Reshifting Na+ from Shoots into Long Roots Is Associated with Salt Tolerance in Two Contrasting Inbred Maize (Zea mays L.) Lines
Abstract
1. Introduction
2. Results
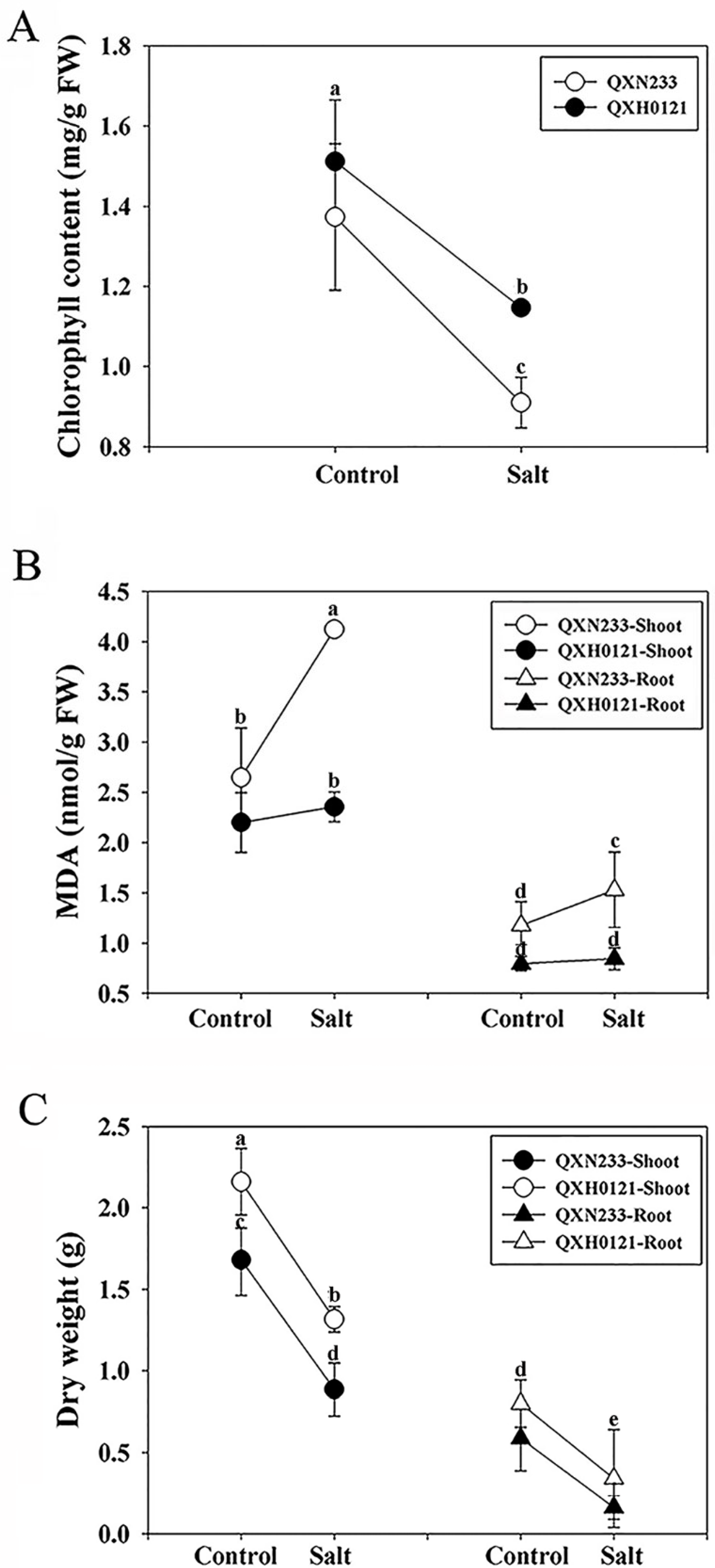
2.1. Salt Tolerance Differences between QXN233 and QXH0121 Inbred Lines
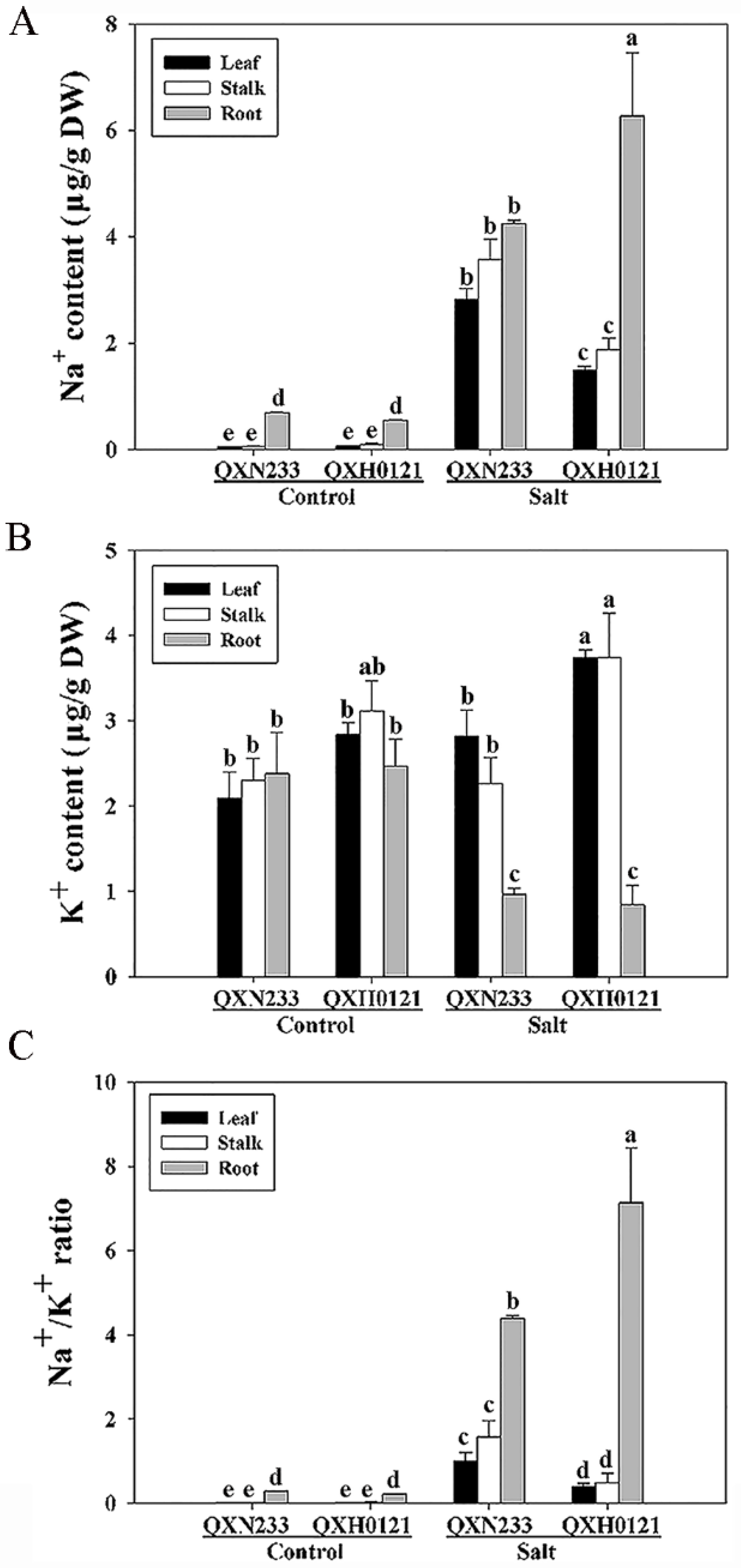
2.2. Na+ and K+ Changes in QXN233 and QXH0121
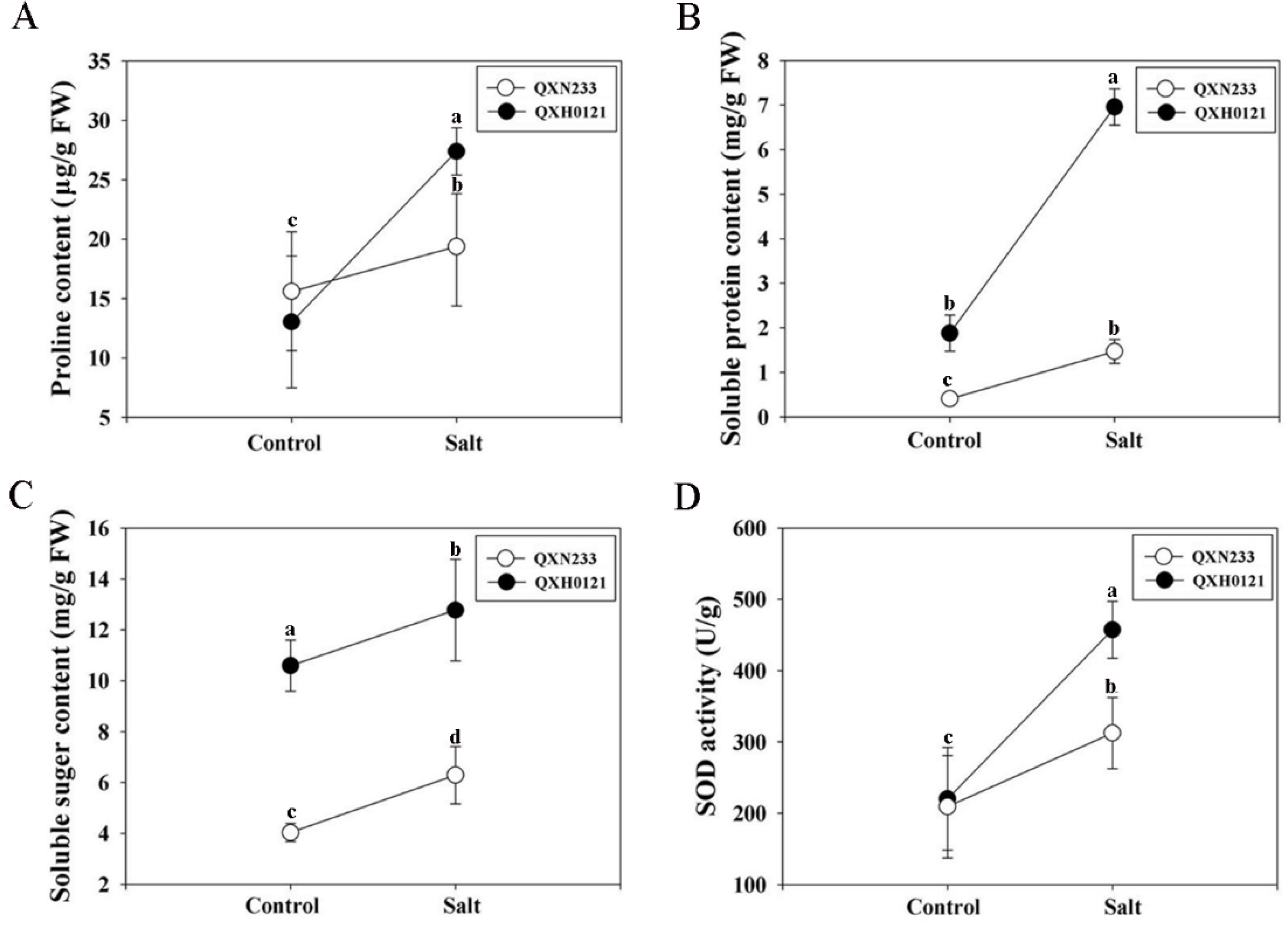
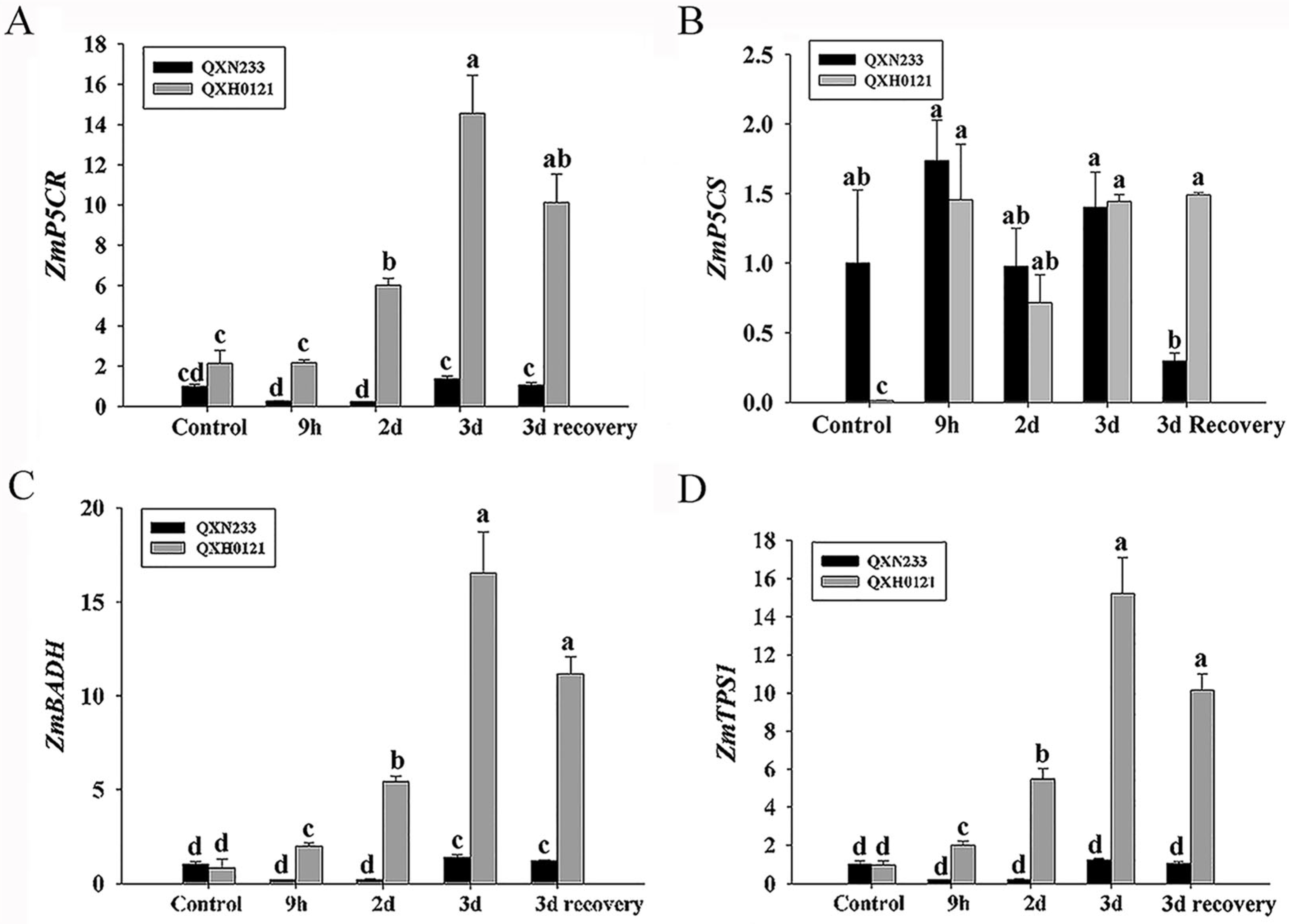
2.3. Alterations in Compatible Solutes in QXN233 and QXH0121
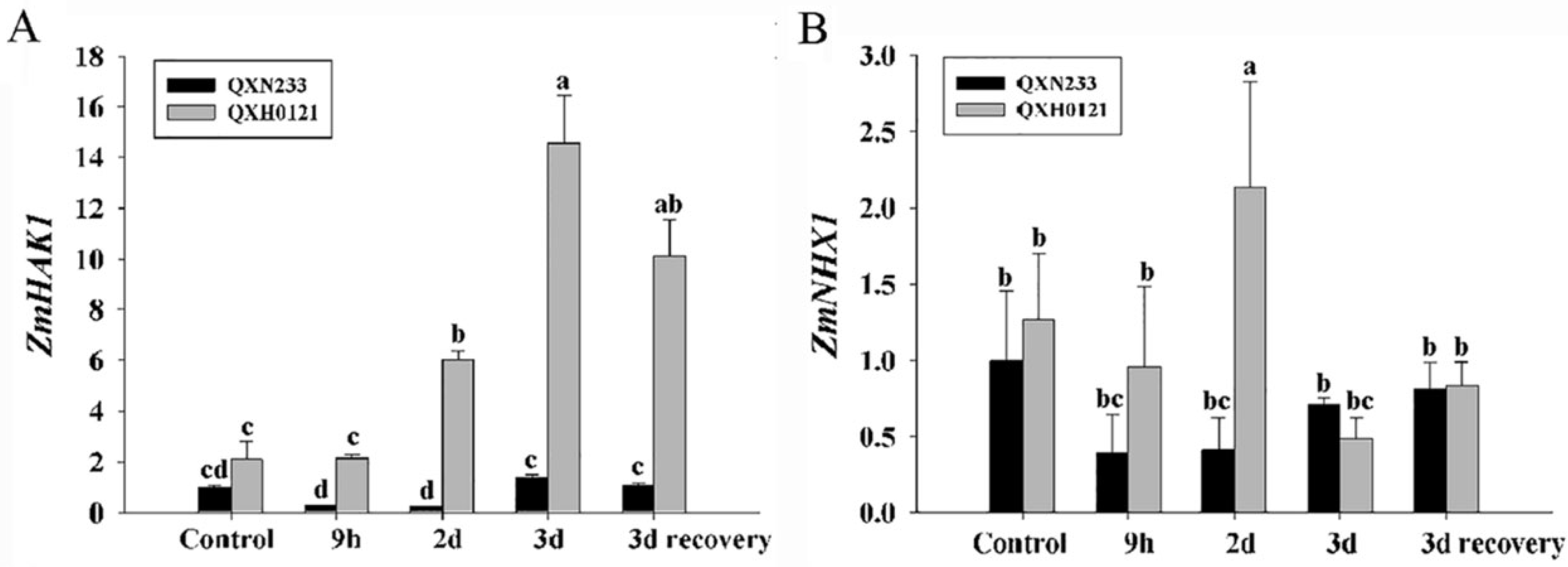
2.4. Expression Patterns of Na+ and K+ Ion Transporter Genes in QXN233 and QXH0121
2.5. Expression Patterns of Some Salt-Responsive Genes in QXN233 and QXH0121
3. Discussion
4. Materials and Methods
4.1. Plant Growth and NaCl Treatments
4.2. Growth Parameters, Chlorophyll, and Malondialdehyde (MDA) Content
4.3. Na+ and K+ Contents
4.4. Proline, Soluble Sugar, Soluble Protein Content and Superoxide Dismutase (SOD) Activity
4.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Machado, R.U.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Park, J.R.; Jang, Y.H.; Kim, E.G.; Kim, M.K. Rice cultivars under salt stress show differential expression of genes related to the regulation of Na+/K+ balance. Front. Plant Sci. 2021, 12, 680131. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kong, X.; Li, C.; Liu, Y.; Ding, Z. Potassium retention under salt stress is associated with natural variation in salinity tolerance among Arabidopsis accessions. PLoS ONE 2015, 10, e0124032. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to remove Na+ and retain K+ correlates with salt tolerance in two maize inbred lines seedlings. Front. Plant Sci. 2016, 7, 1716. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Hassan, F.A.S. How salt stress responsive proteins regulate plant adaptation to saline conditions. Plant Mol. Biol. 2022, 108, 175–224. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Wu, H.H.; Bose, J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef]
