Phenotypic and Genetic Variation in Morphophysiological Traits in Huanglongbing-Affected Mandarin Hybrid Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. HLB Visual Grading
2.3. PCR Detection of CLas
2.4. Measurement of LAI
2.5. Leaf Physiological Measurements
2.6. Statistical Analysis
3. Results
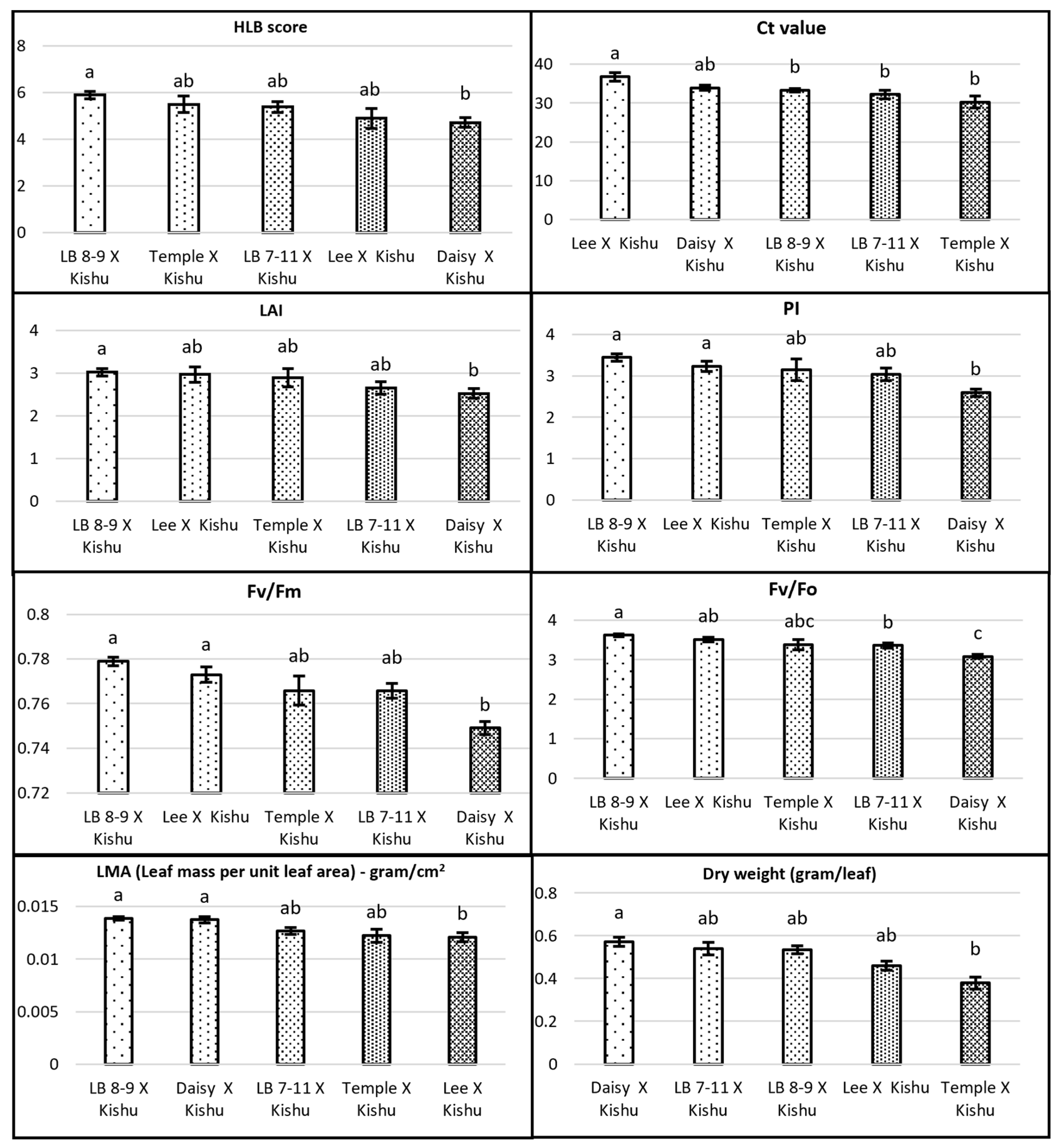
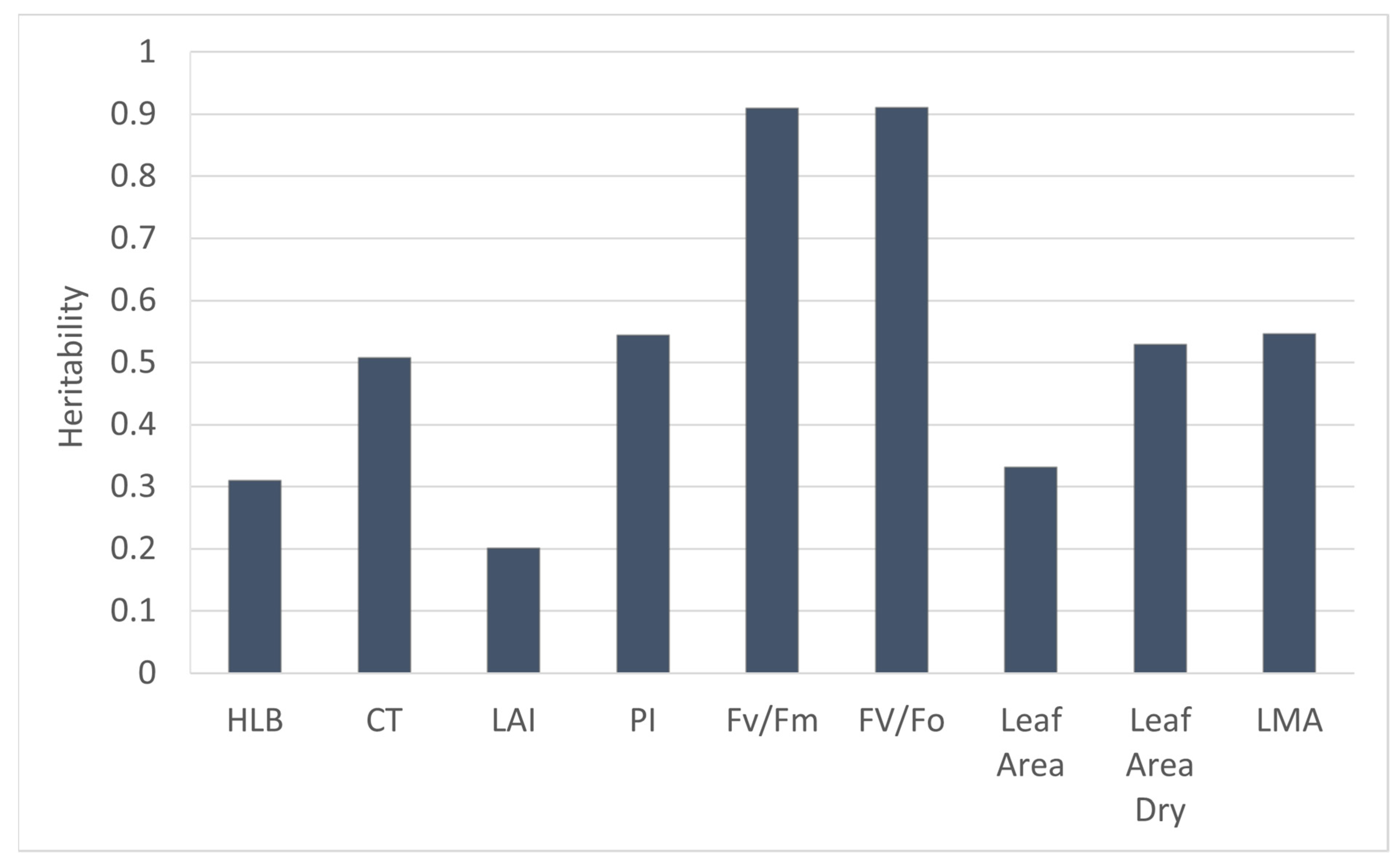
3.1. Genetic Effect on HLB-Tolerant Traits
3.2. HLB Score and Ct Value
3.3. Leaf Morphophysiological Traits
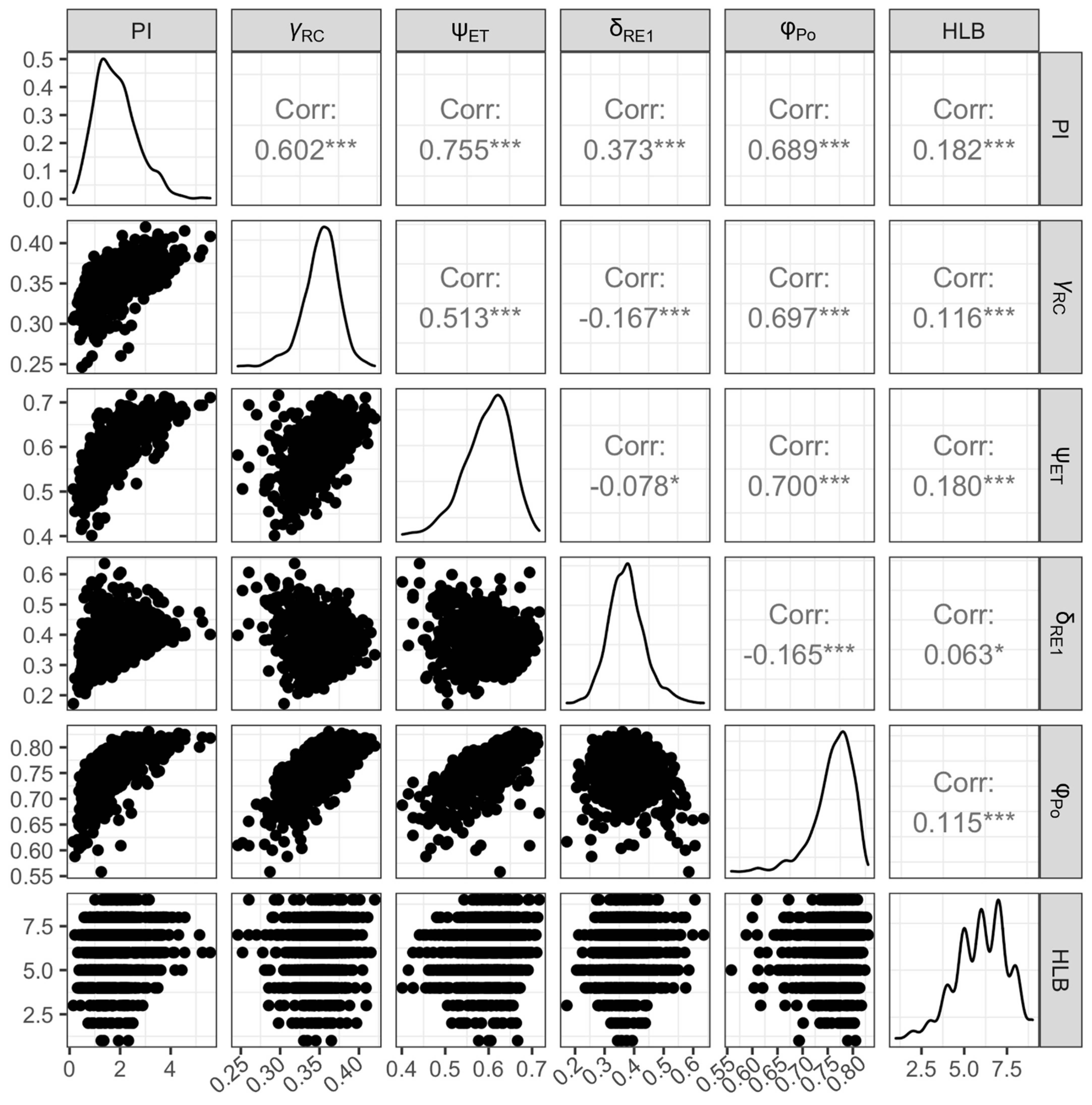
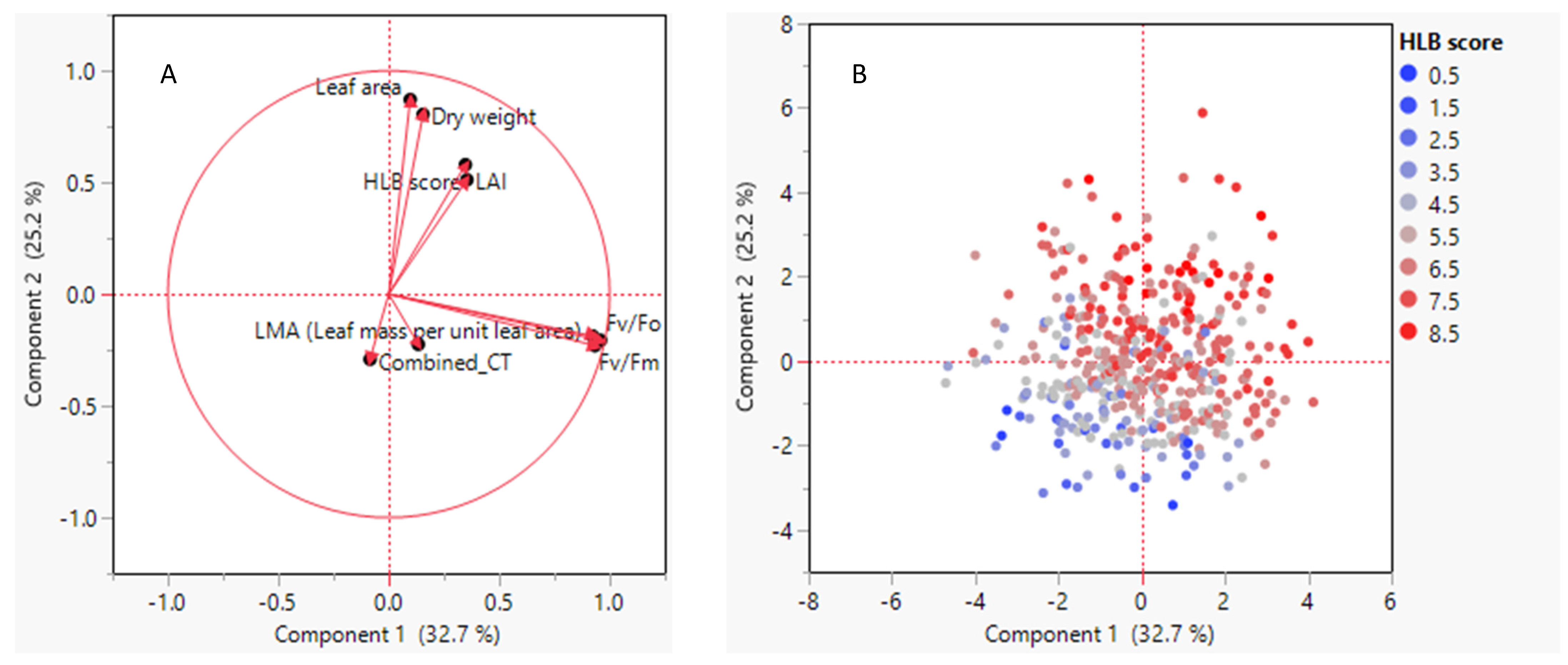
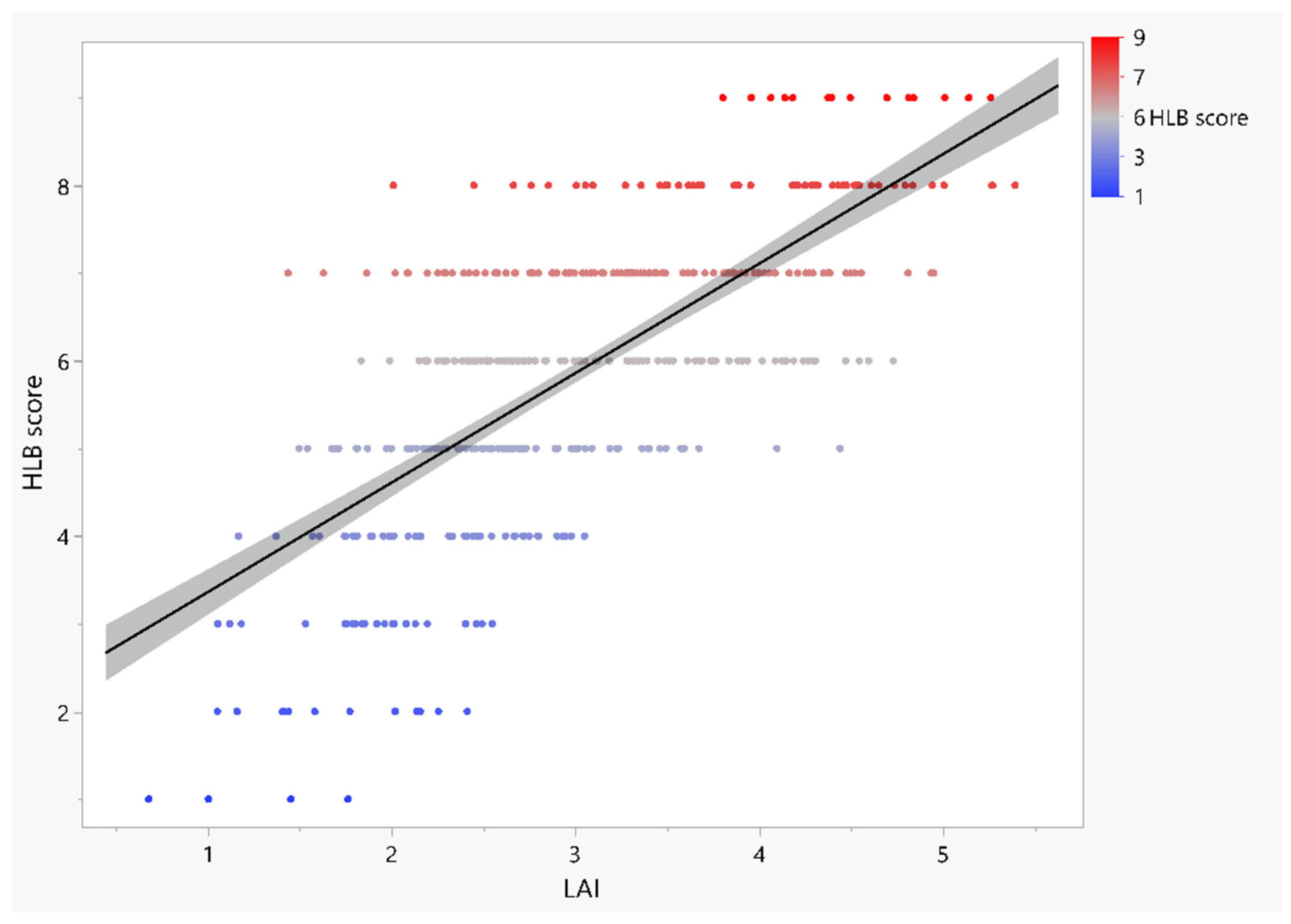
3.4. Relationships between HLB Score and Leaf Morpho-Physiological Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.; Hendrich, C.; Huang, Y.; et al. Citrus huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Stover, E.; Gmitter, F.G.; Grosser, J.; Baldwin, E.; Wu, G.A.; Bai, J.; Wang, Y.; Chaires, P.; Motamayor, J.C. Rationale for Reconsidering Current Regulations Restricting Use of Hybrids in Orange Juice. Hortic. Res. