Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation
Abstract
1. Introduction
2. Results
2.1. Experiment 1: Conditions for Thalli Maintenance Prior Inoculation
2.2. Experiment 2: Effect of Locality on SAD Formation
3. Discussion
4. Materials and Methods
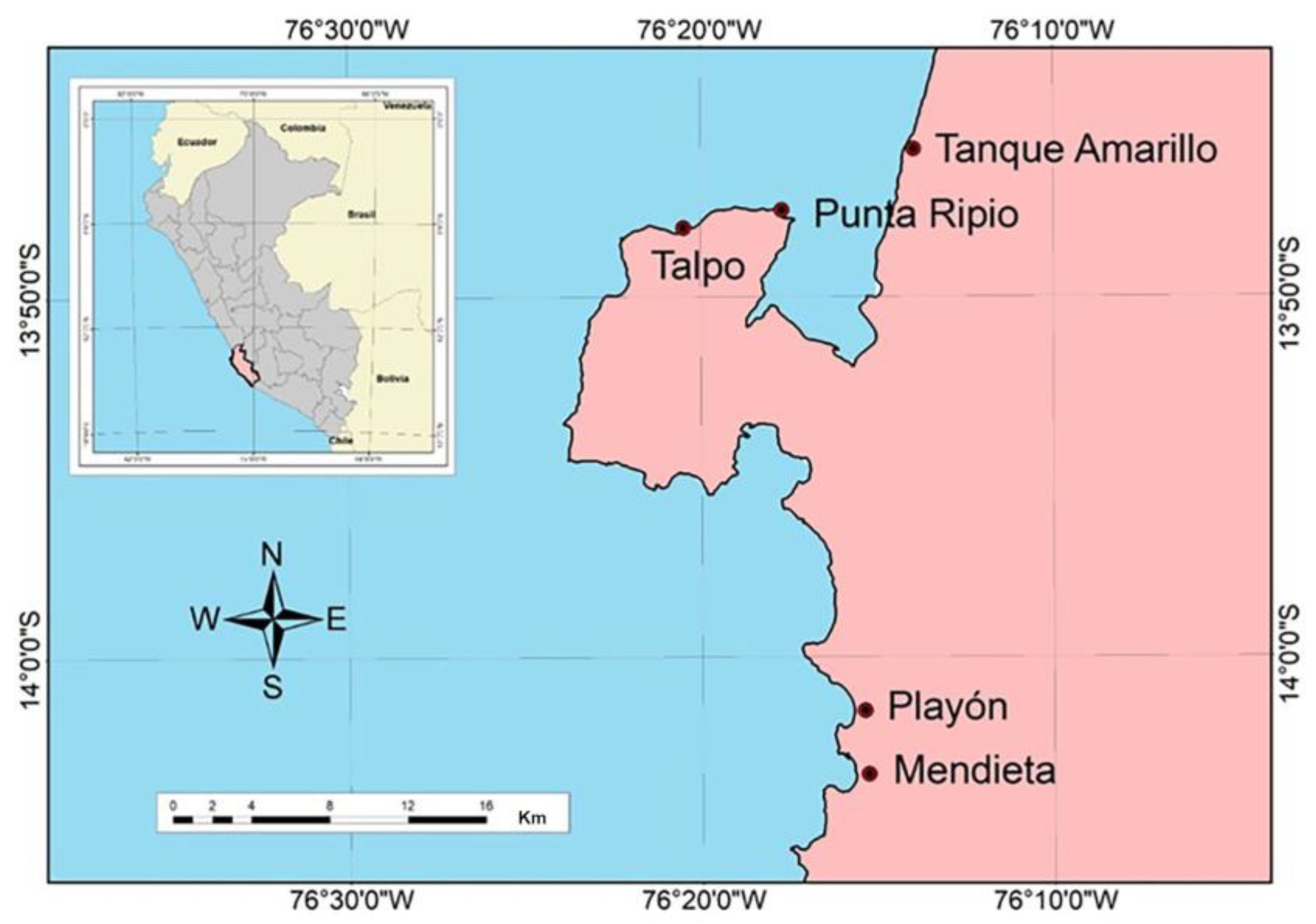
4.1. Sampling Sites
4.2. Experiment 1
4.3. Experiment 2
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arakaki, N.; Carbajal, P.; Marquez-Corigliano, D.; Suárez Alarcón, S.; Gil-Kodaka, P.; Perez-Araneda, K.; Tellier, F. Genética de macroalgas en el Perú: Diagnóstico, guía metodológica y casos de estudio. Inf. Inst. Mar. Perú 2021, 48, 594–609. [Google Scholar]
- Yang, M.Y.; Macaya, E.C.; Kim, M.S. Molecular evidence for verifying the distribution of Chondracanthus chamissoi and C. teedei (Gigartinaceae, Rhodophyta). Bot. Mar. 2015, 58, 103–113. [Google Scholar] [CrossRef]
- Arakaki, N.; Suárez-Alarcón, S.; Márquez-Corigliano, D.; Gil-Kodaka, P.; Tellier, F. The widely distributed, edible seaweeds in Peru, Chondracanthus chamissoi and Chondracanthus chamissoi f. glomeratus (Gigartinaceae, Rhodophyta), are morphologically diverse but not phylogenetically distinct. J. World Aquac. Soc. 2021, 52, 1290–1311. [Google Scholar] [CrossRef]
- Bulboa, C.; Macchiavello, J. Cultivo de frondas cistocárpicas, tetraspóricas y vegetativas de Chondracanthus chamissoi (Rhodophyta, Gigartinales) en dos localidades del norte de Chile. Investig. Mar. 2006, 34, 109–112. [Google Scholar] [CrossRef]
- Calderón, M.; Ramírez, M.E.; Bustamante, D. Notas sobre tres especies de Gigartinaceae (Rhodophyta) del litoral peruano. Rev. Peru. Biol. 2010, 17, 115–122. [Google Scholar] [CrossRef]
- Rodríguez, C.Y.; Tellier, F.; Pérez-Araneda, K.; Otaíza, R.D. Taxonomic position of the two sympatric forms of Chondracanthus chamissoi (f. lessonii and f. chauvinii) (Rhodophyta, Gigartinaceae) by using two molecular markers. Lat. Am. J. Aquat. Res. 2021, 49, 182–187. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland; Available online: http://www.algaebase.org (accessed on 5 April 2023).
- Acosta, J. Nombres Vulgares y Usos de las Algas en el Perú; Departamento de Botánica, Museo de Historia Natural Javier Prado: Jesús María, Perú, 1977; Volume 7, pp. 1–9. [Google Scholar]
- Donnan, C.B.; McClelland, D. Moche burials at Pacatnamu. In The Pacatnamu Papers. The Moche Occupation; Donnan, C.B., Cock, G.A., Eds.; Fowler Museum of Cultural History, University of California: Los Angeles, CA, USA, 1997; Volume 2, pp. 17–187. [Google Scholar]
- Noriega, C. Algas Comestibles del Perú. Pan del Futuro; Universidad San Martín de Porres: Lima, Peru, 2010; pp. 1–180. [Google Scholar]