- Hernández, J.A.; Almansa, M.S. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 2002, 115, 251–257. [Google Scholar] [CrossRef]
- Feng, J.P.; Ma, W.Y.; Ma, Z.B.; Ren, Z.Y.; Zhou, Y.; Zhao, J.J.; Li, W.; Liu, W. GhNHX3D, a vacuolar-localized Na+/H+ antiporter, positively regulates salt response in upland cotton. Int. J. Mol. Sci. 2021, 22, 4047. [Google Scholar] [CrossRef]
- Jabeen, Z.; Irshad, F.; Hussain, N.; Han, Y.; Zhang, G.P. NHX-type Na+/H+ antiporter gene expression under different salt levels and allelic diversity of HvNHX in wild and cultivated barleys. Front. Genet. 2022, 12, 809–988. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.D.; Liang, X.Y.; Lu, M.H.; Lai, J.S.; Song, W.B.; Jiang, C.F. A teosinte-derived allele of an HKT1 family sodium transporter improves salt tolerance in maize. Plant Biotechnol. J. 2022, 21, 97–108. [Google Scholar] [CrossRef]
- Pandolfi, C.; Azzarello, E.; Mancuso, S.; Shabala, S. Acclimation improves salt stress tolerance in Zea mays plants. J. Plant Physiol. 2016, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Zhang, J.W.; Chen, Y.J.; Li, R.F.; Wang, H.Z.; Wei, J.H. Genome wide analysis and identification of HAK potassium transporter gene family in maize (Zea mays L.). Mol. Biol. Rep. 2012, 39, 846573. [Google Scholar] [CrossRef]
- Qin, Y.J.; Wu, W.H.; Wang, Y. ZmHAK5 and ZmHAK1 function in K+ uptake and distribution in maize under low K+ conditions. J. Integr. Plant Biol. 2019, 61, 691–705. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, X.Y.; Wang, L.M.; Cao, Y.B.; Song, W.B.; Shi, J.P.; Lai, J.S.; Jiang, C.F. A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 2019, 5, 12971308. [Google Scholar] [CrossRef] [PubMed]
