Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Common Bunt Inoculation
2.3. Experimental Design
2.4. Statistical Analysis of All the Studied Traits
3. Results
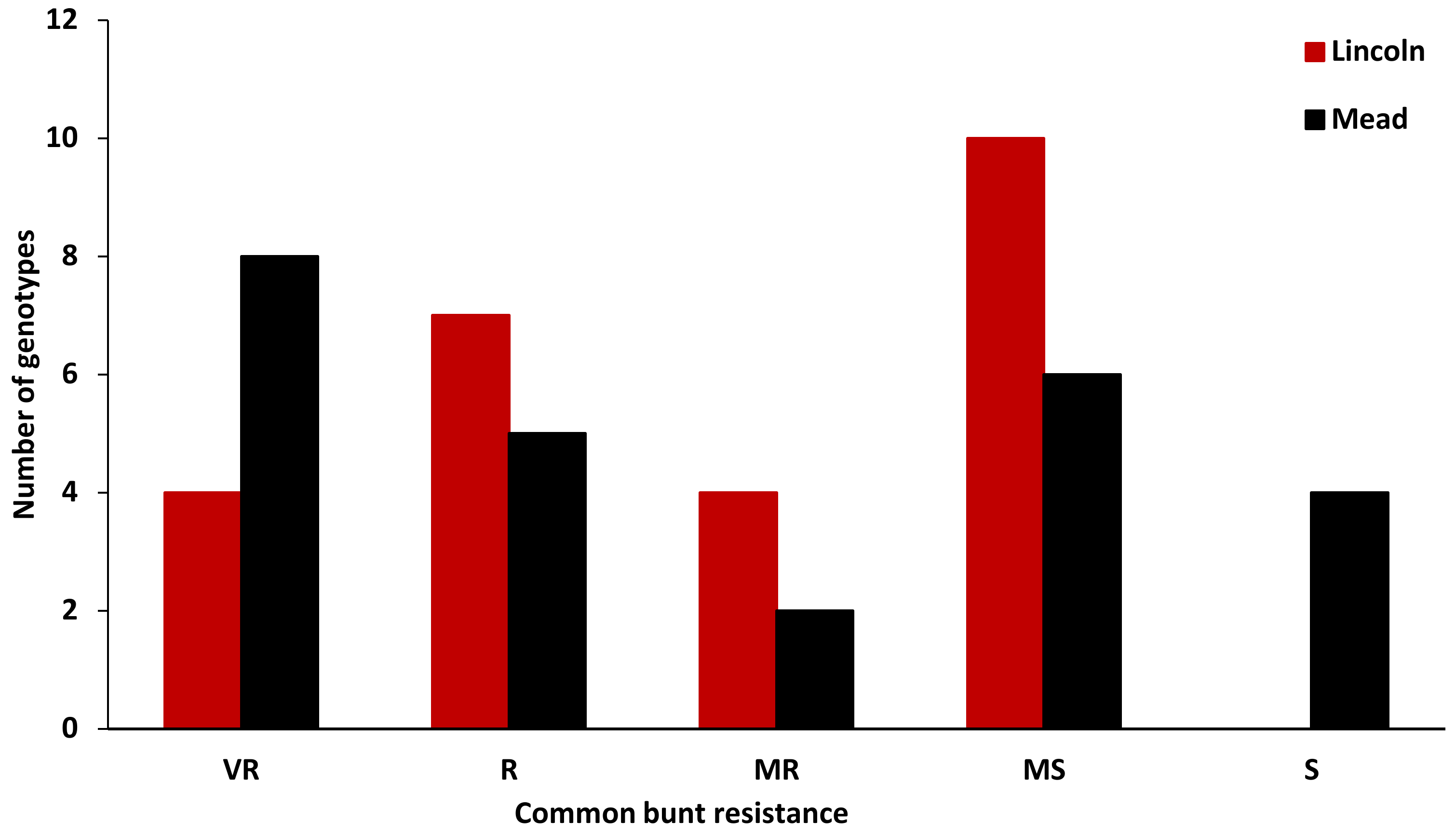
3.1. Evaluating the Differential Lines and Susceptible Checks
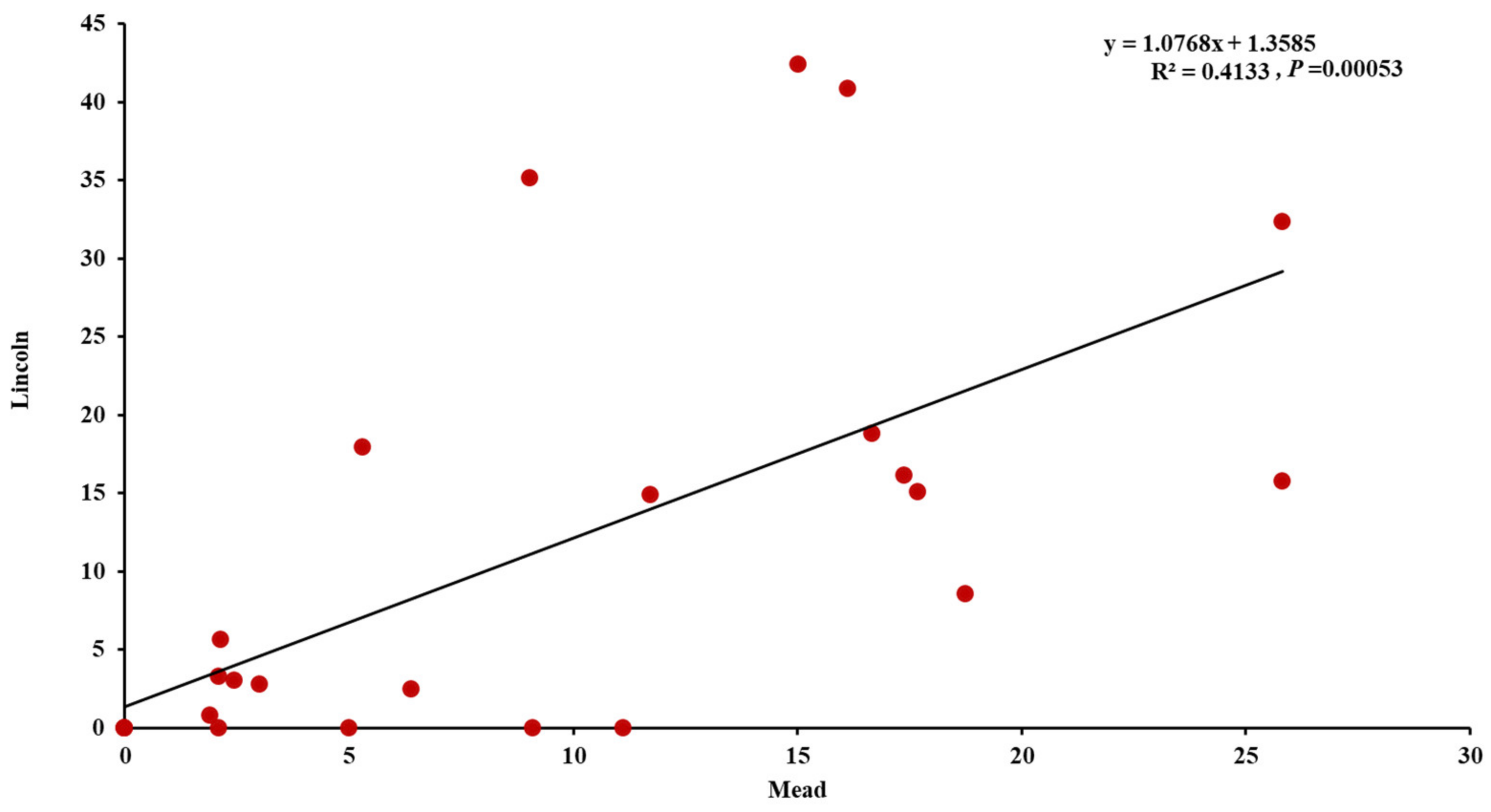
3.2. Evaluation of the Winter Synthetic Wheat Genotypes
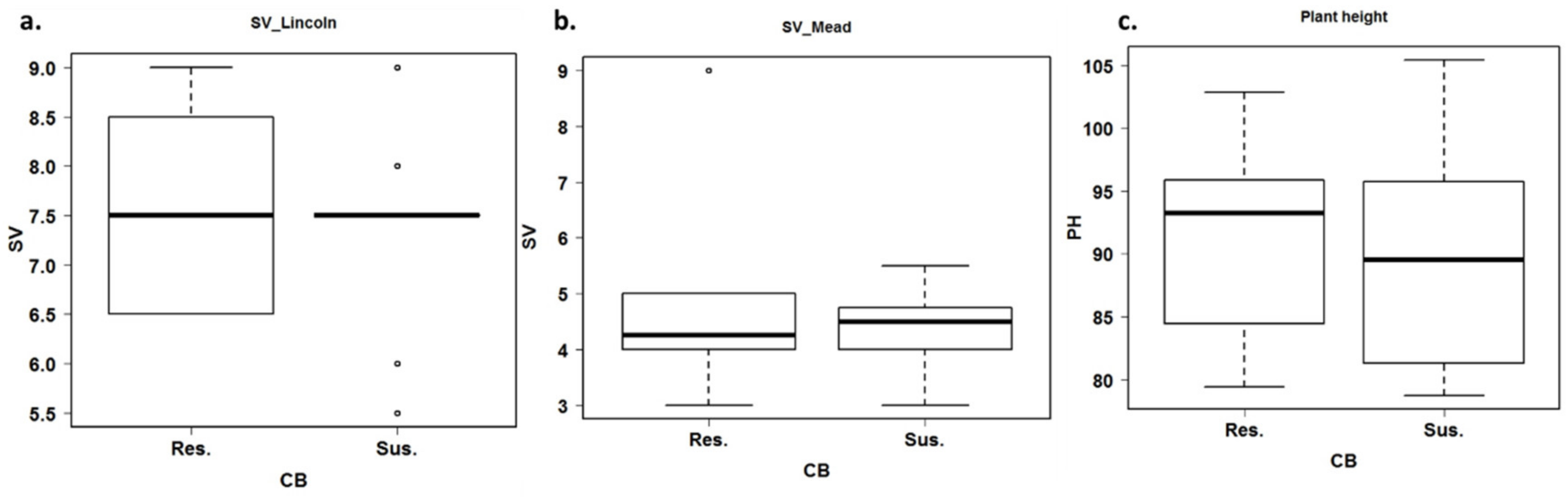
3.3. Estimation of Agronomic Traits under Common Bunt Infection
4. Discussion
4.1. Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat
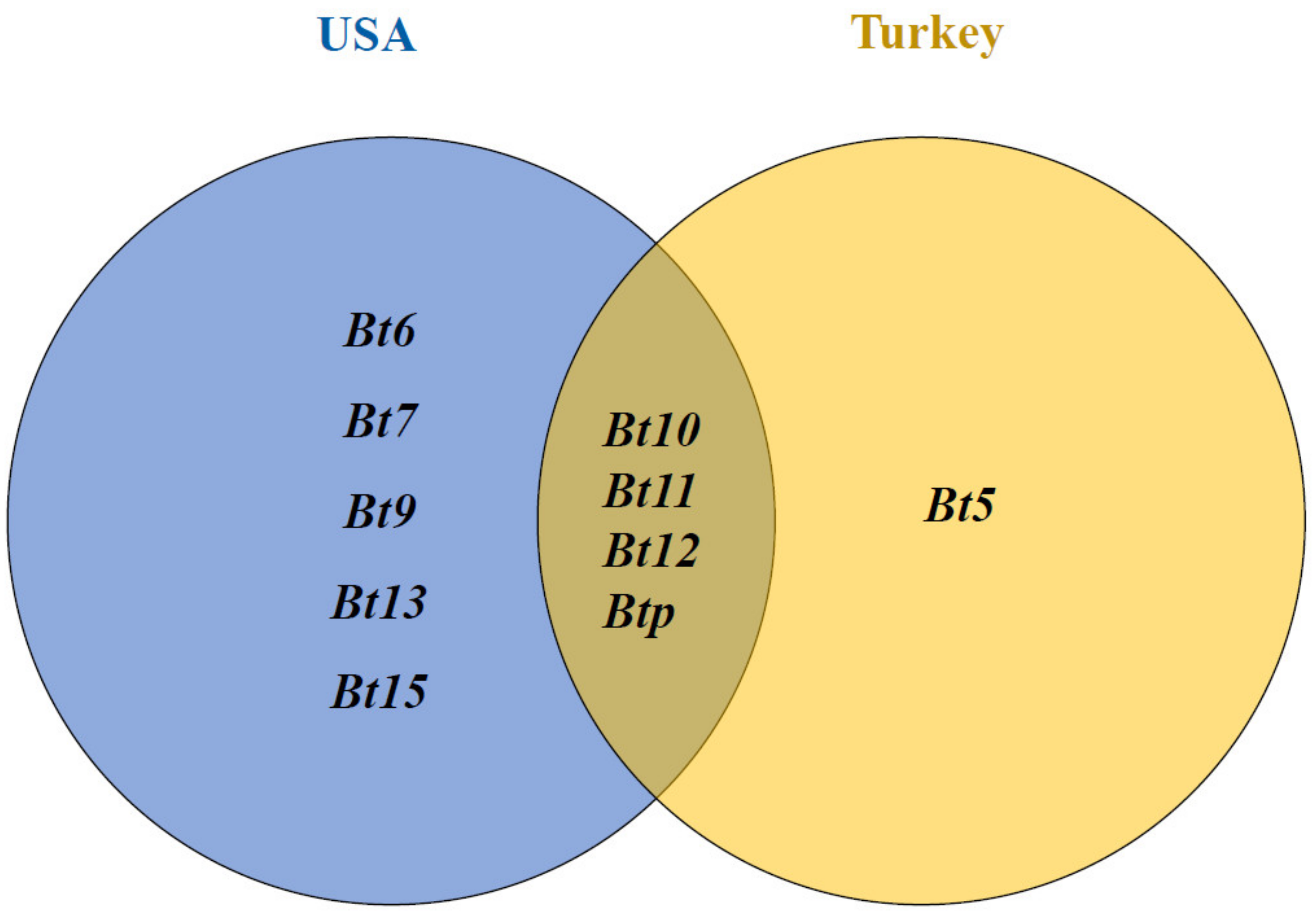
4.2. Expected Resistance Genes in the Studied Synthetic Wheat Genotypes
