Establishment of Highly Efficient Plant Regeneration, Callus Transformation and Analysis of Botrytis cinerea-Responsive PR Promoters in Lilium brownii var. viridulum
Abstract
1. Introduction
2. Materials and Methods
2.1. The Induction and Proliferation of Embryogenic Calli
2.2. Shoot Induction, Proliferation and Rooting Induction
2.3. Callus Sensitivity to Different Antibiotics
2.4. Agrobacterium-Mediated Callus Transformation
2.5. GUS Staining and PCR Analysis of Transgenic Calli
2.6. Vector Construction and Transformation of PR Promoters
2.7. Inducible GUS Activity Assay
2.8. Molecular Identification by Quantitative Real-Time PCR
2.9. Data Statistics and Analysis
3. Results
3.1. Induction and Proliferation of Embryogenic Calli
3.2. Effects of Plant Growth Regulators on Shoot Induction and Proliferation
3.3. Effects of Macroelements and PGRs on Shoot Rooting Induction
3.4. Sensitivity Test of the Callus to Antibiotics
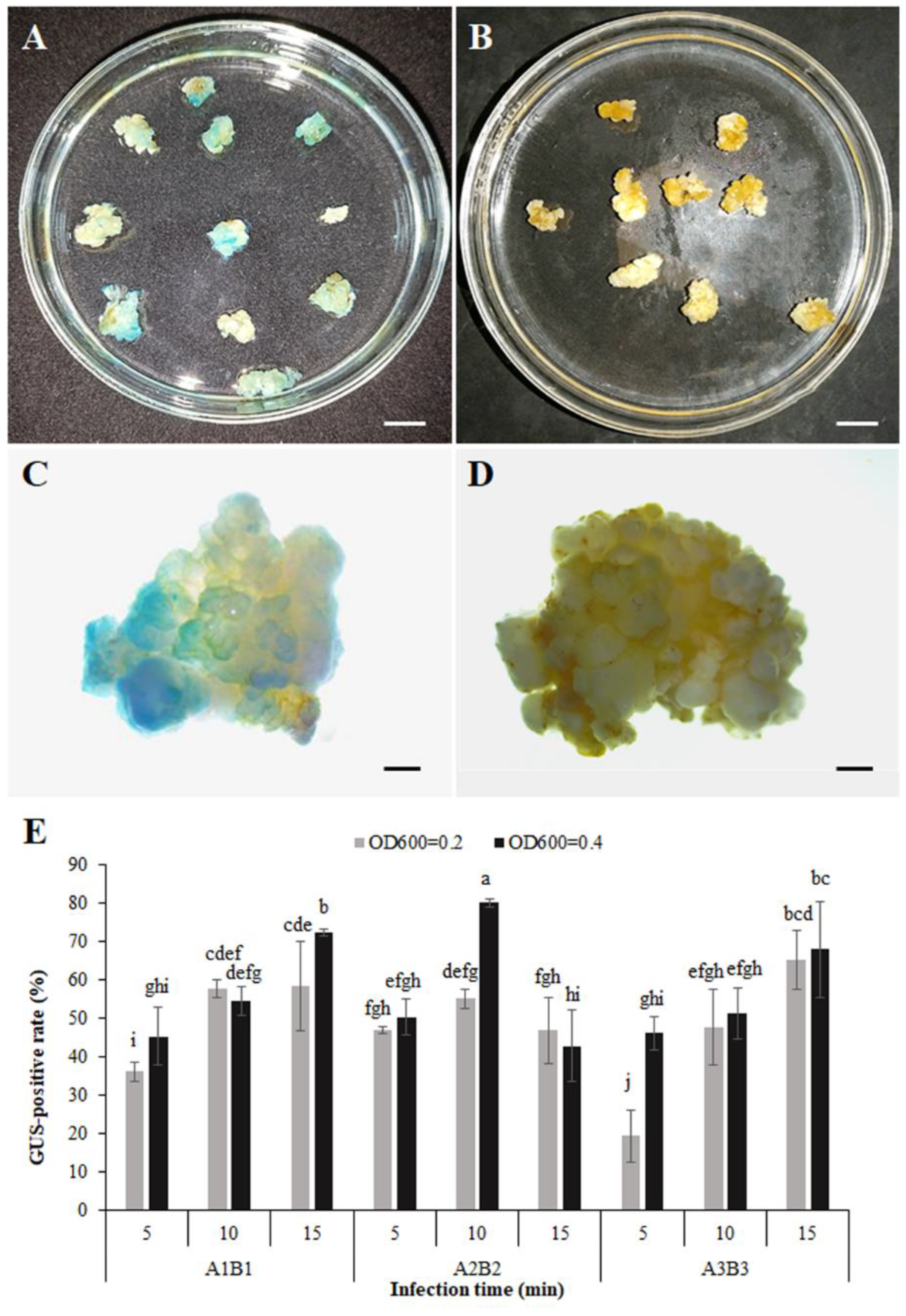
3.5. Transformation System and Obtaining Transgenic Calli
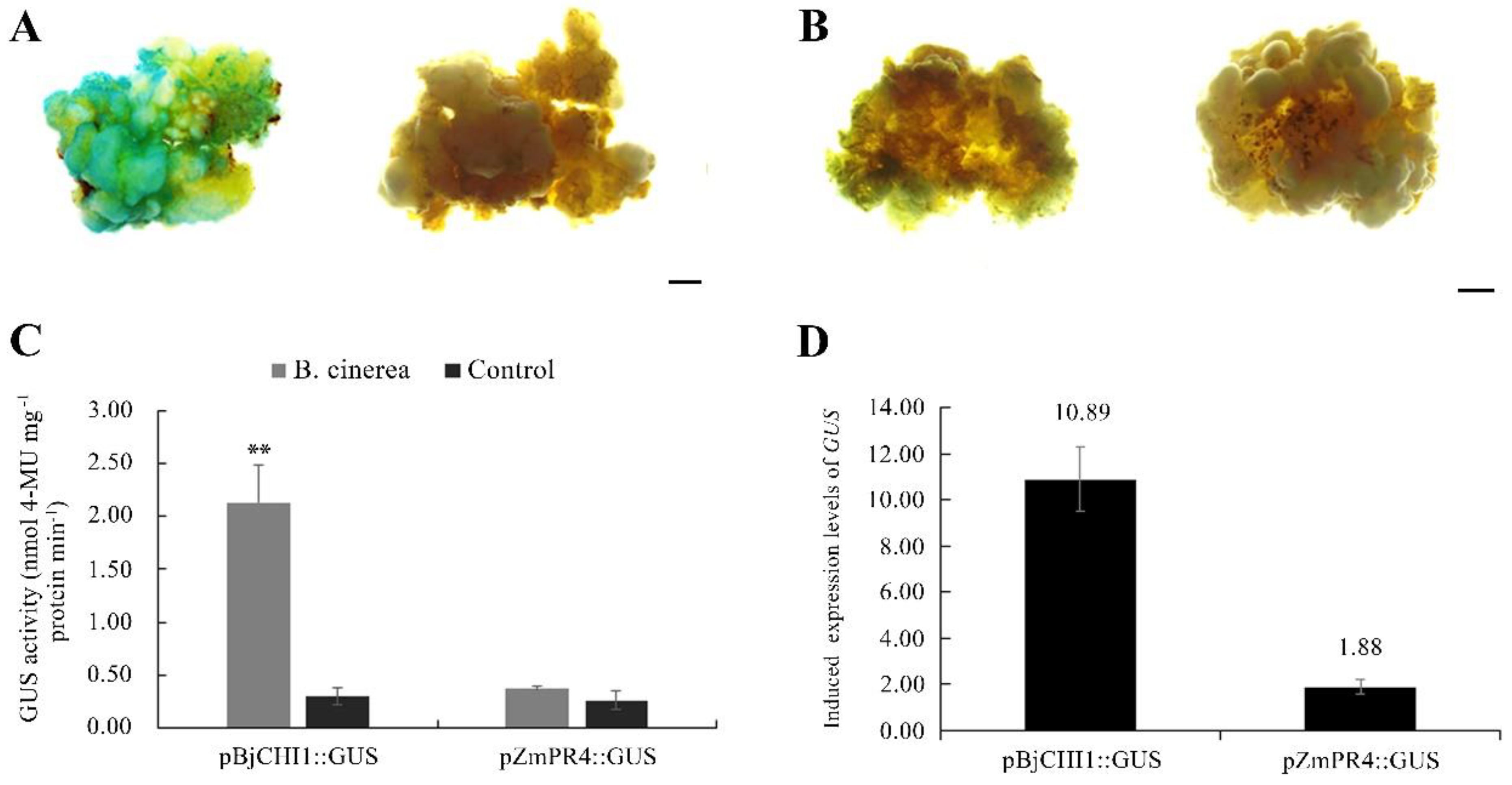
3.6. Expression Responses to B. cinerea of PR Promoter-GUS in Transgenic Calli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, L.P.; Fu, Y.Y.; Fan, J.H.; Huang, Y. Morphological features and karyotype analysis of three different local varieties of Lilium brownii var. viridulum. Plant Sci. J. 2019, 37, 559–568. (In Chinese) [Google Scholar]
- Zhao, X.Y.; Wang, W.H. Status and Development Proposals of Lily Industry in China//Papers (Abstracts) of the 12th Symposium of Chinese Society of Flower Bulbs; Beijing Zhonglv Garden Science Research Institute: Beijing, China, 2017; pp. 61–69. (In Chinese) [Google Scholar]
- Wu, L.H.; Li, R.G.; Liao, Z.J.; Yi, M. Isolation and identification of grey mold disease pathogen Botrytis cinerea from Lilium brownii var. viridulum. Hunan Agric. Sci. 2021, 7, 7–10. (In Chinese) [Google Scholar]
- Fu, Y.Y.; Li, J.; Wu, H.; Jiang, S.J.; Zhu, Y.Y.; Liu, C.Y.; Xu, W.J.; Li, Q.; Yang, L.P. Analyses of Botrytis cinerea-responsive LrWRKY genes from Lilium regale reveal distinct roles of two LrWRKY transcription factors in mediating responses to B. cinerea. Plant Cell Rep. 2022, 41, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Naderi, D. Biotechnology in ornamental plants: Future prospects and approaches. In Proceedings of the 1st International Conference on New Ideas in Agriculture. Islamic Azad University Khorasgan Branch, Isfahan, Iran, 26–27 January 2014. [Google Scholar]