- Riedelsberger, J.; Miller, J.K.; Valdebenito-Maturana, B.; Piñeros, M.A.; González, W.; Dreyer, I. Plant HKT channels: An updated view on structure, function and gene regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.L.; Li, R.K.; Zhou, Y.C.; Chen, Y.L.; Hou, H.Y.; Dai, Q.G. Transcriptome-wide analysis revealed the potential of the high-affinity potassium transporter (HKT) gene family in rice salinity tolerance via ion homeostasis. Bioengineering 2022, 9, 410. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Song, G.S.; Shan, X.H.; Wei, Z.Y.; Liu, Y.Z.; Jiang, C.; Jiang, Y.; Jin, F.X.; Li, Y.D. Association analysis and identification of ZmHKT1;5 variation with salt stress tolerance. Front. Plant Sci. 2018, 9, 1485. [Google Scholar] [CrossRef]
- Venkataraman, G.; Shabala, S.; Véry, A.A.; Hariharan, G.N.; Somasundaram, S.; Pulipati, S.; Sellamuthu, G.; Harikrishnan, M.; Kumari, K.; Shabala, L.; et al. To exclude orto accumulate? Revealing the role of the sodium HKT1;5 transporter in plant adaptive responses to varying soil salinity. Plant Physiol. Biochem. 2021, 169, 333–342. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.B.; Wang, Z.P.; Wang, Z.Q.; Shi, J.P.; Liang, X.Y.; Song, W.B.; Chen, Q.J.; Lai, J.S.; Jiang, C.F. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [CrossRef]
- Cao, Y.B.; Liang, X.Y.; Yin, P.; Zhang, M.; Jiang, C.F. A domestication-associated reduction in K+-preferring HKT transporter activity underlies maize shoot K+ accumulation and salt tolerance. New Phytol. 2019, 222, 301–317. [Google Scholar] [CrossRef]
- Paul, A.; Chatterjee, A.; Subrahmanya, S.; Shen, G.X.; Mishra, N. NHX gene family Camellia sinensis: In-silico Genome-wide identification, expression profiles, and regulatory network analysis. Front. Plant Sci. 2021, 12, 777884. [Google Scholar] [CrossRef]
- Sun, Y.L.; Mu, C.H.; Liu, X. Key factors identified by proteomic analysis in maize (Zea mays L.) seedlings’ response to long-term exposure to different phosphate levels. Proteome Sci. 2018, 16, 19. [Google Scholar] [CrossRef]