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Achor, D.; Exteberria, E.; Yu, Q.; Du, D.; Stanton, D.; Liang, G.; Gmitter, F.G., Jr. Phloem regeneration is a mechanism for Huanglongbing-tolerance of “Bearss” lemon and “LB8-9” Sugar belle® Mandarin. Front. Plant Sci. 2019, 10, 277. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the responses of different genotypes of citrus to Huanglongbing (Citrus greening) under different conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-term field evaluation reveals Huanglongbing resistance in citrus relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.P.; Stover, E.; Ramadugu, C.; Keremane, M.L.; Lee, R.F. Apparent tolerance to Huanglongbing in citrus and citrus-related germplasm. HortScience 2017, 52, 31–39. [Google Scholar] [CrossRef]
- Sagaram, M.; Burns, J.K. Leaf chlorophyll fluorescence parameters and Huanglongbing. J. Am. Soc. Hortic. Sci. 2009, 134, 194–201. [Google Scholar] [CrossRef]
- Weng, H.; Liu, Y.; Captoline, I.; Li, X.; Ye, D.; Wu, R. Citrus huanglongbing detection based on polyphasic chlorophyll a fluorescence coupled with machine learning and model transfer in two citrus cultivars. Comput. Electron. Agric. 2021, 187, 106289. [Google Scholar] [CrossRef]
- Wu, J.; Johnson, E.G.; Gerberich, K.M.; Bright, D.B.; Graham, J.H. Contrasting canopy and fibrous root damage on Swingle citrumelo caused by ‘Candidatus Liberibacter asiaticus’ and Phytophthora nicotianae. Plant Pathol. 2017, 67, 202–209. [Google Scholar] [CrossRef]
- Vincent, C.; Guha, A.; Killiny, N.; Diepenbrock, L. Understory environment promotes photosynthetic efficiency and mitigates severity and function of an introduced, vectored pathosystem: A study of a feral citrus population in Central Florida. Trees 2021, 35, 1711–1725. [Google Scholar] [CrossRef]