4.3. Genetic Variation in Some Agronomic Traits under Common Bunt Infection
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madenova, A.; Sapakhova, Z.; Bakirov, S.; Galymbek, K.; Yernazarova, G.; Kokhmetova, A.; Keishilov, Z. Screening of wheat genotypes for the presence of common bunt resistance genes. Saudi J. Biol. Sci. 2021, 28, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Goates, B.J. Bunt and Smut Diseases of Wheat; Wilcoxson, R.D., Saari, E.E., Ballantyne, B., Burnett, P.A., Nielsen, J., Saari, E.E., Eds.; CIMMYT: Veracruz, Mexico, 1996; ISBN 9686923373. [Google Scholar]
- Yorgancilar, A. Screening Turkish and IWWIP Germplasm (International Winter Wheat Improvement Program) for Common Bunt (Tilletia foetida (wallr.) Liro, Tilletia caries (D.C.) tul.) Resistance under Eskisehir Field Conditions. In Proceedings of the XIX International Workshop on Smuts and Bunts, Izmir, Turkey, 3–6 May 2016; pp. 54–55. [Google Scholar]
- Ciucǎ, M.; Sǎulescu, N.N. Screening Romanian winter wheat germplasm for presence of Bt10 bunt resistance gene, using molecular markers. Rom. Agric. Res. 2008, 25, 1–5. [Google Scholar]
- Bonman, J.M.; Bockelman, H.E.; Goates, B.J.; Obert, D.E.; McGuire, P.E.; Qualset, C.O.; Hijmans, R.J. Geographic distribution of common and dwarf bunt resistance in landraces of Triticum aestivum subsp. aestivum. Crop. Sci. 2006, 46, 1622–1629. [Google Scholar] [CrossRef]
- Dumalasová, V.; Bartoš, P. Wheat Screening for Resistance to Common Bunt and Dwarf Bunt. In Proceedings of the Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs 2, Gumpenstein, Austria, 25–26 November 2013; pp. 51–54. [Google Scholar]
- Liatukas, Ž.; Ruzgas, V. Genetic resources for organic wheat breeding: Impact on resistance to common bunt. Biologija 2005, 51, 62–64. [Google Scholar]
- Watkins, J.E.; Prentice, L.J.; Watkins, N.L. EC97-1874 Diseases Affecting Grain and Seed Quality in Wheat Diseases Affecting Grain and Seed Quality in Wheat. 1997. Available online: https://core.ac.uk/download/pdf/17223265.pdf (accessed on 15 March 2017).
