Molecular Insights into Abiotic Stresses in Mango
Abstract
1. Introduction
2. Abiotic Stresses in Mango
2.1. Drought/Water Deficit Stress
2.2. Cold Stress
2.3. Salinity Stress
3. Abiotic Stress-Induced Physiological Activities in Mango
4. Role of Abiotic Stress-Responsible Genes
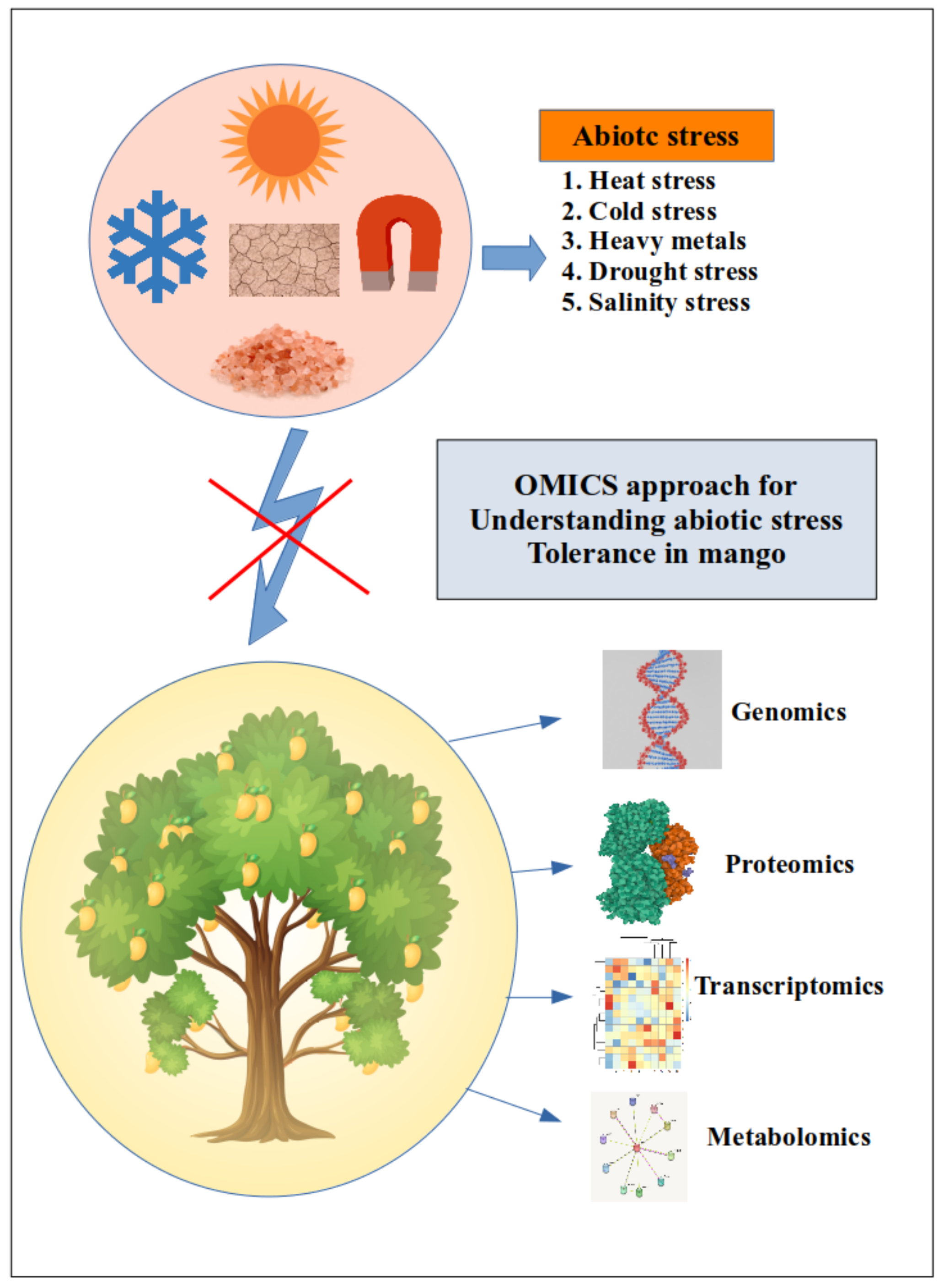
5. Omics Approaches to Dissect the Abiotic Stress-Resistant Mechanisms in Mango
5.1. Using Genomics to Investigate Abiotic Stress Tolerance in Mango
5.2. Transcriptomics Approach for Studying Abiotic Stress Resistance in Mango
5.3. Proteomics Approach for Revealing Abiotic Stress Tolerance in Mango
5.4. Role of Metabolomics in Understanding the Abiotic Stress Tolerance in Mango
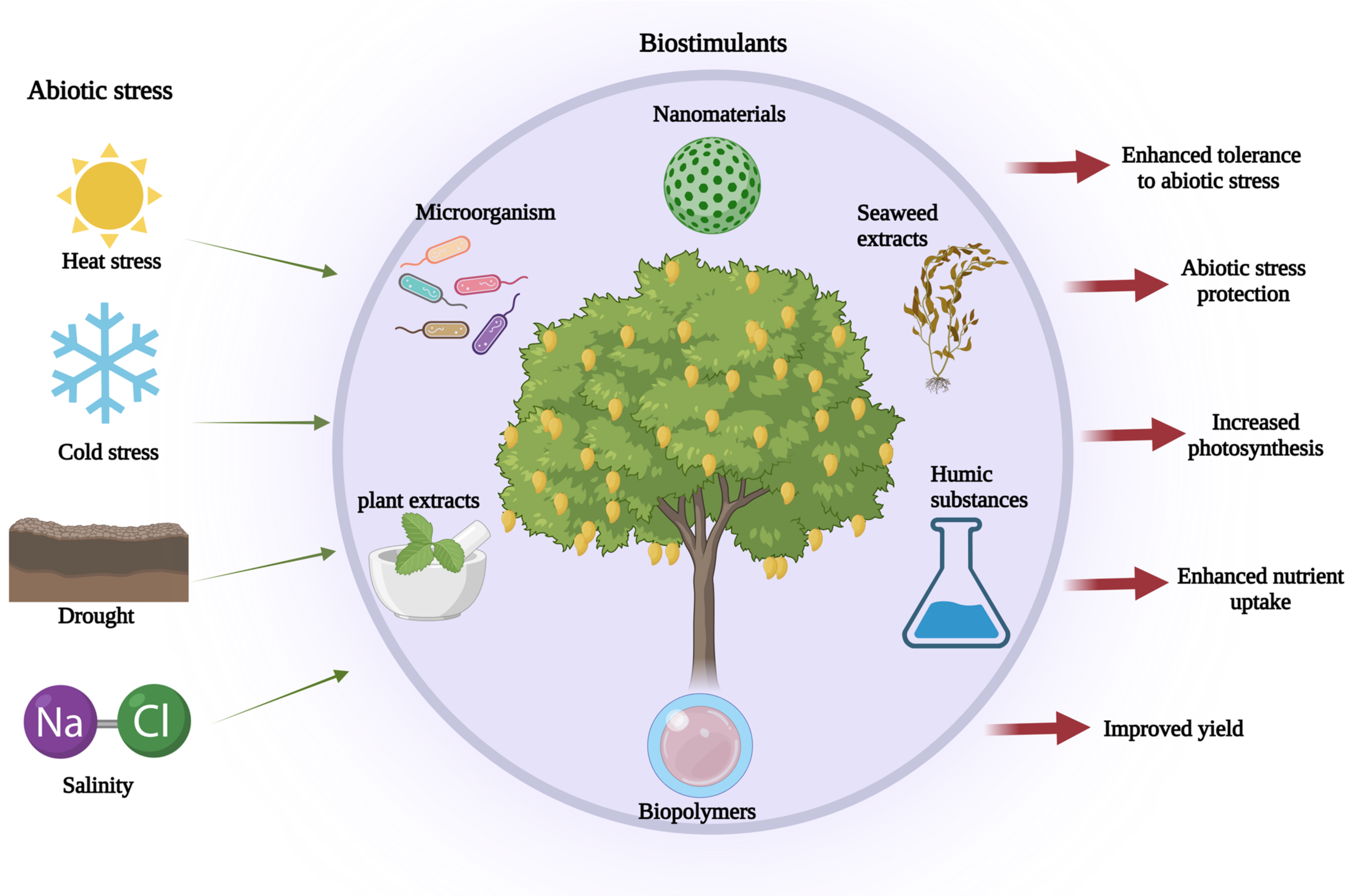
6. Role and Types of Biostimulants in the Alleviation of Abiotic Stress
6.1. Biostimulants
6.2. Biostimulants and Abiotic Stresses in Plants
6.3. Types of Biostimulants
6.3.1. Seaweed Extracts
6.3.2. Plant Extracts
6.3.3. Humic Substances
6.3.4. Chitosan and Other Biopolymers
6.3.5. Microorganisms
6.3.6. Inorganic Compounds and Nanomaterials
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kole, C. The Mango Genome; Springer International Publishing: Cham, Switzerland, 2021; pp. 7–12. [Google Scholar]
- Viruel, M.A.; Escribano, P.; Barbieri, M.; Ferri, M.; Hormaza, J.I. Fingerprinting, Embryo Type and Geographic Differentiation in Mango (Mangifera indica L., Anacardiaceae) with microsatellites. Mol. Breed 2005, 15, 383–393. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, R.K.; Pathak, G.; Goel, R.; Asif, M.H.; Sane, A.P.; Sane, V.A. Comparative Transcriptome Analysis of Unripe and Mid-Ripe Fruit of Mangifera indica (var. “Dashehari”) Unravels Ripening Associated Genes. Sci. Rep. 2016, 6, 32557. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S. Mango: The King of Fruits. In The Mango Genome; Springer: Cham, Switzerland, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Khanum, Z.; Tiznado-Hernández, M.E.; Ali, A.; Musharraf, S.G.; Shakeel, M.; Khan, I.A. Adaptation Mechanism of Mango Fruit (Mangifera indica L. cv. Chaunsa White) to Heat Suggest Modulation in Several Metabolic Pathways. RSC Adv. 2020, 10, 35531–35544. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Shin, H.; Adarshan, S.; Jeyasri, R.; Priya, A.; Chen, J.T.; Ramesh, M. Molecular Insights into Freezing Stress in Peach Based on Multi-Omics and Biotechnology: An Overview. Plants 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Faria, L.N.; Donato, S.L.; Santos, M.R.D.; Castro, L.G. The Effects of Irrigation Management on Floral Induction of ‘Tommy Atkins’ Mango in Bahia Semiarid. Eng. Agric. 2016, 36, 387–398. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Shanthala, L.; Ramachandra, R.K.; Mohan Kumar, S.; Swamy, G.S.K.; Honnabyraiah, M.K.; Pallavi, H.M. Evaluation of Mango (Mangifera indica L.) Rootstocks under Induced Moisture Stress Condition for Various Morpho-Physiological Traits. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 143–148. [Google Scholar] [CrossRef]
- Mathiazhagan, M.; Padala, S.; Doddahejjaji, S.G.C.; Murugan, S.; Makki, D.R.; Kundapura, R.V. Omics of Mango: A Tropical Fruit Tree. Omics Hortic. Crops 2022, 427–448. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M. Drought Stress in Plants: A Review on Morphological Characteristics and Pigment Composition. Int. J. Agric. Biol. 2008, 11, 100–105. [Google Scholar]