- Huang, M.; Roose, M.L.; Yu, Q.; Du, D.; Yu, Y.; Zhang, Y.; Deng, Z.; Stover, E.; Gmitter, F.G. Construction of high-density genetic maps and detection of QTLs associated with Huanglongbing tolerance in citrus. Front. Plant Sci. 2018, 9, 1694. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zhori, A.; Meco, M.; Brandl, H.; Bachofen, R. In situ chlorophyll fluorescence kinetics as a tool to quantify effects on photosynthesis in Euphorbia cyparissias by a parasitic infection of the rust fungus Uromyces pisi. BMC Res. Notes 2015, 8, 698. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Futch, D.B.; Shilts, T.; Folimonova, S.Y.; Reyes-De-Corcuera, J.I. GC–MS metabolomic differentiation of selected citrus varieties with different sensitivity to Citrus huanglongbing. Plant Physiol. Biochem. 2012, 53, 69–76. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, C.; Du, D.; Huang, M.; Yao, J.; Yu, F.; Brlansky, R.H.; Gmitter, F.G. Reprogramming of a defense signaling pathway in rough lemon and sweet orange is a critical element of the early response to ‘Candidatus Liberibacter asiaticus’. Hortic. Res. 2017, 4, 17063. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Yu, Q.; Brlansky, R.H.; Li, Z.-G.; Gmitter, F.G. Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by “Candidatus Liberibacter asiaticus”. Physiol. Plant. 2011, 143, 235–245. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Yu, Q.; Khalaf, A.; Achor, D.S.; Brlansky, R.H.; Moore, G.A.; Li, Z.-G.; Gmitter, F.G. Comparative transcriptional and anatomical analyses of tolerant rough lemon and susceptible sweet orange in response to ‘Candidatus Liberibacter asiaticus’ infection. Mol. Plant-Microbe Interact. 2012, 25, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Vashisth, T. Citrus Canopy Health Is Highly Important for HLB Tolerance. Citrus Industry. 2021. Available online: https://citrusindustry.net/2021/08/09/citrus-canopy-health-is-highly-important-for-hlb-tolerance/ (accessed on 1 December 2022).