- Wegulo, N.S. Loose Smut and Common Bunt of Wheat. Available online: http://www.ianrpubs.unl.edu/live/g1978/build/g1978.pdf (accessed on 20 April 2017).
- Wegulo, S. Common Bunt (Stinking Smut) in Wheat; University of Nebraska–Lincoln: Lincoln, NE, USA, 2015. [Google Scholar]
- Muellner, A.E.; Eshonkulov, B.; Hagenguth, J.; Pachler, B.; Michel, S.; Buerstmayr, M.; Hole, D.; Buerstmayr, H. Genetic mapping of the common and dwarf bunt resistance gene Bt12 descending from the wheat landrace PI119333. Euphytica 2020, 216, 83. [Google Scholar] [CrossRef]
- Muellner, A.E.; Buerstmayr, M.; Eshonkulov, B.; Hole, D.; Michel, S.; Hagenguth, J.F.; Pachler, B.; Pernold, R.; Buerstmayr, H. Comparative mapping and validation of multiple disease resistance QTL for simultaneously controlling common and dwarf bunt in bread wheat. Theor. Appl. Genet. 2020, 134, 489–503. [Google Scholar] [CrossRef]
- Jighly, A.; Alagu, M.; Makdis, F.; Singh, M.; Singh, S.; Emebiri, L.C.; Ogbonnaya, F.C. Genomic regions conferring resistance to multiple fungal pathogens in synthetic hexaploid wheat. Mol. Breed. 2016, 36, 127. [Google Scholar] [CrossRef]
- Rosyara, U.; Kishii, M.; Payne, T.; Sansaloni, C.P.; Singh, R.P.; Braun, H.-J.; Dreisigacker, S. Genetic Contribution of Synthetic Hexaploid Wheat to CIMMYT’s Spring Bread Wheat Breeding Germplasm. Sci. Rep. 2019, 9, 12355. [Google Scholar] [CrossRef]
- Morgounov, A.; Abugalieva, A.; Akan, K.; Akln, B.; Baenziger, S.; Bhatta, M.; Dababat, A.A.; Demir, L.; Dutbayev, Y.; El Bouhssini, M.; et al. High-yielding winter synthetic hexaploid wheats resistant to multiple diseases and pests. Plant Genet. Resour. Charact. Util. 2017, 16, 273–278. [Google Scholar] [CrossRef]
- Goates, B.J. Identification of New Pathogenic Races of Common Bunt and Dwarf Bunt Fungi, and Evaluation of Known Races Using an Expanded Set of Differential Wheat Lines. Plant Dis. 2012, 96, 361–369. [Google Scholar] [CrossRef][Green Version]
- Goates, B.J.; Bockelman, H.E. Identification of new sources of high levels of resistance to dwarf bunt and common bunt among winter wheat landraces in the USDA-ARS national small grains collection. Crop. Sci. 2012, 52, 2595–2605. [Google Scholar] [CrossRef]
- Schmidt, J.; Morris, R.; Johnson, V. Monosomic analysis for bunt resistance in derivatives of Turkey and Oro wheats. Crop. Sci. 1969, 9, 286–288. [Google Scholar] [CrossRef]
- Dumalasová, V.; Simmonds, J.; Bartoš, P.; Snape, J. Location of genes for common bunt resistance in the European winter wheat cv. Trintella. Euphytica 2012, 186, 257–264. [Google Scholar] [CrossRef]