- Ugás, R. 40 Verduras Viejas y Nuevas Para Diversificar Tu Alimentación y Nutrirte Mejor; Universidad Nacional Agraria La Molina: Lima, Peru, 2014; pp. 1–116. [Google Scholar]
- Bonavia, D.; Vasquez, V.F.; Rosales Tham, T.; Dillehay, T.D.; Netherly, P.J.; Benson, K. Plant remains. In Where the Land Meets the Sea: Fourteen Millennia of Human History at Huaca Prieta, Peru; Dillehay, T.D., Ed.; University of Texas Press: Austin, TX, USA, 2017; pp. 367–433. [Google Scholar]
- Icochea, E. Bases biológicas para el Manejo del Recurso Chondracanthus chamissoi en el Litoral Marino de Huanchaco, Departamento La Libertad, Perú. Master’s Thesis, Universidad Nacional de Trujillo, Trujillo, Peru, 2008. [Google Scholar]
- Hayashi, L.; Bulboa, C.; Kradolfer, P.; Soriano, G.; Robledo, D. Cultivation of red seaweeds: A Latin American perspective. J. Appl. Phycol. 2014, 26, 719–727. [Google Scholar] [CrossRef]
- Alemañ, A.E.; Robledo, D.; Hayashi, L. Development of seaweed cultivation in Latin America: Current trends and future prospects. Phycologia 2019, 58, 462–471. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Padilla-Vallejos, J. The seaweed resources of Peru. Bot. Mar. 2020, 62, 381–394. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Villena-Sarmiento, G. Analysis of Peruvian seaweed exports during the period 1995–2020 using trade data. Bot. Mar. 2022, 65, 209–220. [Google Scholar] [CrossRef]
- Acleto, C. Algas Marinas del Perú de Importancia Económica; Departamento de Botánica, Museo de Historia Natural Javier Prado: Jesús María, Perú, 1986; Volume 5, pp. 1–107. [Google Scholar]
- Diaz Ruíz, J.T.; Fretell Timoteo, W.J.; Baltazar Guerrero, P.M.; Castañeda Franco, M.; Meza Balvin, S.J.; Ordoñez Suñiga, C.A. Factibilidad económica de la producción de Chondracanthus chamissoi, cultivo vía esporas en laboratorio, San Andrés-Pisco, Perú. Arnaldoa 2021, 28, 163–182. [Google Scholar]
- Castañeda, M.; Arbaiza, S.; Diaz, F.; Castillo, Y.; Baltazar, P.; Advíncula, O. Evaluación del fotoperiodo en el asentamiento de tetraesporas de Chondracanthus chamissoi sobre cuerdas de polipropileno en condiciones semi-controladas de laboratorio. Anales Científicos 2018, 79, 459–465. [Google Scholar] [CrossRef]
- Arbaiza, S.; Gil-Kodaka, P.; Arakaki, N.; Alveal, K.; Arbaiza, S.; Gil-Kodaka, P.; Arakaki, N.; Alveal, K. Primeros estadios de cultivo a partir de carpósporas de Chondracanthus chamissoi de tres localidades de la costa peruana. Rev. Biol. Mar. Oceanogr. 2019, 54, 204–213. [Google Scholar] [CrossRef]