- Cotrim, C.E.; Coelho-Filho, M.A.; Coelho, E.F.; Ramos, M.M.; Cecon, P.R. Regulated Deficit Irrigation and Tommy Atkins Mango Orchard Productivity under Micro-Sprinkling in Brazilian Semi-Arid. Eng. Agric. 2011, 31, 1052–1063. [Google Scholar]
- Schaffer, B.; Whiley, A.W.; Crane, J.H. Mango. In Handbook of Environmental Physiology of Fruit Crops, Volume 2, Sub Tropical and Tropical Crops; Schaffer, B., Andersen, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 165–197. [Google Scholar]
- Tahir, F.M.; Ibrahim, M.; Hamid, K. Effect of Drought Stress on Vegetative and Reproductive Growth Behaviour of Mango (Mangifera indica L.). Asian J. Plant Sci. 2003, 2, 116–118. [Google Scholar] [CrossRef]
- Santos, M.R.; Martinez, M.A.; Donato, S.L.R. Gas Exchanges of “Tommy Atkins” Mango Trees under Different Irrigation Treatments. J. Biosci. 2013, 29, 1141–1153. [Google Scholar]
- Santos, M.R.; Martinez, M.A.; Donato, S.L.R.; Coelho, E.F. Tommy Atkins Mango Yield and Photosynthesis under Hydric Deficit in Semiarid Region of Bahia. Rev. Bras. Eng. Agricola Ambient. 2014, 18, 899–907. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Hoseiny, H.; El-Sheery, N.I.; Rastogi, A.; Kalaji, H.M. Regulation and Physiological Role of Silicon in Alleviating Drought Stress of Mango. Plant Physiol. Biochem. 2017, 118, 31–44. [Google Scholar] [CrossRef]
- Silva, M.A.D.; Cavalcante, Í.H.; Mudo, L.E.; Paiva Neto, V.B.D.; Cunha, J.G.D. Biostimulant Alleviates Abiotic Stress of Mango Grown in Semiarid Environment. Rev. Bras. Eng. Agricola Ambient. 2020, 24, 457–464. [Google Scholar] [CrossRef]
- Gadallah, F.M.; El-Yazal, S.; Abdel-Samad, G.A. Physiological Changes in Leaves of Some Mango Cultivars as Response to Exposure to Low Temperature Degrees. Hortic. Int. J. 2019, 3, 266–273. [Google Scholar] [CrossRef]
- Vega-Alvarez, M.; Salazar-Salas, N.Y.; López-Angulo, G.; Pineda-Hidalgo, K.V.; López-López, M.E.; Vega-García, M.O.; Delgado-Vargas, F.; López-Valenzuela, J.A. Metabolomic Changes in Mango Fruit Peel Associated with Chilling Injury Tolerance Induced by Quarantine Hot Water Treatment. Postharvest Biol. Technol. 2020, 169, 111299. [Google Scholar] [CrossRef]
- Li, P.; Zheng, X.; Liu, Y.; Zhu, Y. Pre-Storage Application of Oxalic Acid Alleviates Chilling Injury in Mango Fruit by Modulating Proline Metabolism and Energy Status under Chilling Stress. Food Chem. 2014, 142, 72–78. [Google Scholar] [CrossRef]
- Cantre, D.; Herremans, E.; Verboven, P.; Ampofo-Asiama, J.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Tissue Breakdown of Mango (Mangifera indica L. cv. carabao) due to Chilling Injury. Postharvest Biol. Technol. 2017, 125, 99–111. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved Cold Tolerance of Mango Fruit with Enhanced Anthocyanin and Flavonoid Contents. Molecules 2018, 23, 1832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, J.; Jiang, W.; Gu, Y.; Zhao, Y. Maturity-Related Chilling Tolerance in Mango Fruit and The Antioxidant Capacity Involved. J. Sci. Food Agric. 2009, 89, 304–309. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased Anthocyanin and Flavonoids in Mango Fruit Peel are Associated with Cold and Pathogen Resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Sela, N.; Feygenberg, O.; Zemach, H.; Maurer, D.; Alkan, N. Transcriptome Dynamics in Mango Fruit Peel Reveals Mechanisms of Chilling Stress. Front. Plant Sci. 2016, 7, 1579. [Google Scholar] [CrossRef]