- Sun, Y.L.; Mu, C.H.; Zheng, H.X.; Lu, S.H.; Zhang, H.; Zhang, X.C.; Liu, X. Exogenous Pi supplementation improved the salt tolerance of maize (Zea mays L.) by promoting Na+ exclusion. Sci. Rep. 2018, 8, 16203. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zheng, H.X. Divergent molecular and physiological response of two maize breeding lines under phosphate deficiency. Plant Mol. Biol. Rep. 2022, 40, 197–207. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, A.S.; Abo El-Maati, M.F.; Desoky, E.M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef]
- Quan, R.D.; Shang, M.; Zhang, H.; Zhao, Y.X.; Zhang, J.R. Improved chilling tolerance by transformation with betA gene for the enhancement of glycine betaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stanley, P.D.; Holmes, E. Application of NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2010, 22, 214–224. [Google Scholar] [CrossRef]
- Henry, C.; Bledsoe, S.W.; Griffiths, C.A.; Kollman, A.; Paul, M.J.; Sakr, S.; Lagrimini, L.M. Differential role for trehalose metabolism in salt-stressed maize. Plant Physiol. 2015, 169, 10721089. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Zeng, W.; Ding, Y.; Zhuang, Z.; Peng, Y. Comparing transcriptome expression profiles to reveal the mechanisms of salt tolerance and exogenous glycine betaine mitigation in maize seedlings. PLoS ONE 2020, 15, e0233616. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Q.; Zhang, Y.; Zhang, M.; Li, Z. Glycine betaine increases salt tolerance in maize (Zea mays L.) by regulating Na+ homeostasis. Front. Plant Sci. 2022, 13, 978304. [Google Scholar] [CrossRef]
- Rohman, M.M.; Islam, M.R.; Monsur, M.B.; Amiruzzaman, M.; Fujita, M.; Hasanuzzaman, M. Trehalose protects maize plants from salt stress and phosphorus deficiency. Plants 2019, 8, 568. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Sandhu, D.; Pudussery, M.V.; Kumar, R.; Pallete, A.; Markley, P.; Bridges, W.C.; Sekhon, R.S. Characterization of natural genetic variation identifies multiple genes involved in salt tolerance in maize. Funct. Integr. Genom. 2019, 20, 261–275. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Qing, C.; Yang, C.; Shen, Y.; Ma, L. Comparative transcriptome analyses of maize seedling root responses to salt stress. Peer J. 2021, 9, e10765. [Google Scholar] [CrossRef]
- Luo, M.J.; Zhao, Y.X.; Wang, Y.D.; Shi, Z.; Zhang, P.P.; Zhang, Y.X.; Song, W.; Zhao, J.R. Comparative proteomics of contrasting maize genotypes provides insights into salt stress tolerance mechanisms. J. Proteome Res. 2018, 17, 141–153. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Peng, Y.; Zeng, W.; Zhao, X.; Ding, Y.; Zhuang, Z.; Gao, Q.; Ren, B. Comparative proteomics of salt-tolerant and salt-sensitive maize inbred lines to reveal the molecular mechanism of salt tolerance. Int. J. Mol. Sci. 2019, 20, 4725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Bai, X.; Jiang, C.F.; Li, Z. Phosphoproteomic analysis of two contrasting maize inbred lines provides insights into the mechanism of salt-stress tolerance. Int. J. Mol. Sci. 2019, 20, 1886. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.Y.; Zhao, Y.M.; Zhao, Y.N.; Song, X.Q.; Yuan, J.C.; Liu, Y.H. Identification of salt stress responsive proteins in maize (Zea may) seedlings using iTRAQ Based proteomic technique. Iran. J. Biotechnol. 2021, 19, e2512. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.A.; Erban, A.; Kopka, J.; Zörb, C. Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci. 2015, 233, 107–115. [Google Scholar] [CrossRef]
- Liang, X.Y.; Liu, S.Y.; Wang, T.; Li, F.R.; Cheng, J.K.; Lai, J.S.; Qin, F.; Li, Z.; Wang, X.F.; Jiang, C.F. Metabolomics driven gene mining and genetic improvement of tolerance to salt-induced osmotic stress in maize. New Phytol. 2021, 23, 2355–2370. [Google Scholar] [CrossRef]