- Bulboa, C. Bases Bio-Tecnológicas para o Cultivo de Chondracanthus chamissoi, Uma Alga Vermelha de Importância Econômica da Costa Chilena. Ph.D. Thesis, São Paulo University, São Paulo, Brazil, 2006. [Google Scholar]
- Bulboa, C.; Véliz, K.; Sáez, F.; Sepúlveda, C.; Vega, L.; Macchiavello, J. A new method for cultivation of the carragenophyte and edible red seaweed Chondracanthus chamissoi based on secondary attachment disc: Development in outdoor tanks. Aquaculture 2013, 410–411, 86–94. [Google Scholar] [CrossRef]
- Sáez, F.; Macchiavello, J. Secondary attachment discs: A new alternative for restoring populations of Chondracanthus chamissoi (Gigartinales, Rhodophyta). Lat. Am. J. Aquat. Res. 2018, 46, 140–146. [Google Scholar] [CrossRef]
- Macchiavello, J.; Sepúlveda, C.; Basaure, H.; Sáez, F.; Yañez, D.; Marín, C.; Vega, L. Suspended culture of Chondracanthus chamissoi (Rhodophyta: Gigartinales) in Caleta Hornos (northern Chile) via vegetative propagation with secondary attachment discs. J. Appl. Phycol. 2018, 30, 1149–1155. [Google Scholar] [CrossRef]
- Oyarzo, S.; Ávila, M.; Alvear, P.; Remonsellez, J.P.; Contreras-Porcia, L.; Bulboa, C. Secondary attachment disc of edible seaweed Chondracanthus chamissoi (Rhodophyta, Gigartinales): Establishment of permanent thalli stock. Aquaculture 2021, 530, 735954. [Google Scholar] [CrossRef]
- Zapata-Rojas, J.C.; Gonzales-Vargas, A.M.; Zevallos-Feria, S.A. Estudio comparativo para propagación vegetativa de Chondracanthus chamissoi, Yuyo, sobre tres tipos de sustrato en ambiente controlado y su viabilidad en la región Moquegua. Enfoque UTE 2020, 11, 37–47. [Google Scholar] [CrossRef]
- Jiksing, C.; Ongkudon, M.M.; Thien, V.Y.; Rodrigues, K.F.; Yong, W.T.L. Recent advances in seaweed seedling production: A review of eucheumatoids and other valuable seaweeds. Algae 2022, 37, 105–121. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Morales, C.; Moreira, W.S.C. Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquac. Res. 2002, 33, 1081–1086. [Google Scholar] [CrossRef]
- Mai, H.; Fotedar, R.; Fewtrell, J. Evaluation of Sargassum sp. as a nutrient-sink in an integrated seaweed-prawn (ISP) culture system. Aquaculture 2010, 310, 91–98. [Google Scholar] [CrossRef]
- Suthar, P.; Gajaria, T.K.; Reddy, C.R.K. Production of quality seaweed biomass through nutrient optimization for the sustainable land-based cultivation. Algal Res. 2019, 42, 101583. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. The evolution road of seaweed aquaculture: Cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Baltazar, P. Personal Communiacation; Universidad Científica del Sur: Lima, Perú, 2022. [Google Scholar]