- Rai, G.K.; Rai, N.P.; Kumar, S.; Yadav, A.; Rathaur, S.; Singh, M. Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. Vitr. Cell. Dev. Biol. -Plant 2012, 48, 565–578. [Google Scholar] [CrossRef]
- Song, S.; Yan, R.; Wang, C.; Wang, J.; Sun, H. Improvement of a genetic transformation system and preliminary study on the function of LpABCB21 and LpPILS7 based on somatic embryogenesis in Lilium pumilum DC. Fisch. Int. J. Mol. Sci. 2020, 18, 6784. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, J.; Xu, B.Y.; Jia, C.H.; Zhang, J.B.; Tan, G.L.; Jin, Z.Q. Regeneration and production of transgenic Lilium longiflorum via Agrobacterium tumefaciens. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 348–356. [Google Scholar] [CrossRef]
- Azadi, P.; Otang, N.V.; Supaporn, H.; Khan, R.S.; Chin, D.P.; Nakamura, I.; Mii, M. Increased resistance to Cucumber mosaic virus (CMV) in Lilium transformed with a defective CMV replicase gene. Biotechnol. Lett. 2011, 33, 1249–1255. [Google Scholar] [CrossRef]
- Núñez de Cáceres González, F.F.; Davey, M.R.; Sanchez, E.C.; Wilson, Z.A. Conferred resistance to Botrytis cinerea in Lilium by overexpression of the RCH10 chitinase gene. Plant Cell Rep. 2015, 34, 1201–1209. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, M.Z.; Li, X.Y.; Li, T.L.; Sun, H.M. Somatic embryogenesis and direct as well as indirect organogenesis in Lilium pumilum DC. Fisch., an endangered ornamental and medicinal plant. Biosci. Biotechnol. Biochem. 2016, 80, 1898–1906. [Google Scholar] [CrossRef]
- Yan, R.; Wang, Z.; Ren, Y.; Li, H.; Liu, N.; Sun, H. Establishment of efficient genetic transformation systems and application of CRISPR/Cas9 genome editing technology in Lilium pumilum DC. Fisch. and Lilium longiflorum White Heaven. Int. J. Mol. Sci. 2019, 20, 2920. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Zheng, Y.; Lyu, Y. The establishment of a genetic transformation system and the acquisition of transgenic plants of oriental hybrid Lily (Lilium L.). Int. J. Mol. Sci. 2023, 24, 782. [Google Scholar] [CrossRef] [PubMed]
- Potenza, C.; Aleman, L.; Sengupta-Gopalan, C. Targeting transgene expression in research, agricultural, and environmental applications: Promoters used in plant transformation. Vitr. Cell. Dev. Biol. -Plant 2004, 40, 1–22. [Google Scholar] [CrossRef]