- Bernardini, C.; Turner, D.; Wang, C.; Welker, S.; Achor, D.; Artiga, Y.A.; Turgeon, R.; Levy, A. Candidatus Liberibacter asiaticus reduces callose and reactive oxygen species production in the phloem. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ginnan, N.A.; Dang, T.; Bodaghi, S.; Ruegger, P.M.; McCollum, G.; England, G.; Vidalakis, G.; Borneman, J.; Rolshausen, P.E.; Roper, M.C. Disease-induced microbial shifts in citrus indicate microbiome-derived responses to Huanglongbing across the disease severity spectrum. Phytobiomes J. 2020, 4, 375–387. [Google Scholar] [CrossRef]
- Vasconcelos, F.N.; Li, J.; Pang, Z.; Vincent, C.; Wang, N. The total population size of ‘Candidatus Liberibacter asiaticus’ inside the phloem of citrus trees and the corresponding metabolic burden related to Huanglongbing disease development. Phytopathology 2021, 111, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on Huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2013, 63, 290–298. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Bassanezi, R.B.; Montesino, L.H.; Gasparoto, M.C.G.; Bergamin, F.A.; Amorim, L. Yield loss caused by Huanglongbing in different sweet orange cultivars in São Paulo, Brazil. Eur. J. Plant Pathol. 2011, 130, 577–586. [Google Scholar] [CrossRef]
- Tang, L.; Singh, S.; Vashisth, T. Association between fruit development and mature fruit drop in Huanglongbing-affected sweet orange. HortScience 2020, 55, 851–857. [Google Scholar] [CrossRef]
- Ampatzidis, Y.; Partel, V.; Meyering, B.; Albrecht, U. Citrus rootstock evaluation utilizing UAV-based remote sensing and artificial intelligence. Comput. Electron. Agric. 2019, 164, 104900. [Google Scholar] [CrossRef]
- Suh, J.W.; Anderson, E.; Ouimet, W.; Johnson, K.M.; Witharana, C. Mapping Relict Charcoal Hearths in New England Using Deep Convolutional Neural Networks and LiDAR Data. Remote Sens. 2021, 13, 4630. [Google Scholar] [CrossRef]
- Lüttge, U.; Escher, P.; Paluch, R.; Pfanz, H.; Wittmann, C.; Rennenberg, H.; Rakowski, K. Variability of photosynthetic capacity and water relations of Pinus sylvestris L. in the field. Biol. Plant. 2011, 55, 90–98. [Google Scholar] [CrossRef]
- Bown, H.E.; Mason, E.G.; Clinton, P.W.; Watt, M.S. Chlorophyll fluorescence response of Pinus radiata clones to nitrogen and phosphorus supply. Cienc. Investig. Agrar. 2009, 36, 451–464. [Google Scholar] [CrossRef]
- Nebauer, S.G.; Renau-Morata, B.; Guardiola, J.L.; Molina, R.-V. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.M.; Bundock, N.; Rolfe, S.A.; Scholes, J.D. Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol. Plant Pathol. 2000, 1, 99–113. [Google Scholar] [CrossRef]
- Brugger, A.; Kuska, M.T.; Mahlein, A.-K. Impact of compatible and incompatible barley—Blumeria graminis f.sp. hordei interactions on chlorophyll fluorescence parameters. J. Plant Dis. Prot. 2017, 125, 177–186. [Google Scholar] [CrossRef]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Défago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]


| Mandarin Cross | Number of Trees | HLB Visual Score | Ct Value |
|---|---|---|---|
| LB 8-9 × Seedless Kishu | 100 | 6 | 33 |
| Daisy × Seedless Kishu | 68 | 5 | 34 |