- Véliz, K.; Chandía, N.; Karsten, U.; Lara, C.; Thiel, M. Geographic variation in biochemical and physiological traits of the red seaweeds Chondracanthus chamissoi and Gelidium lingulatum from the south east Pacific coast. J. Appl. Phycol. 2019, 31, 665–682. [Google Scholar] [CrossRef]
- Winberg, P.; Skropeta, D.; Ullrich, A. Seaweed Cultivation Pilot Trials: Towards Culture Systems and Marketable Products; Australian Government Rural Industries Research and Development Corporation (RIRDC): Canberra, Australia, 2011; pp. 1–184.
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–565. [Google Scholar]
- Ferreira, L.B.; Barufi, J.B.; Plastino, E.M. Growth of red and green strains of the tropical agarophyte Gracilaria cornea J. Agardh (Gracilariales, Rhodophyta) in laboratory. Rev. Bras. Bot. 2006, 29, 187–192. [Google Scholar] [CrossRef]
- Mansilla, A.; Rodriguez, J.P.; Souza, J.M.; Rosenfeld, S.; Ojeda, J.; Yokoya, N.S. Growth responses to temperature, salinity and nutrient variations, and biomass variation and phenology of Ahnfeltia plicata (Rhodophyta, Ahnfeltiales): A commercially interesting agarophyte from the Magellanic Region, Chile. J. Appl. Phycol. 2014, 26, 1133–1139. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Ting, S.H.; Yong, Y.S.; Thien, V.Y.; Wong, S.H.; Chin, W.L.; Anton, A. Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J. Appl. Phycol. 2014, 26, 1597–1606. [Google Scholar] [CrossRef]
- Alveal, K.; Romo, H.; Werlinger, C.; Oliveira, E. Mass cultivation of the agar-producing alga Gracilaria chilensis (Rhodophyta) from spores. Aquaculture 1997, 148, 77–83. [Google Scholar] [CrossRef]
- Pacheco-Ruiz, I.; Zertuche-González, J.A.; Arroyo-Ortega, E.; Valenzuela-Espinoza, E. Agricultural fertilizers as alternative culture media for biomass production of Chondracanthus squarrulosus (Rhodophyta, Gigartinales) under semi-controlled conditions. Aquaculture 2004, 240, 201–209. [Google Scholar] [CrossRef]
- Romo, H.; Ávila, M.; Candía, A.; Nuñez, M.; Oyarzo, C.; Gallegillos, F.; Cáceres, J. Manual de Técnicas de Cultivo de Luche (Porphyra sp.); Proyecto FONDEF D01 I 1148; IFOP: New York, NY, USA, 2005; pp. 1–32. [Google Scholar]
- Arbaiza, S.; Castañeda, M.; Gerónimo, G.; Munayco, P.; Reynaga, R.; Advíncula, O. Efecto del fotoperiodo y nutriente foliar comercial en el crecimiento (biomasa) de cochayuyo Porphyra spp. bajo condiciones semicontroladas de cultivo. In Proceedings of the Annual Meeting of the Latin American & Caribbean Aquaculture Societies, Bogota, Colombia, 23–26 October 2018. [Google Scholar]