- Gurr, S.J.; Rushton, P.J. Engineering plants with increased disease resistance: How are we going to express it? Trends Biotechnol. 2005, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Montero, M.; Company, N.; Badosa, E.; Messeguer, J.; Montesinos, L.; Montesinos, E.; Pla, M. Constitutive expression of transgenes encoding derivatives of the synthetic antimicrobial peptide BP100: Impact on rice host plant fitness. BMC Plant Biol. 2012, 12, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, H.; Szankowski, I.; Fischer, T.C.; Richter, K.; Peil, A.; Höfer, M.; Dörschel, C.; Schmoock, S.; Gau, A.E.; Halbwirth, H.; et al. Transgenic apple plants overexpressing the Lc gene of maize show an altered growth habit and increased resistance to apple scab and fire blight. Planta 2010, 231, 623–635. [Google Scholar] [CrossRef]
- Keller, H.; Pamboukdjian, N.; Ponchet, M.; Poupet, A.; Delon, R.; Verrier, J.L.; Roby, D.; Ricci, P. Pathogen-induced elicitin production in transgenic tobacco generates a hypersensitive response and nonspecific disease resistance. Plant Cell 1999, 11, 223–235. [Google Scholar] [CrossRef]
- Malnoy, M.; Reynoird, J.P.; Borejsza-Wysocka, E.E.; Aldwinckle, H.S. Activation of pathogen-inducible Gst1 promoter of potato after elicitation by Venturia inaequla and Erwinia amylovora in transgenic apple (Malus × domestica). Transgenic Res. 2006, 15, 83–93. [Google Scholar] [CrossRef]
- Barbosa-Mendes, J.M.; Mourao Filho, F.A.A.; Bergamin Filho, A.; Harakavab, R.; Beer, S.V.; Januzzi Mendesd, B.M. Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci. Hortic. 2009, 122, 109–115. [Google Scholar] [CrossRef]
- Zou, X.P.; Song, E.L.; Peng, A.H.; He, Y.R.; Xu, L.Z.; Lei, T.G.; Yao, L.X.; Chen, S.C. Activation of three pathogen-inducible promoters in transgenic citrus (Citrus sinensis Osbeck) after Xanthomonas axonopodis pv. citri infection and wounding. Plant Cell Tissue Organ Cult. 2014, 117, 85–98. [Google Scholar] [CrossRef]
- Moreno, A.B.; Peñas, G.; Rufat, M.; Bravo, J.M.; Estopà, M.; Messeguer, J.; Segundo, B.S. Pathogen-induced production of the antifungal AFP protein from Aspergillus giganteus confers resistance to the blast fungus Magnaporthe grisea in transgenic rice. Mol. Plant-Microbe Interact. 2005, 18, 960–972. [Google Scholar] [CrossRef]
- Wang, A.Y.; Zhuang, H.T.; Zhang, Z.Y.; Zhang, X.W.; Du, L.P.; Ye, X.G. Cloning and activity analysis of Zea mays ZmPR4 Promoter in wheat immature embryonic calli. Acta Agron. Sin. 2010, 36, 1605–1609. (In Chinese) [Google Scholar]
