842 | 330,647,336 | 1 |
| TdPR1.2 | QEQ43326.1 | TRITD_7Bv1G043610 | Chr7B | + | MK570869.1 | 120,612,592 | 120,613,116 | 1 |
| TdPR1.3 | VAI82177.1 | TRITD_7Av1G281080 | ChrA | − | LT934123.1 | 727,376,831 | 727,377,454 | 1 |
| TdPR1.4 | VAI38046.1 | TRITD_5Bv1G217120 | Chr5B | + | LT934120.1 | 613,827,887 | 613,828,453 | 1 |
| TdPR1.5 | VAI61858.1 | TRITD6Bv1G202080 | Chr6B | + | LT934122.1 | 630,324,650 | 630,325,183 | 1 |
| TdPR1.6 | VAI38037.1 | TRITD_5Bv1G216890 | Chr5B | + | LT934120.1 | 613,290,494 | 613,290,994 | 1 |
| TdPR1.7 | VAI71684.1 | TRITD7Av1G051340 | Chr7A | + | LT934123.1 | 113,022,659 | 113,023,153 | 1 |
| TdPR1.8 | VAI38042.1 | TRITD5Bv1G217000 | Chr5B | − | LT934120.1 | 613,580,189 | 613,580,695 | 1 |
| TdPR1.9 | VAI38038.1 | TRITD_5Bv1G216900 | Chr5B | + | LT934120.1 | 613,295,645 | 613,296,202 | 1 |
| TdPR1.10 | VAI23305.1 | TRITD_5Av1G219320 | Chr5A | + | LT934119.1 | 582,431,569 | 582,432,072 | 1 |
| TdPR1.11 | VAI82381.1 | TRITD_7Bv1G001260 | Chr7B | − | LT934124.1 | 2,277,012 | 2,277,524 | 1 |
| TdPR1.12 | VAI23307.1 | TRITD_5Av1G219360 | Chr5A | + | LT934119.1 | 582,698,401 582,703,182 | 582,698,778 582,703,304 | 2 |
| Gene Name | Locus/Protein Id | Cleavage Site Position | Sequence Position | Probability | Signal Peptide (Sec/SPI) | Other |
|---|---|---|---|---|---|---|
| TdPR1.1 | VAI31614.1 | 24–25 | SQA-QN | 0.8626 | 0.996896 | 0.003104 |
| TdPR1.2 | QEQ43326.1 | 25–26 | VSA-QN | 0.9374 | 0.997416 | 0.002584 |
| TdPR1.3 | VAI82177.1 | - | - | - | 0.089198 | 0.910802 |
| TdPR1.4 | VAI38046.1 | - | - | - | 0.260482 | 0.739518 |
| TdPR1.5 | VAI61858.1 | 23–24 | CNA-AF | 0.5384 | 0.791296 | 0.208704 |
| TdPR1.6 | VAI38037.1 | 23–24 | ATA-QN | 0.4766 | 0.9964 | 0.0036 |
| TdPR1.7 | VAI71684.1 | 24–25 | SEA-QN | 0.8419 | 0.998760 | 0.001240 |
| TdPR1.8 | VAI38042.1 | 23–24 | VTA-QN | 0.5392 | 0.993040 | 0.006960 |
| TdPR1.9 | VAI38038.1 | 24–25 | VTA-QN | 0.8174 | 0.994069 | 0.005931 |
| TdPR1.10 | VAI23305.1 | 24–25 | VTA-QN | 0.6873 | 0.995200 | 0.004800 |
| TdPR1.11 | VAI82381.1 | 30–31 | CAA-QN | 0.5826 | 0.975437 | 0.024563 |
| TdPR1.12 | VAI23307.1 | 23–24 | VTA-QN | 0.5527 | 0.991300 | 0.008700 |
| Gene Name | Locus/Protein Id | Length | MW | pI | Aliphatic Index | Gravy |
|---|---|---|---|---|---|---|
| TdPR1.1 | VAI31614.1 | 164 | 17,634.89 | 8.74 | 72.07 | −0.236 |
| TdPR-1.2 | QEQ43326.1 | 174 | 18,836.12 | 9.02 | 65.11 | −0.238 |