- Salazar-Salas, N.Y.; Chairez-Vega, D.A.; Vega-Alvarez, M.; González-Nuñez, D.G.; Pineda-Hidalgo, K.V.; Chávez-Ontiveros, J.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Proteomic Changes in Mango Fruit Peel Associated with Chilling Injury Tolerance Induced by Quarantine Hot Water Treatment. Postharvest Biol. Technol. 2022, 186, 111838. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V. Salt Tolerance of Plants. J. Appl. Agric. Res. 1986, 1, 12–26. [Google Scholar]
- Jindal, P.C.; Singh, J.P.; Gupta, O.P. Screening of Mango Seedlings for Salt Tolerance. J. Hortic. Sci. 1975, 4, 112–115. [Google Scholar]
- Dubey, A.K.; Srivastav, M.; Singh, R.; Pandey, R.N.; Deshmukh, P.S. Response of Mango (Mangifera indica) Genotypes to Graded Levels of Salt Stress. Indian J. Agric. Sci. 2006, 76, 670–672. [Google Scholar]
- Srivastav, M.; Dubey, A.K.; Pandey, R.N.; Deshmukh, P.S. Effect of Soil Salinity on Survival, Growth and Chlorophyll Contents of ‘Kurukkan’ Mango (Mangifera indica). Indian J. Agric. Sci. 2007, 77, 685–688. [Google Scholar]
- Kishor, A.; Srivastav, M.; Dubey, A.K.; Singh, A.K.; Sairam, R.K.; Pandey, R.N.; Dahuja, A.; Sharma, R.R. Paclobutrazol Minimises The Effects of Salt Stress in Mango (Mangifera indica L.). J. Hortic. Sci. Biotech. 2009, 48, 459–465. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, A.K.; Dubey, A.K.; Dahuja, A. Biochemical and Salt Ion Uptake Responses of Seven Mango (Mangifera indica L.) Rootstocks to NaCl Stress. J. Hortic. Sci. Biotech. 2014, 89, 367–372. [Google Scholar] [CrossRef]
- Mahouachi, J. Long-Term Salt Stress Influence on Vegetative Growth and Foliar Nutrient Changes in Mango (Mangifera indica L.) Seedlings. Sci. Hortic. 2018, 234, 95–100. [Google Scholar] [CrossRef]
- Shalan, A.M. Inducing Salinity Tolerance in Mango (Mangifera indica L.) Cv.“El-Gahrawey” by Sodium Silicate Pentahydrate and Glycine Betaine. J. Plant Prod. 2020, 11, 541–549. [Google Scholar] [CrossRef]
- Rao, N.S.; Shivashankara, K.S.; Laxman, R.H. Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Laxman, R.H.; Annapoornamma, C.J.; Biradar, G. Mango. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 169–181. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Huang, F.; Liu, Z.; An, Z.; Dong, L.; He, X. Identification and Characterization of The Mango eIF Gene Family Reveals Mieif1a-a, Which Confers Tolerance to Salt Stress in Transgenic Arabidopsis. Sci. Hortic. 2019, 248, 274–281. [Google Scholar] [CrossRef]
- Lobo, J.T.; de Sousa, K.d.S.M.; de Paiva Neto, V.B.; Pereira, R.N.; dos Santos Silva, L.; Cavalcante, Í.H.L. Biostimulants on Fruit Yield and Quality of Mango cv. Kent Grown in Semiarid. J. Am. Pomol. Soc. 2019, 73, 152–160. [Google Scholar]
- Gunjate, R.T.; Tare, S.J.; Rangwala, A.D.; Limaye, V.P. Calcium Content in Alphonso Mango Fruits in Relation to Occurrence of Spongy Tissue [India]. J. Maharashtra Agric. Univ. 1979, 4, 159–161. [Google Scholar]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit Ripening Phenomena—An Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Oak, P.; Jha, V.; Deshpande, A.; Tanpure, R.; Dawkar, V.; Mundhe, S.; Ghuge, S.; Prabhudesai, S.; Krishanpal, A.; Jere, A.; et al. Transcriptional and Translational Perturbation in Abiotic Stress Induced Physiological Activities and Metabolic Pathway Networks in Spongy Tissue Disorder of Mango Fruit. Postharvest Biol. Technol. 2022, 188, 111880. [Google Scholar] [CrossRef]