| B6R9T131 × Ortanique | 40 | 6 | 33 |
| LB-5-19 × DPI Minneola | 23 | 7 | 32 |
| Lee × Seedless Kishu | 20 | 5 | 37 |
| LB 7-11 × Seedless Kishu | 19 | 5 | 33 |
| LB9-24 × Orlando | 13 | 7 | 32 |
| LB-5-19 × Ortanique | 13 | 6 | 33 |
| Temple × Seedless Kishu | 13 | 6 | 31 |
| LB8-8 × Fortune | 12 | 6 | 34 |
| King × Seedless Kishu | 11 | 6 | 30 |
| LB-8-4 × Vermilian | 10 | 6 | 32 |
| LB 8-9 × Paperrind | 6 | 7 | 31 |
| LB 8-9 × Ortanique | 6 | 8 | 28 |
| LB 2-2 × Seedless Kishu | 5 | 5 | 27 |
| LB 8-9 × Orlando Tangelo | 5 | 6 | 31 |
| B3R13T51 × Seedless Kishu | 4 | 6 | 32 |
| DPI Fortune × Minneola | 4 | 4 | 28 |
| LB9-24 × US 119 | 4 | 5 | 30 |
| Lee × Fairchild | 4 | 6 | 36 |
| LB 8-8 × 9-11 USDA | 3 | 7 | 33 |
| LB 8-8 × Seedless Kishu | 3 | 6 | 30 |
| LB-7-1 × Vermilian Hong | 3 | 7 | 31 |
| Fallglo × Fairchild | 2 | 7 | 29 |
| LB 3-10 × USDA 9-11 | 2 | 7 | 30 |
| LB5-19 × Sunburst | 2 | 8 | 29 |
| LB-7-1 × Bendizao | 2 | 6 | 31 |
| Robinson × Paperrind | 2 | 7 | 29 |
| Robinson × US 119 | 2 | 6 | 33 |
| Temple × Orlando | 2 | 6 | 30 |
| Mandarin Cross | Number of Trees | HLB Visual Score | Ct Value |
|---|---|---|---|
| Delta DPI 118 | 1 | 7 | 31 |
| UF609 | 1 | 4 | 25 |
| UF950 | 1 | 8 | 24 |
| Anna (SRA) | 1 | 7 | 30 |
| Daisey | 1 | 6 | 29 |
| DPI King H4 × Marsh | 1 | 7 | 31 |
| Fallglo × Murcott | 1 | 5 | 29 |
| Fallglo/Cleo | 1 | 5 | 29 |
| Fortune × Halls | 1 | 7 | 28 |
| Fortune × Ortanique | 1 | 3 | 31 |
| Fortune × Paperrind | 1 | 8 | 35 |
| Fortune × Royal | 1 | 5 | 40 |
| Fortune × Valencia | 1 | 5 | 32 |
| Fortune/Carrizo | 1 | 6 | 40 |
| Fortune/Czo | 1 | 8 | 36 |
| Fujian Tankan | 1 | 7 | 31 |
| Kara (SRA) | 1 | 5 | 31 |
| Kawabata | 1 | 7 | 33 |
| Lakeland Limequat | 1 | 5 | 28 |
| LB 1-2 × Paperrind | 1 | 7 | 29 |
| LB-7-11 | 1 | 7 | 29 |
| LB 7-5 × Imperial | 1 | 8 | 26 |
| LB 8-8/Carrizo | 1 | 9 | 27 |
| LB 8-9/Swingle | 1 | 9 | 31 |
| LB 8-9 × Big Tangelo | 1 | 6 | 31 |
| LB 8-9 × Orlando | 1 | 9 | 29 |
| LB 9-11/Carrizo | 1 | 8 | 29 |
| Lee × Murcott | 1 | 6 | 37 |
| Lee/Swingle | 1 | 8 | 40 |
| Minneola Tangelo/Swi | 1 | 7 | 27 |
| Naartje Redskin (SRA) | 1 | 8 | 29 |
| Noir/Volklmer lemon | 1 | 7 | 29 |
| Ortanique/Cleo | 1 | 7 | 32 |
| Ota/Cleo | 1 | 6 | 30 |
| Ponkan | 1 | 6 | 30 |
| Robinson × Fairchild | 1 | 6 | 38 |
| Robinson × Paperrind | 1 | 8 | 31 |
| Satsuma × Minneola | 1 | 7 | 28 |
| Satsuma × Sunburst | 1 | 5 | 31 |
| Seedless Kishu | 1 | 6 | 30 |
| Shiranui/Flying Dragon | 1 | 3 | 27 |
| Sue Linda/Carrizo | 1 | 4 | 31 |
| Tamami | 1 | 6 | 32 |
| Tankan Tangor 38752/Swi | 1 | 8 | 25 |
| Temple/Cleo | 1 | 8 | 28 |
| HLB Score | Number of Trees | LAI | Leaf Area (cm2/Leaf) | Dry Weight (g) | PIabs | Fv/Fm | Fv/Fo | LMA | Ct Value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1.23 ± 0.24 d | 43.6 ± 3.5 b | 0.58 ± 0.07 ab | 2.85 ± 0.50 abc | 0.75 ± 0.03 a | 3.25 ± 0.41 a | 0.013 a | 34.15 a |
| 2 | 12 | 1.73 ± 0.13 d | 37.95 ± 4.20 b | 0.50 ± 0.05 ab | 2.73 ± 0.23 abc | 0.76 ± 0.01 a | 3.24 ± 0.12 a | 0.014 a | 33.37 a |
| 3 | 21 | 1.9 ± 0.09 d | 40.99 ± 3.60 b | 0.57 ± 0.05 ab | 2.59 ± 0.18 bc | 0.75 ± 0.01 a | 3.13 ± 0.12 a | 0.014 a | 32.96 a |