- Luo, X.; Wang, B.C.; Gao, S.; Zhang, F.; Terzaghi, W.; Dai, M.Q. Genome-wide association study dissects the genetic bases of salt tolerance in maize seedlings. J. Integr. Plant Biol. 2019, 61, 658–674. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.C.; Zou, C.Y.; Yang, C.; Pan, G.T.; Ma, L.L.; Shen, Y.O. Integrated analysis of long noncoding RNAs and mRNAs reveals the regulatory network of maize seedling root responding to salt stress. BMC Genom. 2022, 23, 50. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.X.; Liu, H.; Liang, Z.J.; Zhang, M.Y.; Zou, C.Y.; Yuan, G.S.; Shibin Gao, S.B.; Pan, G.T.; Shen, Y.O.; et al. A combination of a genome-wide association study and a transcriptome analysis reveals circRNAs as new regulators involved in the response to salt stress in maize. Int. J. Mol. Sci. 2022, 23, 9755. [Google Scholar] [CrossRef]
- Kotula, L.; Caparros, G.P.; Zörb, C.; Colmer, T.D.; Flowers, T.J. Improving crop salt tolerance using transgenic approaches: An update and physiological analysis. Plant Cell Environ. 2020, 43, 2932–2956. [Google Scholar] [CrossRef]
- Steppuhn, H.; Asay, K.H. Emergence, height, and yield of tall, NewHy, and green wheatgrass forage crops grown in saline root zones. Can. J. Plant Sci. 2005, 85, 863–875. [Google Scholar] [CrossRef]
- Omoto, E.; Iwasaki, Y.; Miyake, H.; Taniguchi, M. Salinity induces membrane structure and lipid changes in maize mesophyll and bundle sheath chloroplasts. Physiol. Plant. 2016, 157, 13–23. [Google Scholar] [CrossRef]
- Fu, L.B.; Shen, Q.F.; Kuang, L.H.; Yu, J.H.; Wu, D.Z.; Zhang, G.P. Metabolite profiling and gene expression of Na+/K+ transporter analyses reveal mechanisms of the difference in salt tolerance between barley and rice. Plant Physiol. Biochem. 2018, 130, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, T.T.; Huang, D.; Liu, R.J.; Xu, B.C.; Zhang, S.Q.; Deng, X.P.; Siddique, K.H.M.; Chen, Y.L. Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 2021, 21, 457. [Google Scholar] [CrossRef]