- Jardine, K.J.; Meyers, K.; Abrell, L.; Alves, E.G.; Serrano, A.M.; Kesselmeier, J.; Karl, T.; Guenther, A.; Vickers, C.; Chambers, J.Q. Emissions of Putative Isoprene Oxidation Products from Mango Branches under Abiotic Stress. J. Exp. Bot. 2013, 64, 3669. [Google Scholar] [CrossRef]
- Luria, N.; Sela, N.; Yaari, M.; Feygenberg, O.; Kobiler, I.; Lers, A.; Prusky, D. De-Novo Assembly of Mango Fruit Peel Transcriptome Reveals Mechanisms of Mango Response to Hot Water Treatment. BMC Genomics 2014, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Da Cunha, J.G.; Cavalcante, H.L.; da Silva, M.A.; e Amariz, R.A.; Carmo, R.N.D.; Lobo, J.T. Proline and Algal Extract to Alleviate the Abiotic Stress in Mango ‘Tommy Atkins’ in the Tropical Semiarid. Erwerbs-Obstbau 2022, 64, 115–126. [Google Scholar] [CrossRef]
- Elsamad, G.A.; Moustafa, A.; Hussein, R.; Hussein, H. Effect of foliar application of potassium silicate and α-tocopherol on mitigating the adverse impacts of low temperature and salinity stresses on young mango trees. Fayoum J. Agric. Res. Dev. 2022, 36, 324–341. [Google Scholar] [CrossRef]
- Pandit, S.S.; Kulkarni, R.S.; Giri, A.P.; Köllner, T.G.; Degenhardt, J.; Gershenzon, J.; Gupta, V.S. Expression Profiling of Various Genes During the Fruit Development and Ripening of Mango. Plant Physiol. Biochem. 2010, 1, 426–433. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Luo, R.; Sun, Y.; Yang, C.; Li, X.; Gao, A.; Pu, J. Genome-Wide Characterization, Identification and Expression Profile of Myb Transcription Factor Gene Family During Abiotic and Biotic Stresses in Mango (Mangifera indica). Plants 2022, 11, 3141. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Jeyasri, R.; Rakkammal, K.; Satish, L.; Shamili, S.; Karthikeyan, A.; Valliammai, A.; Priya, A.; Selvaraj, A.; Gowri, P.; et al. Multi-Omics and Integrative Approach Towards Understanding Salinity Tolerance in Rice: A Review. Biology 2022, 11, 1022. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis Among Eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0, A Portal for the Functional and Evolutionary Study of Plant Transcription Factors. Nucleic Acids Res. 2014, 42, 1182–1187. [Google Scholar] [CrossRef]
- De Lucena, C.C.; de Siqueira, D.L.; Martinez, H.E.P.; Cecon, P.R. Salt Stress Change Chlorophyll Fluorescence in Mango. Rev. Bras. Frutic. 2012, 34, 1245–1255. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Liang, R.; Lan, M.; Yu, H.; Guo, Y.; Chen, S.; Lu, T.; Mo, X.; He, X. Genome-Wide Identification of The Mango Constans (Co) Family and Functional Analysis of Two Micol9 Genes in Transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 1028987. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; He, X.; Huang, X.; Yu, H.; Lu, T.; Xie, X.; Zeng, X.; Zhu, J.; Luo, C. Genome-Wide Identification and Expression Analysis of The 14-3-3 Gene Family in Mango (Mangifera indica L.). Int. J. Mol. Sci. 2022, 23, 1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Luo, C.; Dong, L.; Van Toan, C.; Wei, P.X.; He, X.H. Molecular Characterization and Expression Analysis of A Gtp-Binding Protein (Mirab5) in Mangifera Indica. Gene 2014, 540, 86–91. [Google Scholar] [CrossRef]