- Wu, X.F.; Wang, C.L.; Xie, E.B.; Gao, Y.; Fan, Y.L.; Liu, P.Q.; Zhao, K.J. Molecular cloning and characterization of the promoter for the multiple stress-inducible gene BjCHI1 from Brassica juncea. Planta 2009, 229, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zan, X.L.; Wu, X.F.; Yao, L.; Chen, Y.L.; Jia, S.W.; Zhao, K.J. Identification of fungus-responsive cis-acting element in the promoter of Brassica juncea chitinase gene, BjCHI1. Plant Sci. 2014, 215–216, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Kondo, M.; Mori, S.; Adachi, Y.; Nakano, M.; Kobayashi, H. Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Rep. 2004, 22, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Chin, D.P.; Kuroda, K.; Khan, R.S.; Mii, M. Macro elements in inoculation and co-cultivation medium strongly affect the efficiency of Agrobacterium-mediated transformation in Lilium. Plant Cell Tissue Organ Cult. 2010, 101, 201–209. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, F.J.; Ma, F.W.; Zhang, Y.Z. Studies on construction of plant expression vector carrying DHAR gene and its genetic transformation in Lilium longiflorum. Acta Agric. Boreali-Occident. Sin. 2009, 18, 225–229. [Google Scholar]
- Li, Q.H.; Hong, B.; Tong, Z.; Yang, Y.J.; Ma, C.; Yu, J.J.; Gao, J.P. Establishment of regeneration system and transformation of Zm401 gene in Lilium longiflorum x L.formosanum. J. Agric. Biotechnol. 2008, 16, 96–102. [Google Scholar]
- Núñez de Cáceres González, F.F.; Davey, M.R.; Wilson, Z.A. A rapid and efficient Agrobacterium-mediated transformation protocol for Lilium. Acta Hortic. 2011, 900, 161–167. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Liu, J.L.; Zhu, Y.Y.; Xu, W.J.; Lei, M.Y.; Yang, L.P. Construction of transformation system and integration of LrCCoAOMT Gene into Lilium lancifolium. Acta Hortic. Sin. 2020, 47, 1345–1358. (In Chinese) [Google Scholar]
- Li, H.Y.; Zhang, J.; Yang, Y.; Jia, N.N.; Wang, C.X.; Sun, H.M. miR171 and its target gene SCL6 contribute to embryogenic callus induction and torpedo-shaped embryo formation during somatic embryogenesis in two lily species. Plant Cell Tissue Organ Cult. 2017, 130, 591–600. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, F.L.; Liu, J.L.; Liu, W.Q.; Zhang, S.L.; Li, D.L.; Song, J.K.; Wang, R.; Yang, Y.J. Establishment of efficient callus genetic transformation system for Pyrus armeniacaefolia. Sci. Hortic. 2021, 289, 110429. [Google Scholar] [CrossRef]