| 4 | 46 | 2.25 ± 0.07 d | 43.72 ± 2.18 b | 0.58 ± 0.03 b | 2.57 ± 0.14 c | 0.75 ± 0.01 a | 3.14 ± 0.08 a | 0.014 a | 32.52 a |
| 5 | 88 | 2.62 ± 0.06 c | 45.27 ± 1.87 b | 0.58 ± 0.02 b | 2.72 ± 0.09 abc | 0.76 ± 0.00 a | 3.23 ± 0.05 a | 0.013 a | 32.42 a |
| 6 | 104 | 3.1 ± 0.07 b | 48.5 ± 1.80 ab | 0.64 ± 0.02 ab | 2.93 ± 0.09 ab | 0.76 ± 0.00 a | 3.31 ± 0.04 a | 0.013 a | 32.36 a |
| 7 | 104 | 3.32 ± 0.07 b | 50.81 ± 1.86 ab | 0.67 ± 0.03 ab | 3.04 ± 0.10 a | 0.76 ± 0.00 a | 3.37 ± 0.05 a | 0.013 a | 32.01 a |
| 8 | 54 | 4.00 ± 0.10 a | 57.40 ± 3.40 a | 0.75 ± 0.05 a | 3.06 ± 0.12 a | 0.76 ± 0.00 a | 3.35 ± 0.06 a | 0.013 a | 31.71 a |
| 9 | 14 | 4.51 ± 0.12 a | 63.73 ± 4.64 a | 0.78 ± 0.05 a | 3.08 ± 0.17 abc | 0.76 ± 0.01 a | 3.29 ± 0.11 a | 0.012 a | 31.38 a |
| Trait | HLB Score | LAI | Leaf Area | Dry Weight | PIabs | Fv/Fm | Fv/Fo | Ct Value |
|---|---|---|---|---|---|---|---|---|
| Whole population | ||||||||
| LAI | 0.704 *** | |||||||
| Leaf area | 0.259 *** | 0.176 | ||||||
| Dry weight | 0.228 *** | 0.136 | 0.929 *** | |||||
| PIabs | 0.168 *** | 0.169 *** | −0.029 | 0.010 | ||||
| Fv/Fm | 0.103 * | 0.124 ** | −0.043 | 0.009 | 0.849 *** | |||
| Fv/Fo | 0.130 ** | 0.147 ** | −0.019 | 0.032 | 0.907 *** | 0.974 *** | ||
| CT | −0.079 | −0.132 ** | −0.158 *** | −0.157 *** | −0.019 | −0.023 | −0.013 | |
| LMA | −0.111 | −0.128 ** | −0.221 *** | 0.126 ** | 0.078 | 0.101 * | 0.101 * | 0.006 |
| Five seedless Kishu families | ||||||||
| HLB score | ||||||||
| LAI | 0.741 *** | |||||||
| Leaf area | 0.104 | 0.055 | ||||||
| Dry weight | 0.122 | 0.064 | 0.876 | |||||
| PIabs | 0.220 *** | 0.164 *** | −0.112 | −0.036 | ||||
| Fv/Fm | 0.228 *** | 0.216 *** | −0.129 | −0.056 | 0.893 *** | |||
| Fv/Fo | 0.226 *** | 0.203 *** | −0.097 | −0.021 | 0.917 *** | 0.989 *** | ||
| CT | 0.010 | −0.006 | −0.231 *** | 0.244 *** | 0.091 | 0.094 | 0.096 | |
| LMA | 0.053 | −0.024 | −0.008 | −0.008 | 0.006 | 0.021 | 0.025 | −0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Dai, F.; Russo, R.; Guha, A.; Pierre, M.; Zhuo, X.; Wang, Y.Z.; Vincent, C.; Gmitter, F.G., Jr. Phenotypic and Genetic Variation in Morphophysiological Traits in Huanglongbing-Affected Mandarin Hybrid Populations. Plants 2023, 12, 42. https://doi.org/10.3390/plants12010042
Yu Q, Dai F, Russo R, Guha A, Pierre M, Zhuo X, Wang YZ, Vincent C, Gmitter FG Jr. Phenotypic and Genetic Variation in Morphophysiological Traits in Huanglongbing-Affected Mandarin Hybrid Populations. Plants. 2023; 12(1):42. https://doi.org/10.3390/plants12010042
Chicago/Turabian StyleYu, Qibin, Fanwei Dai, Riccardo Russo, Anirban Guha, Myrtho Pierre, Xiaokang Zhuo, Yuanzhi Zimmy Wang, Christopher Vincent, and Frederick G. Gmitter, Jr. 2023. "Phenotypic and Genetic Variation in Morphophysiological Traits in Huanglongbing-Affected Mandarin Hybrid Populations" Plants 12, no. 1: 42. https://doi.org/10.3390/plants12010042
APA StyleYu, Q., Dai, F., Russo, R., Guha, A., Pierre, M., Zhuo, X., Wang, Y. Z., Vincent, C., & Gmitter, F. G., Jr. (2023). Phenotypic and Genetic Variation in Morphophysiological Traits in Huanglongbing-Affected Mandarin Hybrid Populations. Plants, 12(1), 42. https://doi.org/10.3390/plants12010042