- Luo, C.; Dong, L.; He, X.H.; Yu, H.X.; Ou, S.J.; Fang, Z.B. Molecular Cloning and Characterisation of A cDNA Encoding An Abscisic Acid-, Stress-, and Ripening-Induced (ASR) Protein in Mango (Mangifera indica L.). J. Hortic. Sci. Biotechnol. 2014, 89, 352–358. [Google Scholar] [CrossRef]
- Luo, C.; He, X.H.; Hu, Y.; Yu, H.X.; Ou, S.J.; Fang, Z.B. Oligo-dT Anchored cDNA–SCoT: A Novel Differential Display Method for Analyzing Differential Gene Expression in Response to Several Stress Treatments in Mango (Mangifera indica L.). Gene 2014, 548, 182–189. [Google Scholar] [CrossRef]
- Bajpai, A.; Muthukumar, M.; Kumar, S. Mango Functional Genomics. In The Mango Genome; Springer: Cham, Switzerland, 2021; Volume 1, pp. 195–217. [Google Scholar] [CrossRef]
- Tan, L.; Salih, H.; Htet, N.N.W.; Azeem, F.; Zhan, R. Genomic Analysis of WD40 Protein Family in the Mango Reveals a Ttg1 Protein Enhances Root Growth and Abiotic Tolerance in Arabidopsis. Sci. Rep. 2021, 11, 2266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; He, X.H.; Li, Y.Z.; Zhang, Y.L.; Yu, H.X.; Xia, L.M.; MO, X.; Zeng, X.M.; Yang, J.H.; Luo, C. Genome-wide Analysis of the Mango SPL Family and Overexpression of MiSPL13 Confers Early Flowering and Stress Tolerance in Transgenic Arabidopsis. Sci. Hortic. 2022, 305, 111363. [Google Scholar] [CrossRef]
- Lu, T.T.; Fan, Z.Y.; He, X.H.; Yu, H.X.; Liang, R.Z.; Huang, X.; Zhang, Y.L.; Zhu, J.W.; Wang, J.Y.; Luo, C. Overexpression of Mango MiMFT Inhibits Seed Germination and Enhances Abiotic Stress Tolerance in Transgenic Arabidopsis. Sci. Hortic. 2023, 307, 111495. [Google Scholar] [CrossRef]
- Kaur, H.; Sidhu, G.S.; Mittal, A.; Yadav, I.S.; Mittal, M.; Singla, D.; Singh, K.; Singh, N.K.; Chhuneja, P. Comparative Transcriptomics in Alternate Bearing Cultivar Dashehari Reveals the Genetic Model of Flowering in Mango. Front. Genet. 2022, 13, 857152. [Google Scholar] [CrossRef]
- Khan, W.; Razzak, S.A.; Kamran Azim, M. Comparative Transcriptomics of Mango (Mangifera indica L.) Cultivars Provide Insights of Biochemical Pathways Involved in Flavor and Color. bioRxiv 2017, 196881. [Google Scholar] [CrossRef]
- Patel, J.; Mishra, A. Plant Aquaporins Alleviate Drought Tolerance in Plants by Modulating Cellular Biochemistry, Root-Architecture, and Photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef]
- Tan, L.; Waqas, M.; Rehman, A.; Rashid, M.A.R.; Fiaz, S.; Manzoor, H.; Azeem, F. Computational Analysis and Expression Profiling of Potassium Transport-Related Gene Families in Mango (Mangifera indica) Indicate Their Role in Stress Response and Fruit Development. Front. Plant Sci. 2022, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, C.; Guo, Y.; Liang, R.; Yu, H.; Chen, S.; He, X. Isolation and Functional Characterization of Two CONSTANS-Like 16 (MiCOL16) Genes from Mango. Int. J. Mol. Sci. 2022, 23, 3075. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, M.K.; Aleem, M.; Mansoor, S.; Khan, M.A.; Rauf, S.; Iqbal, S.; Siddique, K.H. Omics and CRISPR-Cas9 Approaches for Molecular Insight, Functional Gene Analysis, and Stress Tolerance Development in Crops. Int. J. Mol. Sci. 2021, 22, 1292. [Google Scholar] [CrossRef] [PubMed]