- Frame, B.; Yu, K.F.; Christie, B.R.; Pauls, K.P. In vitro selection for resistance to verticillium wilt in alfalfa (Medicago sativa L.) using a fungal culture filtrate. Physiol. Mol. Plant Pathol. 1991, 38, 325–340. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Report. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cohen, A.; Meredith, C.P. Agrobacterium-mediated transformation of Lilium. Acta Hortic. 1992, 325, 611–618. [Google Scholar] [CrossRef]
- Langeveld, S.A.; Gerrits, M.M.; Derks, A.F.L.M.; Boonekamp, P.M.; Bol, J.F. Transformation of lily by Agrobacterium. Euphytica 1995, 85, 97–100. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Khosravi, S.; Azadi, P.; Bagheri, H.; van Tuyl, J.M. Biotechnological advances in Lilium. Plant Cell Rep. 2016, 35, 1799–1826. [Google Scholar] [CrossRef]
- Mercuri, A.; Benedetti, L.D.; Bruna, S.; Bregliano, R.; Bianchini, C.; Foglia, G.; Schiva, T. Agrobacterium-mediated transformation with rol genes of Lilium longiflorum Thunb. Acta Hortic. 2003, 612, 129–136. [Google Scholar] [CrossRef]
- Ault, J.R.; Siqueira, S.S. Morphogenetic response of Lilium michiganense to four auxin-type plant growth regulators in vitro. HortScience 2008, 43, 1922–1924. [Google Scholar] [CrossRef]
- Kȩdra, M.; Bach, A. Morphogenesis of Lilium martagon L. explants in callus culture. Acta Biol. Crac. 2005, 47, 65–73. [Google Scholar]
- Tang, Y.P.; Liu, X.Q.; Gituru, R.W.; Chen, L.Q. Callus induction and plant regeneration from in vitro cultured leaves, petioles, and scales of Lilium leucanthum (Baker) Baker. Biotechnol. Biotechnol. Equip. 2010, 24, 2071–2076. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.Z.; Liu, S.L.; Chen, Y.; Shen, L.J. Embryonic callus induction and plant regeneration of Lilium leucanthum. Bull. Bot. Res. 2019, 39, 338–346. (In Chinese) [Google Scholar] [CrossRef]
- Fu, Y.Y.; Qin, M.; Chen, Y.; Huang, M.; Xu, W.J.; Lei, M.Y.; Yang, L.P. A method of callus induction and proliferation for Lilium lancifolium Thunb. Mol. Plant Breed. 2022, 20, 3029–3037. (In Chinese) [Google Scholar]
- Liu, R.; Xue, Y.Q.; Ci, H.T.; Gao, J.; Wang, S.L.; Zhang, X.X. Establishment of Highly Efficient Plant Regeneration of Paeonia ostii ‘Fengdan’ through Optimization of Callus, Adventitious Shoot, and Rooting Induction. Hortic. Plant J. 2022, 8, 777–786. [Google Scholar] [CrossRef]
- Ren, J.H.; Wang, P.L.; Yin, L.N.; Zhao, J. Callus induction of Lilium brownii var. viridulum by orthogonal test. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2017, 37, 189–193. (In Chinese) [Google Scholar]