- Werlinger, C.; Mansilla, A.; Villarroel, A.; Palacios, M. Effects of photon flux density and agricultural fertilizers on the development of Sarcothalia crispata tetraspores (Rhodophyta, Gigartinales) from the Strait of Magellan, Chile. J. Appl. Phycol. 2008, 20, 757–765. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Varela, D.; Cifuentes, M.; Carmen Hernández-González, M.C.; Henríquez, L.; Westermeier, R.; Correa, J.A. Experimental indoor cultivation of the carrageenophytic red alga Gigartina skottsbergii. Aquaculture 2004, 241, 357–370. [Google Scholar] [CrossRef]
- Mansilla, A.; Palacios, M.; Navarro, N.P.; Avila, M. Growth and survival performance of the gametophyte of Gigartina skottsbergii (Rhodophyta, Gigartinales) under defined nutrient conditions in laboratory culture. In Proceedings of the 19th International Seaweed Symposium, Kobe, Japan, 26–31 March 2007. [Google Scholar]
- Arbaiza, S. Viabilidad Reproductiva para el Cultivo de Chondracanthus chamissoi Proveniente de Tres Poblaciones Del Litoral Peruano. Master’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2016. [Google Scholar]
- Fernández-Linares, L.; Durán-Páramo, E.; Guerrero-Barajas, C. A scale-up evaluation of a semicontinuous culture of Scenedesmus sp. in a raceway under greenhouse conditions using a commercial fertilizer as culture medium. Biofuels 2021, 12, 1291–1299. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Harrison, P.J.; Hurd, C.L. Nutrient physiology of seaweeds: Application of concepts to aquaculture. Cah. Biol. Mar. 2001, 42, 71–82. [Google Scholar]
- Demetropoulos, C.L.; Langdon, C.J. Enhanced production of Pacific dulse (Palmaria mollis) for co-culture with abalone in a land-based system: Effects of seawater exchange, pH, and inorganic carbon concentration. Aquaculture 2004, 235, 457–470. [Google Scholar] [CrossRef]
- Matos, J.; Costa, S.; Rodrigues, A.; Pereira, R.; Sousa-Pinto, I. Experimental integrated aquaculture of fish and red seaweeds in Northern Portugal. Aquaculture 2006, 252, 31–42. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Grote, B. Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): Cultivation and uses. Rev. Aquac. 2019, 11, 25–41. [Google Scholar]
- Nagler, P.L.; Glenn, E.P.; Nelson, S.G.; Napolean, S. Effects of fertilization treatment and stocking density on the growth and production of the economic seaweed Gracilaria parvispora (Rhodophyta) in cage culture at Molokai, Hawaii. Aquaculture 2003, 219, 379–391. [Google Scholar] [CrossRef]
- Msuya, F. The effect of stocking density on the performance of the seaweed Ulva reticulata as a biofilter in earthen pond channels, Zanzibar, Tanzania. West. Indian Ocean J. Mar. Sci. 2008, 6, 65–72. [Google Scholar] [CrossRef]