- Owusu Adjei, M.; Ma, J.; Luo, R.; Huang, J.; Zhao, Z.; Wang, Y.; Gao, A. Transcriptome analyses revealed chilling response genes in mango (Mangifera indica L. cv. Keitt) leaf. J. Plant Interact. 2023, 18, 217–226. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant Proteome Changes Under Abiotic Stress—Contribution of Proteomics Studies to Understanding Plant Stress Response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, P.; Yun, Z.; Jiang, G.; Zhang, Z.; Jiang, Y. β-aminobutyric Acid Priming Acquisition and Defense Response of Mango Fruit to Colletotrichum Gloeosporioides Infection Based on Quantitative Proteomics. Cells 2019, 8, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, F. The Multifunctions of WD40 Proteins in Genome Integrity and Cell Cycle Progression. J. Genom. 2015, 3, 40. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef]
- Oak, P.; Deshpande, A.; Giri, A.; Gupta, V. Metabolomic Dynamics Reveals Oxidative Stress in Spongy Tissue Disorder During Ripening of Mangifera Indica L. Fruit. Metabolites 2019, 9, 255. [Google Scholar] [CrossRef]
- Dawid, C.; Hille, K. Functional Metabolomics—A Useful Tool to Characterize Stress-Induced Metabolome Alterations Opening New Avenues Towards Tailoring Food Crop Quality. Agronomy 2018, 8, 138. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative Tool for Enhancing Plant Nutrition in Organic Farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; de Pascale, S.; Colla, G. Improving Vegetable Quality in Controlled Environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; de Pascale, S.; Bonini, P. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multi-Level Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant Activity of Phosphate in Horticulture. Sci Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant Activity of Silicon in Horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of Abiotic Stress on Plants: A Systems Biology Perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Huang, J.; Levine, A.; Wang, Z. Plant Abiotic Stress. Sci. World J. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Cavalcante, Í.; dos Santos, G.N.F.; da Silva, M.A.; Martins, R.D.S.; Lima, A.M.N.; Modesto, P.I.R.; Beckmann-Cavalcante, M.Z. A New Approach to Induce Mango Shoot Maturation in Brazilian Semi-Arid Environment. J. Appl. Bot. Food Qual. 2018, 91, 281–286. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Ferrante, A. Evaluation of Borage Extracts as Potential Biostimulant Using a Phenomic, Agronomic, Physiological, and Biochemical Approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact Under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Bradáčová, K.; Weber, N.F.; Morad-Talab, N.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), Seaweed Extracts, and Plant Growth-Promoting Bacteria As Cold-Stress Protectants in Maize. Chem. Biol. Technol. Agric. 2016, 3, 19. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Marroig, R.G. Effect of the commercial extract of the brown alga Ascophyllum nodosum Mont. on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva in situ submitted to lethal temperatures. J. Appl. Phycol. 2014, 26, 629–634. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of Seaweed Extract-Based Cytokinins and Zeatin Riboside on Creeping Bentgrass Heat Tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Mohamed, A.Y.; El-Sehrawy, O.A. Effect of Seaweed Extract on Fruiting of Hindy Bisinnara Mango Trees. J. Am. Sci. 2013, 9, 537–544. [Google Scholar]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for Production, Characterization and Application of Protein-Based Biostimulants in Agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops Under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- El-Sharony, T.F.; El-Gioushy, S.F.; Amin, O.A. Effect of Foliar Application with Algae and Plant Extracts on Growth, Yield and Fruit Quality of Fruitful Mango Trees cv. Fagri Kalan. J. Hortic. 