- Mii, M.; Yuzawa, Y.; Suetomi, H.; Motegi, T.; Godo, T. Fertile plant regeneration from protoplasts of a seed-propagated cultivar of Lilium×formolongi by utilizing meristematic nodular cell clumps. Plant Sci. 1994, 100, 221–226. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wu, L.F.; Wu, X.W.; Cui, G.F.; Ding, D.; Wang, J.H. Embryonic callus inducement and plantlet regeneration of oriental lily. Acta Agric. Jiangxi 2008, 20, 33–36, 39. (In Chinese) [Google Scholar]
- Zhang, X.H.; Wang, D.; Liang, Z.X.; Sun, M.Y.; Zhang, J.Z.; Shi, L. Callus induction and establishment of a plant regeneration system with Lilium martagon. Chin. Bull. Bot. 2018, 53, 840–847. (In Chinese) [Google Scholar]
- Ma, Y.D. Studies on Efficient Regeneration System and Regulation of Callus Proliferation in Wild Lilies. Master’s Thesis, Zhejiang University, Hangzhou, China, 2015; pp. 1–61. (In Chinese). [Google Scholar]
- Li, H.Y.; Liu, F.S.; Song, S.L.; Wang, C.X.; Sun, H.M. Highly effective organogenesis and somatic embryogenesis of Clivia. Sci. Hortic. 2022, 306, 111443. [Google Scholar] [CrossRef]
- Lu, Q.N. Study on tissue culture and rapid propagation of Lilium brownii var. viridulum. Acta Agric. Jiangxi 2002, 14, 43–46. (In Chinese) [Google Scholar]
- Chen, H.; Wang, D.; Wang, F.; Wang, J. Optimization of tissue culture technology system of Lilium brownii var. viridulum. Tianjin Agric. Sci. 2021, 27, 6–12. (In Chinese) [Google Scholar]
- Feyissa, T.; Welander, M.; Negash, L. In vitro regeneration of Hagenia abyssinica (Bruce) J.F. Gmel. (Rosaceae) from leaf explants. Plant Cell Rep. 2005, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Teixeira da Silva, J.A.; Wu, G. Direct adventitious shoot formation from apical shoot explants of Euphorbia tirucalli. J. Plant Growth Regul. 2011, 30, 114–116. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Sun, H.M. Floral organs tissue culture and rapid propagation technology of Lilium longiflorum × L. asiatic Hybrid ‘Eyeliner’. Acta Bot. Boreali-Occident. Sin. 2014, 34, 1894–1899. (In Chinese) [Google Scholar]
- Huang, J.; Liu, X.H.; Guan, J.; Lu, Y.M. Progress in molecular breeding of lily. Acta Hortic. Sin. 2012, 39, 1793–1808. [Google Scholar]
- Li, Y. Studies on In Vitro Regeneration and Genetic Transformation of Lilium brownii var. giganteum. Master’s Thesis, Zhejiang University, Hangzhou, China, 2017; pp. 1–100. (In Chinese). [Google Scholar]
- Kamo, K.; Han, B.H. Biolistic-mediated Transformation of Lilium longiflorum cv. Nellie White. Hortscience 2008, 43, 1864–1869. [Google Scholar] [CrossRef]