- Aleman, F.; Nieves-Cordones, M.; Martinez, V.; Rubio, F. Root K+ acquisition in plants: The Arabidopsis thaliana model. Plant Cell Physiol. 2011, 52, 1603–1612. [Google Scholar] [CrossRef]
- Ma, W.; Ren, Z.; Zhou, Y.; Zhao, J.; Zhang, F.; Feng, J.; Liu, W.; Ma, X. Genome-Wide Identification of the Gossypium hirsutum NHX genes reveals that the endosomal-type GhNHX4A is critical for the salt tolerance of cotton. Int. J. Mol. Sci. 2020, 21, 7712. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under c hanging environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Arias-Baldrich, C.; Bosch, N.; Begines, D.; Feria, A.B.; Monreal, J.A.; García-Mauriño, S.J. Proline synthesis in barley under iron deficiency and salinity. Plant Physiol. 2015, 183, 121–129. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, Y.F.; Zhang, Y.F.; Li, C.X.; Gong, S.C.; Yan, S.Q.; Li, G.L.; Hu, G.H.; Ren, H.L.; Yang, J.F.; et al. Comparative transcriptome analysis of salt-sensitive and salt-tolerant maize reveals potential mechanisms to enhance salt resistance. Genes Genom. 2019, 41, 781801. [Google Scholar] [CrossRef]
- Sun, Y.L.; Mu, C.H.; Chen, Y.; Kong, X.P.; Xu, Y.C.; Zheng, H.X.; Zhang, H.; Wang, Q.C.; Xue, Y.F.; Li, Z.X.; et al. Comparative transcript profiling of maize inbreds in response to long-term phosphorus deficiency stress. Plant Physiol. Biochem. 2016, 109, 467481. [Google Scholar] [CrossRef]
- Liu, X.; Su, S. Growth and physiological response of Viola tricolor L. to NaCl and NaHCO3 stress. Plants 2023, 12, 178. [Google Scholar] [CrossRef]
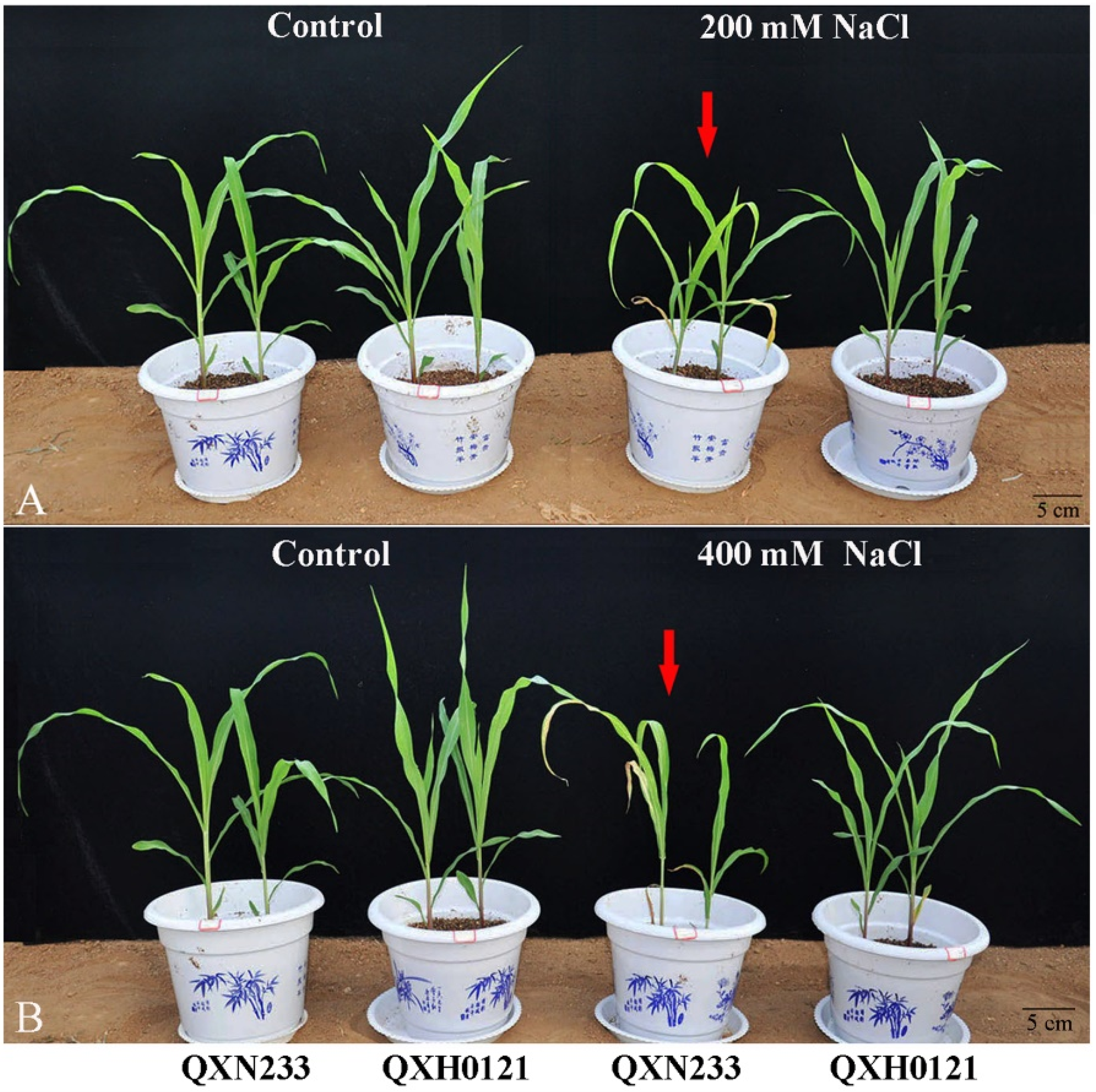

| Plants | Treatments | Plant Height (cm) | Leaf Width (cm) | Leaf Length (cm) | Root Length (cm) |
|---|---|---|---|---|---|
| QXN233 | Control | 59.75 ± 0.30 a | 2.65 ± 0.30 a | 50.75 ± 0.30 a | 41.00 ± 0.90 b |