2015, 2, 162. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.D.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From Lab to Field: Role of Humic Substances Under Open-Field and Greenhouse Conditions as Biostimulant and Biocontrol Agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Rajan, R.K.; Mali, P.C.; Haldankar, P.M.; Haldavanekar, P.C.; Potphode, P.D. Effect of Humic Acid on Growth of Mango (Mangifera indica L.) Nursery Grafts Cv. Alphonso. J. Pharmacogn. Phytochem. 2018, 7, 2778–2780. [Google Scholar]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; dos Reis, C.O.; Karam, D.; Doriguetto, A.C.; dos Santos, M.H.; dos Santos Santos Filho, P.R.; de Souza, T.C. The Foliar Application of A Mixture of Semisynthetic Chitosan Derivatives Induces Tolerance to Water Deficit in Maize, Improving the Antioxidant System and Increasing Photosynthesis and Grain Yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X. Physiological Effects of Chitosan Coating on Wheat Growth and Activities of Protective Enzyme with Drought Tolerance. Open J. Soil Sci. 2012, 2, 282. [Google Scholar] [CrossRef]
- Zagzog, O.A.; Gad, M.M.; Hafez, N.K. Effect of Nano-Chitosan on Vegetative Growth, Fruiting and Resistance of Malformation of Mango. Trends Hortic. Res. 2017, 6, 673–681. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Kitir, N.; Unek, C.; Nikerel, E.; Ozdemir, B.S.; Güne¸s, A.; Mokhtari, N.E.P. Beneficial Role of Plant Growth-Promoting Bacteria in Vegetable Production Under Abiotic Stress. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 151–166. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on Plant Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
| Abiotic Stress | Genotype Used | Abiotic Stress-Responsive Genes | Type of Study Involved | Plant Tissue/Stage Showing Upregulation | Role/Function of Genes | References |
|---|---|---|---|---|---|---|
| K+ depletion, salinity, and PEG treatment | Guire 82 | MiHAK genes (1–18) MiHAK14 exhibit tolerance to K+ depletion, salinity | Isolation and characterization and expression profile analysis of MiHAKs, overexpression of MiHAK14 in A. thaliana | Upregulation of various MiHAKs in root tissues under abiotic stress | MiHAK genes belong to KT/HAK/KUP family that encodes K+ transporters which provide resistance to salinity, drought, and heavy metal stress | [51] |
| Salinity and drought | Jin Huang | MiCOL genes | Genome-wide identification of CO genes, expression pattern analysis of MiCOL genes, and overexpression of MiCOL9 genes in A. thaliana | Higher expression of MiCOL9A and MiCOL9B genes in leaves after 12 h of abiotic stress treatment | COL candidate gene in the photoperiod pathway played a role in the regulation of flowering and abiotic stress response | [56] |