- Dong, W.; Mao, Y.F.; Li, W. Factors influencing T-DNA transferring into pollen of lily in vitro. Russ. J. Plant Physiol. 2007, 54, 420–425. [Google Scholar] [CrossRef]
- McDowell, J.M.; Woffenden, B.J. Plant disease resistance genes: Recent insights and potential applications. Trends Biotechnol. 2003, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Saranya, M.; Kanchana, M. Promoter diversity in plants—A review. Int. J. Appl. Adv. Sci. Res. 2016, 1, 209–217. [Google Scholar]
- Ouyang, L.J.; Huang, Z.C.; Sha, Y.E.; Zeng, F.H. Construction of a plant inducible expression vector and its transient expression in tobacco. Acta Agric. Boreali-Sin. 2013, 28, 41–45. (In Chinese) [Google Scholar]
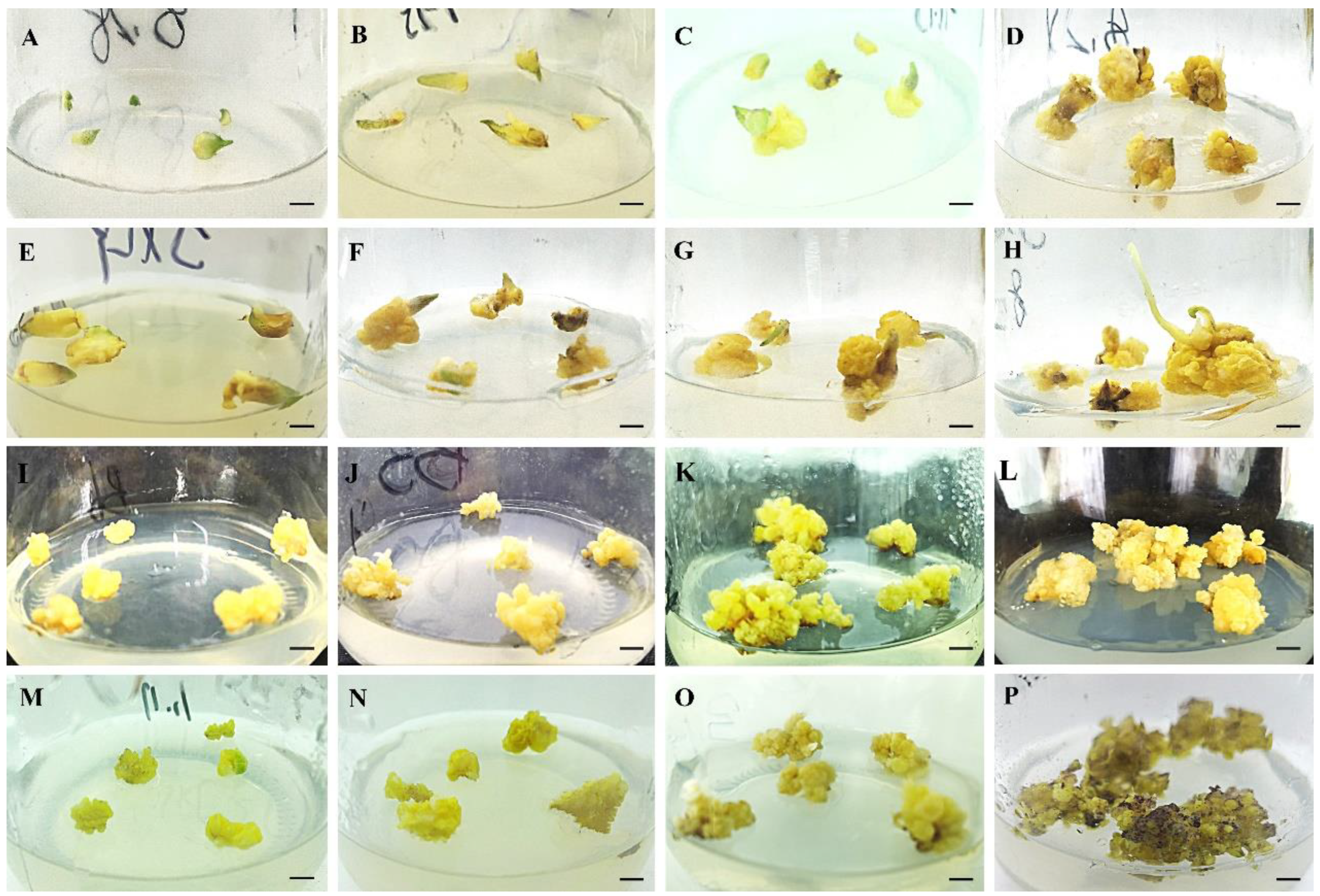


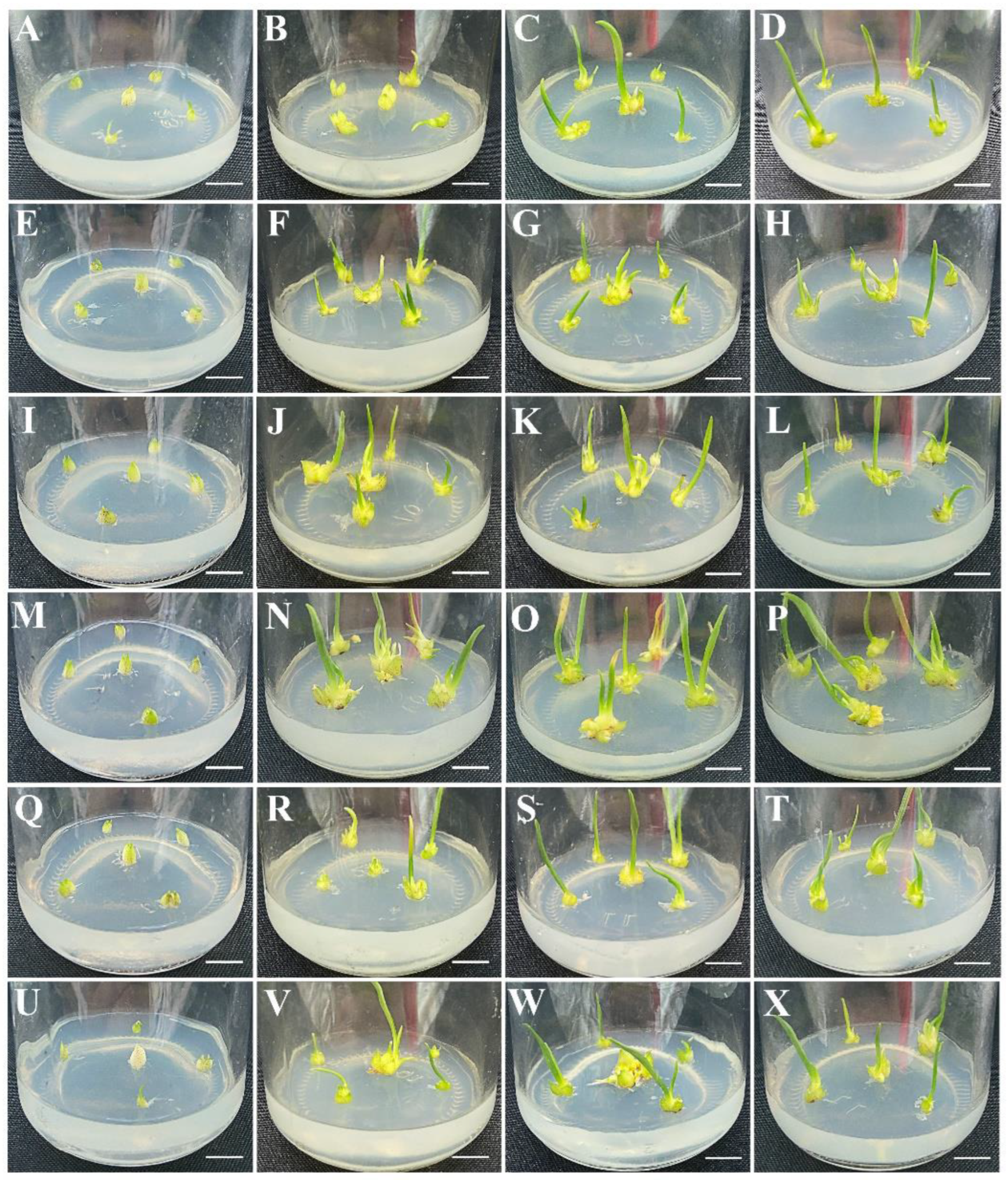

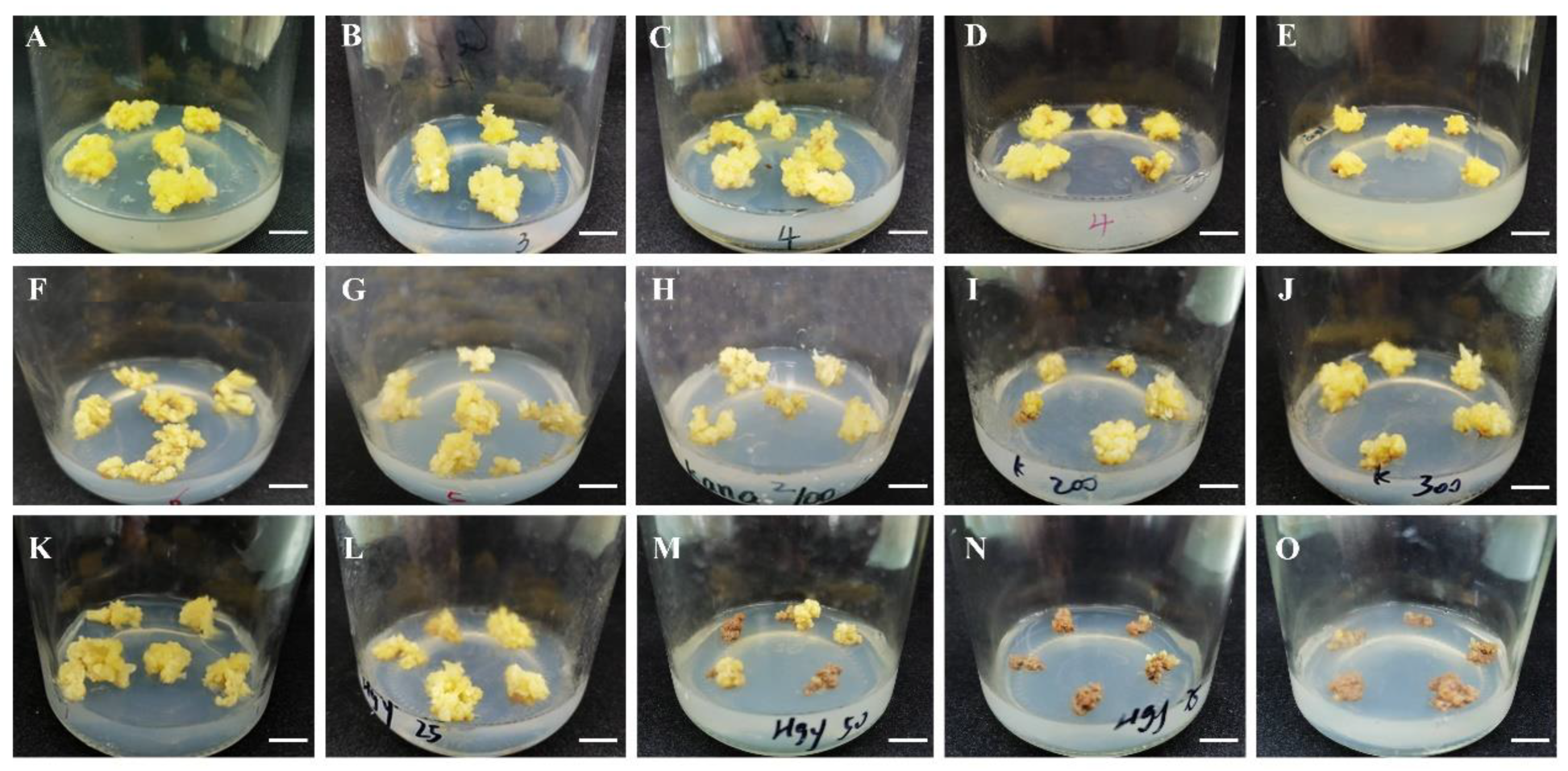

| MS/(mg·L−1) | Scale Number | Number of Scales Inducing Embryogenic Callus | Frequency of Scales Inducing Embryogenic Callus (%) | |
|---|---|---|---|---|
| PIC | NAA | |||
| 0.5 | 0.2 | 58.33 ± 2.19 a | 10.00 ± 2.00 c | 17.28 ± 3.10 de |
| 0.5 | 0.5 | 50.67 ± 9.33 a | 6.33 ± 2.85 c | 12.78 ± 4.34 e |
| 1.0 | 0.2 | 51.67 ± 10.93 a | 22.67 ± 3.84 b | 45.00 ± 2.89 b |
| 1.0 | 0.5 | 47.00 ± 8.50 a | 14.67 ± 3.28 bc | 31.08 ± 2.66 c |
| 2.0 | 0.2 | 48.00 ± 7.57 a | 9.67 ± 2.73 c | 23.56 ± 1.09 cd |
| 2.0 | 0.5 | 49.00 ± 6.66 a | 8.00 ± 1.53 c | 16.01 ± 1.30 de |
| * 1.0 | 0.2 | 50.00 ± 0.00 a | 32.33 ± 1.67 a | 64.67 ± 3.33 a |
| MS/(mg·L−1) | Callus Number | Number of Shooting Callus | Total Shooting Number | Callus Shooting Rate (%) | Callus Shooting Coefficient | Shoots Features | ||
|---|---|---|---|---|---|---|---|---|
| TDZ NAA | Texture | Colour | ||||||
| 0.5 | 0.2 | 30.00 | 25.33 ± 1.09 ab | 85.33 ± 9.98 b | 84.44 ± 3.64 ab | 2.84 ± 0.33 b | Most short, less long | Tender green or light-yellow |
| 0.5 | 0.4 | 30.00 | 22.67 ± 1.36 b | 68.00 ± 7.76 b | 75.55 ± 4.55 b | 2.27 ± 0.26 b | Most short, less long | Light-yellow |
| 1.0 | 0.2 | 30.00 | 25.00 ± 1.53 ab | 77.00 ± 10.44 b | 83.33 ± 5.09 ab | 2.56 ± 0.35 b | All short | Tender green or green |
| 1.0 | 0.4 | 30.00 | 24.67 ± 1.42 ab | 71.00 ± 8.28 b | 82.22 ± 4.75 ab | 2.37 ± 0.28 b | All short | Tender green or green |
| 2.0 | 0.2 | 30.00 | 25.33 ± 1.67 ab | 68.33 ± 8.38 b | 84.45 ± 3.88 ab | 2.28 ± 0.28 b | All short | Tender green or light-yellow |
| 2.0 | 0.4 | 30.00 | 25.11 ± 0.52 a | 117.33 ± 13.50 a | 92.22 ± 2.42 a | 3.91 ± 0.45 a | Most short, less long | Tender green or green |
| Media/(mg·L−1) | Inoculated Clustered Shoots | Number of Rooting Shoots | Total Induced Roots Number | Root Induction Rate (%) | Rooting Coefficient | Root Features | |