- Msuya, F. Effects of stocking density and additional nutrients on growth of the commercially farmed seaweeds Eucheuma denticulatum and Kappaphycus alvarezii in Zanzibar, Tanzania. TaJONAS 2013, 4, 605–612. [Google Scholar]
- Corey, P.; Kim, J.K.; Duston, J.; Garbary, D.J. Growth and nutrient uptake by Palmaria palmata integrated with Atlantic halibut in a land-based aquaculture system. Algae 2014, 29, 35. [Google Scholar] [CrossRef]
- Alveal, K. Estrategias Reproductivas de Rhodophyta y Sus Nexos Con la Biodiversidad. In Sustentabilidad de la Biodiversidad. Un Problema Actual: Bases Científico-Técnicas. Teorizaciones y Proyecciones; Alveal, K., Antezana, T., Eds.; Universidad de Concepción: Concepción, Chile, 2001; pp. 367–388. [Google Scholar]
- Bulboa, C.R.; Macchiavello, J.E.; Oliveira, E.C.; Fonck, E. First attempt to cultivate the carrageenan-producing seaweed Chondracanthus chamissoi (C. Agardh) Kützing (Rhodophyta; Gigartinales) in Northern Chile. Aquac. Res. 2005, 36, 1069–1074. [Google Scholar] [CrossRef]
- Fonck, E.; Martínez, R.; Vásquez, J.; Bulboa, C. Factors that affect the re-attachment of Chondracanthus chamissoi (Rhodophyta, Gigartinales) thalli. J. Appl. Phycol. 2007, 20, 311–314. [Google Scholar] [CrossRef]
- Sáez, F.; Macchiavello, J.; Fonck, E.; Bulboa, C. The role of the secondary attachment disc in the vegetative propagation of Chondracanthus chamissoi (Gigartinales, Rhodophyta). Aquat. Bot. 2008, 89, 63–65. [Google Scholar] [CrossRef]
- Rodríguez, C.Y.; Otaíza, R. Factors affecting morphological transformation and secondary attachment of apexes of Chondracanthus chamissoi (Rhodophyta, Gigartinales). J. Appl. Phycol. 2018, 30, 1157–1166. [Google Scholar] [CrossRef]
- Maggs, C.A.; Callow, M.E. Algal spores. Encyclopedia of Life Sciences; Nature Publishing Group: London, UK, 2002; pp. 1–6. [Google Scholar]
- Bischof, K.; Rautenberger, R. Seaweed responses to environmental stress: Reactive oxygen and antioxidative strategies. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: Berlin, Germany, 2012; pp. 109–132. [Google Scholar]
- Rydgren, K.; Økland, R.H. Short-term Costs of Sexual Reproduction in the Clonal Moss Hylocomium splendens. Bryologist 2003, 106, 212–220. [Google Scholar] [CrossRef]
- Halling, C.; Aroca, G.; Cifuentes, M.; Buschmann, A.H.; Troell, M. Comparison of spore inoculated and vegetative propagated cultivation methods of Gracilaria chilensis in an integrated seaweed and fish cage culture. Aquac. Int. 2005, 13, 409–422. [Google Scholar] [CrossRef]
- Guillemin, M.L.; Valenzuela, P.; Gaitán-Espitia, J.D.; Destombe, C. Evidence of reproductive cost in the triphasic life history of the red alga Gracilaria chilensis (Gracilariales, Rhodophyta). J. Appl. Phycol. 2014, 26, 569–575. [Google Scholar] [CrossRef]