| Low temperature, drought, and salinity | Siji | Family members of Mi14-3-3 gene (Mi14-3-3-A1, -A2, -B1, -B2, -C1, -C2, -D1, -D2, -E1, -E2, -I1, -I2, -6A, -6B, -7A, -7B) | Genome identification and gene expression profiling using qRT-PCR | Mi14-3-3-A1- young stems Mi14-3-3-6A, Mi14-3-3-C1 and Mi14-3-3-D1—adult leaves Mi14-3-3-E1—flowers Mi14-3-3-I2—buds Mi14-3-3-A2, Mi14-3-3-D2 and Mi14-3-3-7B—young leaves | Opening of stomata, root movement, plant growth and development, hormone signaling, morpho-physiological metabolisms, and stress responses | [57] |
| Heat | Chaunsa White | MiGAD, MiNRX1, MiGI, MiGSTF6MiWun1, MiCAT1 and MiPER42 | RNA-Seq analysis, gene expression analysis using qRT-PCR | Mango fruits after 79 days of flowering (79DAF) | The enzymatic and non-enzymatic antioxidant activity involved in ROS homeostasis and circadian rhythm control | [6] |
| Cold, osmotic, and salinity | Siji | MieIF genes (particularly MieIF1A-α, MieIF3sB, and MieIF5 were more strongly expressed during salinity, cold and osmotic stress, respectively) | Transcriptome analysis, functional analysis by overexpression of MieIF1A-α in A. thaliana | Leaves of one-year-old seedlings at various time points (0, 6, 12, 24, 48, 72 h) | Protein synthesis, translation initiation, virus resistance, vegetative and reproductive growth, and stress responses | [40] |
| Cold, drought, and salinity | Siji | MiRab5 | Isolation, characterization, and gene expression analysis of MiRab5 | Higher expression in younger leaves and stems as well as in later stages of fruit ripening | Regulate the fusion of vesicles with target membranes via conformational changes, fruit ripening, and stress responses | [58] |
| Cold, drought, and salinity | Siji | MiASR | Molecular cloning, characterization, and qRT-PCR analysis of MiASR gene | leaves and stems at various time points (0, 24, 48, and 72 h) | Plant development, fruit ripening, post-harvest storage, biotic and abiotic stress responses | [59] |
| Cold, salinity, drought, and heavy metal | Siji | Transcript-derived fragments viz., TDF4, 7, 23, 45, 49, 50, 57, 91 and 92 | Oligo-dT cDNAstart codon targeted marker (cDNA–SCoT) analysis and gene expression analysis using qRT-PCR | leaves and stems at various time points (0, 24, 48, and 72 h) | Fruit ripening, post-harvest storage, energy metabolism metabolite transport, post-transcriptional regulation of genes, flowering time control, plant defense, and abiotic stress responses | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthuramalingam, P.; Muthamil, S.; Shilpha, J.; Venkatramanan, V.; Priya, A.; Kim, J.; Shin, Y.; Chen, J.-T.; Baskar, V.; Park, K.; et al. Molecular Insights into Abiotic Stresses in Mango. Plants 2023, 12, 1939. https://doi.org/10.3390/plants12101939
Muthuramalingam P, Muthamil S, Shilpha J, Venkatramanan V, Priya A, Kim J, Shin Y, Chen J-T, Baskar V, Park K, et al. Molecular Insights into Abiotic Stresses in Mango. Plants. 2023; 12(10):1939. https://doi.org/10.3390/plants12101939
Chicago/Turabian StyleMuthuramalingam, Pandiyan, Subramanian Muthamil, Jayabalan Shilpha, Varadharajan Venkatramanan, Arumugam Priya, Jinwook Kim, Yunji Shin, Jen-Tsung Chen, Venkidasamy Baskar, Kyoungmi Park, and et al. 2023. "Molecular Insights into Abiotic Stresses in Mango" Plants 12, no. 10: 1939. https://doi.org/10.3390/plants12101939
APA StyleMuthuramalingam, P., Muthamil, S., Shilpha, J., Venkatramanan, V., Priya, A., Kim, J., Shin, Y., Chen, J.-T., Baskar, V., Park, K., & Shin, H. (2023). Molecular Insights into Abiotic Stresses in Mango. Plants, 12(10), 1939. https://doi.org/10.3390/plants12101939