|---|---|---|---|---|---|---|---|
| Texture | Color | ||||||
| 1/2 MS + 0.5 NAA + 0.2 6-BA | 30 | 29.00 ± 0.58 a | 107.67 ± 14.94 b | 96.67 ± 2.03 a | 3.70 ± 0.46 b | A few, sturdy | Yellow |
| 1/2 MS + 0.2 NAA + 0.5 6-BA | 30 | 23.00 ± 1.53 b | 82.00 ± 8.54 c | 76.67 ± 4.91 b | 3.58 ± 0.33 b | A few, slim | Light yellow |
| 1/2 MS + 0.3 6-BA | 30 | 21.33 ± 0.67 b | 32.00 ± 4.16 e | 71.00 ± 2.00 b | 1.49 ± 0.16 d | A few, sturdy | Yellow |
| 1/2 MS + 0.3 NAA | 30 | 28.67 ± 0.88 a | 110.67 ± 9.13 b | 95.67 ± 2.96 a | 3.85 ± 0.21 b | A few, slim | Light yellow |
| MS + 0.5 NAA + 0.2 6-BA | 30 | 28.33 ± 0.67 a | 116.67 ± 5.55 b | 94.67 ± 2.33 a | 4.12 ± 0.13 b | Much, sturdy | Yellow |
| MS + 0.2 NAA + 0.5 6-BA | 30 | 22.67 ± 0.88 b | 61.67 ± 5.23 cd | 75.67 ± 2.96 b | 2.74 ± 0.28 c | A few, slim | Light yellow |
| MS + 0.3 NAA | 30 | 29.67 ± 0.33 a | 158.67 ± 9.60 a | 99.00 ± 1.00 a | 5.34 ± 0.27 a | Much, sturdy | Yellow |
| MS + 0.3 6-BA | 30 | 23.33 ± 0.88 b | 48.00 ± 3.46 de | 77.67 ± 2.91 b | 2.05 ± 0.07 cd | A few, sturdy | Light yellow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Shu, L.; Li, H.; Zhang, X.; Liu, X.; Ou, Z.; Liang, X.; Qi, X.; Yang, L. Establishment of Highly Efficient Plant Regeneration, Callus Transformation and Analysis of Botrytis cinerea-Responsive PR Promoters in Lilium brownii var. viridulum. Plants 2023, 12, 1992. https://doi.org/10.3390/plants12101992
Fu Y, Shu L, Li H, Zhang X, Liu X, Ou Z, Liang X, Qi X, Yang L. Establishment of Highly Efficient Plant Regeneration, Callus Transformation and Analysis of Botrytis cinerea-Responsive PR Promoters in Lilium brownii var. viridulum. Plants. 2023; 12(10):1992. https://doi.org/10.3390/plants12101992
Chicago/Turabian StyleFu, Yongyao, Liling Shu, Hanyi Li, Xingming Zhang, Xuan Liu, Zhengying Ou, Xiaomeng Liang, Xiangying Qi, and Liping Yang. 2023. "Establishment of Highly Efficient Plant Regeneration, Callus Transformation and Analysis of Botrytis cinerea-Responsive PR Promoters in Lilium brownii var. viridulum" Plants 12, no. 10: 1992. https://doi.org/10.3390/plants12101992
APA StyleFu, Y., Shu, L., Li, H., Zhang, X., Liu, X., Ou, Z., Liang, X., Qi, X., & Yang, L. (2023). Establishment of Highly Efficient Plant Regeneration, Callus Transformation and Analysis of Botrytis cinerea-Responsive PR Promoters in Lilium brownii var. viridulum. Plants, 12(10), 1992. https://doi.org/10.3390/plants12101992