- Deza, K.; Gil-Kodaka, P.; Fernández, E.; Mendo, J. Efecto del tamaño de corte sobre la tasa de crecimiento y cobertura de la macroalga Chondracanthus chamissoi “yuyo” de la zona submareal de Playa Mendieta, Paracas, Pisco. In Proceedings of the Memorias I Jornada Científica Reserva Nacional Paracas, Lima, Peru, 28–31 March 2001. [Google Scholar]
- Gil-Kodaka, P.; Mendo, J.; Fernández, E. Diversidad de macroalgas del submareal en la Reserva Nacional de Paracas y notas sobre su uso potencial. In Proceedings of the Memorias I Jornada Científica Reserva Nacional Paracas, Lima, Peru, 28–31 March 2001. [Google Scholar]
- Sullivan, G.M.; Feinn, R. Using effect size-or why the P value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVA. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Los Angeles, CA, USA, 2019; pp. 1–608. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression Models for Count Data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- Jackman, S. pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory. United States Studies Centre, University of Sydney, Sydney, New South Wales, Australia. R Package Version 1.5.5. 2020. Available online: https://github.com/atahk/pscl/ (accessed on 12 February 2023).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 1–510. [Google Scholar]
- Sellke, T.; Bayarri, M.J.; Berger, J.O. Calibration of p values for testing precise null hypotheses. Am. Stat. 2001, 55, 62–71. [Google Scholar] [CrossRef]
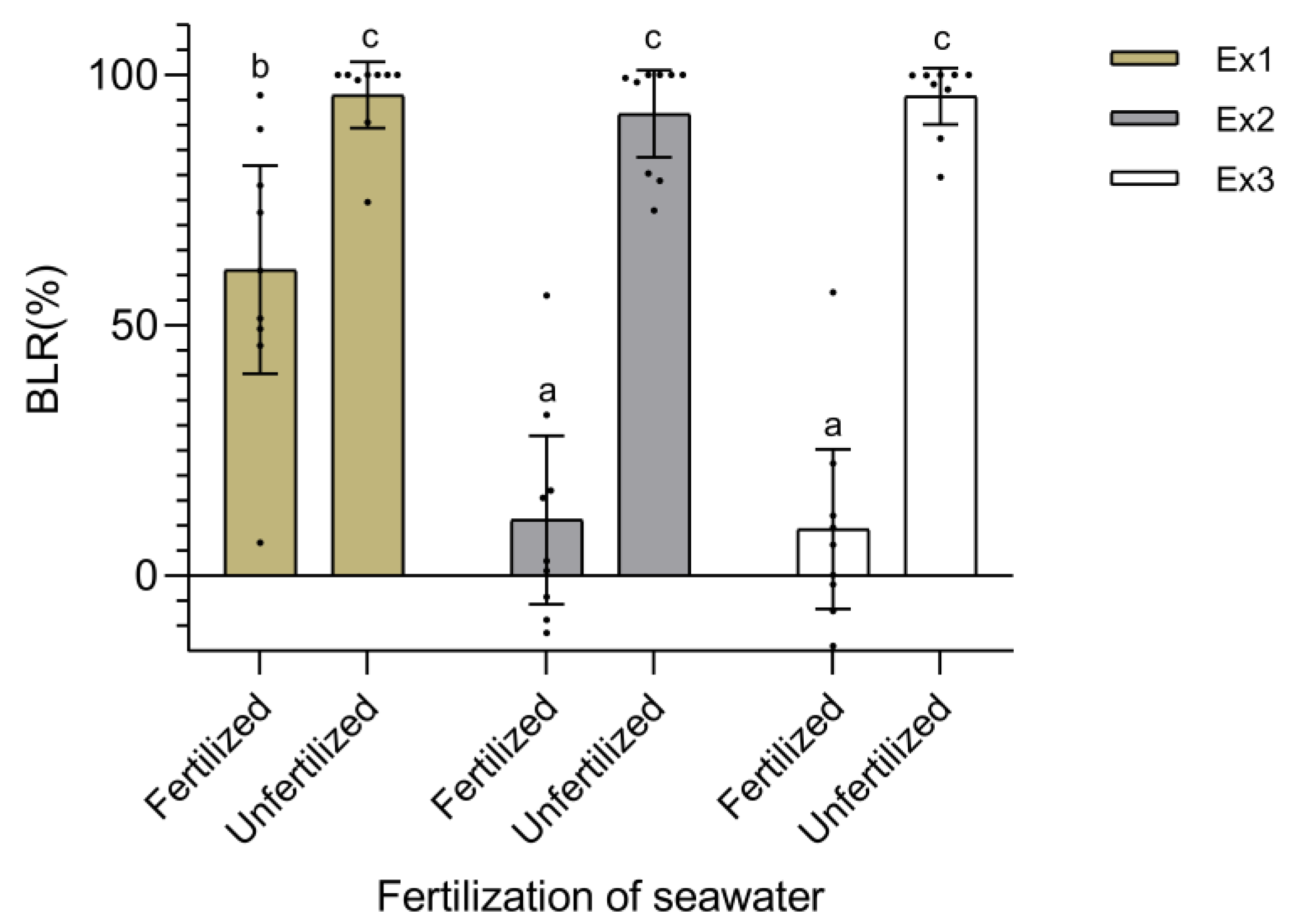





| Effects | df * | F * | p * | ω2 * |
|---|---|---|---|---|
| Fertilization of seawater (A) | 1 | 232.94 | <0.0001 | 0.86 |
| Medium exchange (B) | 2 | 15.90 | <0.0001 | 0.43 |
| Inoculum density (C) | 2 | 2.71 | 0.080 | NS |
| A × B | 2 | 13.70 | <0.0001 | 0.39 |
| A × C | 2 | 1.46 | 0.25 | NS |
| B × C | 4 | 1.61 | 0.19 | NS |
| A × B × C | 4 | 1.66 | 0.18 | NS |
| Fertilization of Seawater * | Medium Exchange ** | Inoculum Density (g L−1) | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|---|---|
| Fertilized | Daily (Ex1) | 3 | 3.44 ± 15.24% a | 14.56 ± 18.08% a | 54.89 ± 23.38% a | 74.44 ± 26.64% a |
| 5 | 1.40 ± 11.30% a | 19.07 ± 15.32% a | 45.67 ± 44.57% a | 56.13 ± 49.44% a | ||
| 7 | −1.10 ± 4.25% a | 8.85 ± 7.54% a | 26.86 ± 4.20% a | 52.81 ± 8.59% a | ||
| Every two days (Ex2) | 3 | −2.89 ± 4.17% a | −1.56 ± 3.30% a | −5.00 ± 6.63% a | 7.00 ± 9.86% a | |
| 5 | −4.20 ± 4.19% a | −4.73 ± 2.44% a | −11.80 ± 2.55% a | −8.13 ± 4.12% a | ||
| 7 | 19.71 ± 21.40% a | 31.90 ± 23.71% a | 30.71 ± 24.62% a | 34.57 ± 23.00% a | ||