| 200 mM NaCl | 37.25 ± 5.50 c | 1.85 ± 0.10 b | 43.00 ± 5.00 b | 18.25± 1.50 d | |
| QXH0121 | Control | 46.00 ± 4.20 b | 1.95 ± 0.07 ab | 56.00 ± 1.40 a | 60.00± 4.50 a |
| 200 mM NaCl | 34.25 ± 0.30 c | 1.80 ± 0.04 b | 43.00 ± 1.70 b | 40.00 ± 2.40 c | |
| QXN233 | Control | 58.95 ± 0.34 a | 2.75 ± 0.36 a | 51.75 ± 0.26 a | 40.00 ± 0.67 b |
| 400 mM NaCl | 35.15 ± 3.40 c | 1.60 ± 0.14 b | 40.12 ± 4.90 b | 17.85 ± 1.34 d | |
| QXH0121 | Control | 45.69 ± 3.68 b | 1.97 ± 0.11 ab | 55.79 ± 1.10 a | 59.46± 4.34 a |
| 400 mM NaCl | 34.75 ± 0.29 c | 1.67 ± 0.13 b | 42.19 ± 1.15 b | 39.14 ± 3.16 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zheng, H.; Wang, M.; Guo, Y.; Wang, Y.; Zheng, C.; Tao, Y.; Sun, X.; Qian, D.; Cao, G.; et al. Reshifting Na+ from Shoots into Long Roots Is Associated with Salt Tolerance in Two Contrasting Inbred Maize (Zea mays L.) Lines. Plants 2023, 12, 1952. https://doi.org/10.3390/plants12101952
Zhao Z, Zheng H, Wang M, Guo Y, Wang Y, Zheng C, Tao Y, Sun X, Qian D, Cao G, et al. Reshifting Na+ from Shoots into Long Roots Is Associated with Salt Tolerance in Two Contrasting Inbred Maize (Zea mays L.) Lines. Plants. 2023; 12(10):1952. https://doi.org/10.3390/plants12101952
Chicago/Turabian StyleZhao, Zhenyang, Hongxia Zheng, Minghao Wang, Yaning Guo, Yingfei Wang, Chaoli Zheng, Ye Tao, Xiaofeng Sun, Dandan Qian, Guanglong Cao, and et al. 2023. "Reshifting Na+ from Shoots into Long Roots Is Associated with Salt Tolerance in Two Contrasting Inbred Maize (Zea mays L.) Lines" Plants 12, no. 10: 1952. https://doi.org/10.3390/plants12101952
APA StyleZhao, Z., Zheng, H., Wang, M., Guo, Y., Wang, Y., Zheng, C., Tao, Y., Sun, X., Qian, D., Cao, G., Zhu, M., Liang, M., Wang, M., Gong, Y., Li, B., Wang, J., & Sun, Y. (2023). Reshifting Na+ from Shoots into Long Roots Is Associated with Salt Tolerance in Two Contrasting Inbred Maize (Zea mays L.) Lines. Plants, 12(10), 1952. https://doi.org/10.3390/plants12101952