| TdPR1.3 | VAI82177.1 | 207 | 22,622.68 | 9.41 | 72.66 | −0.229 |
| TdPR1.4 | VAI38046.1 | 188 | 20,172.30 | 4.23 | 76.76 | −0.043 |
| TdPR1.5 | VAI61858.1 | 177 | 19,162.68 | 8.83 | 65.20 | −0.106 |
| TdPR1.6 | VAI38037.1 | 166 | 17,702.37 | 4.47 | 64.10 | −0.330 |
| TdPR1.7 | VAI71684.1 | 164 | 17,592.72 | 8.21 | 70.30 | −0.230 |
| TdPR1.8 | VAI38042.1 | 168 | 17,921.57 | 4.28 | 64.46 | −0.320 |
| TdPR1.9 | VAI38038.1 | 185 | 19,868.14 | 5.18 | 73.84 | −0.186 |
| TdPR1.10 | VAI23305.1 | 167 | 17,801.64 | 4.82 | 70.12 | −0.265 |
| TdPR1.11 | VAI82381.1 | 170 | 18,449.72 | 7.53 | 71.24 | −0.062 |
| TdPR1.12 | VAI23307.1 | 166 | 17,657.44 | 4.58 | 68.19 | −0.184 |
| Gene Name | Locus/Protein Id | TMHMM | SOSUI | TMDET | Percentage (%) |
|---|---|---|---|---|---|
| TdPR1.1 | VAI31614.1 | 0 | 1 | 1 | 66.66 |
| TdPR-1.2 | QEQ43326.1 | 1 | 1 | 1 | 100 |
| TdPR1.3 | VAI82177.1 | 0 | 1 | 1 | 66.66 |
| TdPR1.4 | VAI38046.1 | 0 | 1 | 1 | 66.66 |
| TdPR1.5 | VAI61858.1 | 1 | 1 | 1 | 100 |
| TdPR1.6 | VAI38037.1 | 0 | 1 | 1 | 66.66 |
| TdPR1.7 | VAI71684.1 | 0 | 0 | 0 | 0 |
| TdPR1.8 | VAI38042.1 | 0 | 1 | 1 | 66.66 |
| TdPR1.9 | VAI38038.1 | 1 | 1 | 1 | 100 |
| TdPR1.10 | VAI23305.1 | 1 | 1 | 1 | 100 |
| TdPR1.11 | VAI82381.1 | 0 | 1 | 0 | 33.33 |
| TdPR1.12 | VAI23307.1 | 0 | 1 | 1 | 66.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zribi, I.; Ghorbel, M.; Haddaji, N.; Besbes, M.; Brini, F. Genome-Wide Identification and Expression Profiling of Pathogenesis-Related Protein 1 (PR-1) Genes in Durum Wheat (Triticum durum Desf.). Plants 2023, 12, 1998. https://doi.org/10.3390/plants12101998
Zribi I, Ghorbel M, Haddaji N, Besbes M, Brini F. Genome-Wide Identification and Expression Profiling of Pathogenesis-Related Protein 1 (PR-1) Genes in Durum Wheat (Triticum durum Desf.). Plants. 2023; 12(10):1998. https://doi.org/10.3390/plants12101998
Chicago/Turabian StyleZribi, Ikram, Mouna Ghorbel, Najla Haddaji, Malek Besbes, and Faiçal Brini. 2023. "Genome-Wide Identification and Expression Profiling of Pathogenesis-Related Protein 1 (PR-1) Genes in Durum Wheat (Triticum durum Desf.)" Plants 12, no. 10: 1998. https://doi.org/10.3390/plants12101998
APA StyleZribi, I., Ghorbel, M., Haddaji, N., Besbes, M., & Brini, F. (2023). Genome-Wide Identification and Expression Profiling of Pathogenesis-Related Protein 1 (PR-1) Genes in Durum Wheat (Triticum durum Desf.). Plants, 12(10), 1998. https://doi.org/10.3390/plants12101998