| Weekly (Ex3) | 3 | −4.00 ± 8.91% a | −2.44 ± 11.22% a | −7.22 ± 14.98% a | 1.11 ± 11.09% a | |
| 5 | −8.67 ± 3.40% a | −1.93 ± 12.03% a | −2.33 ± 10.24% a | 0.60 ± 14.44% a | ||
| 7 | 21.29 ± 28.31% a | 27.38 ± 30.44% a | 23.67 ± 32.58% a | 26.38 ± 32.16% a | ||
| Unfertilized | Daily (Ex1) | 3 | 13.67 ± 11.51% a | 67.89 ± 35.67% a | 81.89 ± 29.50% a | 91.22 ± 16.23% a |
| 5 | 7.40 ± 20.77% a | 80.47 ± 31.14% a | 92.00 ± 14.71% a | 96.87 ± 6.15% a | ||
| 7 | 2.29 ± 9.54% a | 97.14 ± 1.54% b | 99.71 ± 0.43% b | 100% b | ||
| Every two days (Ex2) | 3 | 1.44 ± 4.29% a | 78.89 ± 26.06% ab | 99.78 ± 0.43% b | 100% b | |
| 5 | 2.13 ± 4.19% a | 12.47 ± 7.63% a | 48.00 ± 22.24% a | 84.00 ± 14.91% a | ||
| 7 | 3.52 ± 2.18% a | 38.14 ± 52.81% ab | 82.72 ± 33.75% ab | 92.76 ± 13.63% b | ||
| Weekly (Ex3) | 3 | 10.88 ± 18.89% a | 59.11 ± 7.55% a | 70.22 ± 17.52% a | 88.99 ± 11.60% a | |
| 5 | −2.66 ± 3.76% a | 95.00 ± 9.80% b | 98.33 ± 3.27% b | 99.99 ± 0.01% b | ||
| 7 | −6.42 ± 11.83% a | 93.76 ± 2.73% b | 96.19 ± 2.29% b | 98.43 ± 1.64% b |
| Locality | Reproductive Stage | Zone | Treatment |
|---|---|---|---|
| Tanque Amarillo | Vegetative | Subtidal | TaVS |
| Punta Ripio | Vegetative | Subtidal | PrVS |
| Cystocarpic | Subtidal | PrCS | |
| Talpo | Vegetative | Subtidal | TVS |
| Cystocarpic | Subtidal | TCS | |
| Playon | Vegetative | Subtidal | PnVS |
| Mendieta | Vegetative | Intertidal | MVI |
| Vegetative | Subtidal | MVS | |
| Tetrasporophytic | Intertidal | MTI | |
| Tetrasporophytic | Subtidal | MTS | |
| Cystocarpic | Intertidal | MCI | |
| Cystocarpic | Subtidal | MCS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbaiza, S.; Avila-Peltroche, J.; Castañeda-Franco, M.; Mires-Reyes, A.; Advíncula, O.; Baltazar, P. Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation. Plants 2023, 12, 1940. https://doi.org/10.3390/plants12101940
Arbaiza S, Avila-Peltroche J, Castañeda-Franco M, Mires-Reyes A, Advíncula O, Baltazar P. Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation. Plants. 2023; 12(10):1940. https://doi.org/10.3390/plants12101940
Chicago/Turabian StyleArbaiza, Samuel, Jose Avila-Peltroche, Max Castañeda-Franco, Arturo Mires-Reyes, Orlando Advíncula, and Paul Baltazar. 2023. "Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation" Plants 12, no. 10: 1940. https://doi.org/10.3390/plants12101940
APA StyleArbaiza, S., Avila-Peltroche, J., Castañeda-Franco, M., Mires-Reyes, A., Advíncula, O., & Baltazar, P. (2023). Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation. Plants, 12(10), 1940. https://doi.org/10.3390/plants12101940