- Sears, E.R.; Schaller, C.W.; Briggs, F.N. Identification of the chromosome carrying the martin gene for resistance of wheat to bunt. Can. J. Genet. Cytol. 1960, 2, 262–267. [Google Scholar] [CrossRef]
- Chen, J.; Guttieri, M.J.; Zhang, J.; Hole, D.; Souza, E.; Goates, B. A novel QTL associated with dwarf bunt resistance in Idaho 444 winter wheat. Theor. Appl. Genet. 2016, 129, 2313–2322. [Google Scholar] [CrossRef]
- Ciucǎ, M. A preliminary report on the identification of SSR markers for bunt (Tilletia sp.) resistance in wheat. Czech J. Genet. Plant Breed. 2011, 47, 142–145. [Google Scholar] [CrossRef]
- Knox, R.E.; Campbell, H.L.; Depauw, R.M.; Gaudet, D.; Puchalski, B.; Clarke, F.C. DNA markers for resistance to common bunt in “McKenzie” wheat. Can. J. Plant Pathol. 2013, 35, 328–337. [Google Scholar] [CrossRef]
- Lara, E.P. Mapping of Genomic Regions Associated with Agronomic Traits and Resistance to Diseases in Canadian Spring Wheat; University of Alberta: Edmonton, AB, Canada, 2017. [Google Scholar]
- Steffan, P.M.; Torp, A.M.; Borgen, A.; Backes, G.; Rasmussen, S.K. Mapping of common bunt resistance gene Bt9 in wheat. Theor. Appl. Genet. 2017, 130, 1031–1040. [Google Scholar] [CrossRef]
- Singh, A.; Knox, R.E.; DePauw, R.M.; Singh, A.K.; Cuthbert, R.D.; Kumar, S.; Campbell, H.L. Genetic mapping of common bunt resistance and plant height QTL in wheat. Theor. Appl. Genet. 2016, 129, 243–256. [Google Scholar] [CrossRef]
- Mourad, A.M.I.; Sallam, A.; Belamkar, V.; Mahdy, E.; Bakheit, B.; El-wafaa, A.A.; Baenziger, P.S. Genetic architecture of common bunt resistance in winter wheat using genome- wide association study. BMC Plant Biol. 2018, 18, 280. [Google Scholar] [CrossRef]
- Steffan, P.; Backes, G.; Rasmussen, S.K.; Borgen, A. Genome Wide Association Study for Common Bunt Resistance in Wheat and Creation of Common Bunt Resistant Composite Cross Populations. In Proceedings of the XVIII Biennial International Workshop on the Smuts and Bunts Copenhagen, Tune Kursuscenter, Copenhagen, Denmark, 3–5 February 2014; pp. 2–3. [Google Scholar]
- Netto, A.T.; Campostrini, E.; De Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Veisz, O.; Szunics, L.; Szunics, L. Effect of common bunt on the frost resistance and winter hardiness of wheat (Triticum aestivum L.) lines containing Bt genes. Euphytica 2000, 114, 159–164. [Google Scholar] [CrossRef]
- Rife, T.W.; Poland, J.A. Field book: An open-source application for field data collection on android. Crop. Sci. 2014, 54, 1624–1627. [Google Scholar] [CrossRef]
- Utz, H. Computer Program for Statistical Analysis for Plant Breeding Experiments; Version 2N; Institute of Plant Breeding, Seed Science and Population Genetics, University of Hohenheim: Stuttgart, Germany, 1997. [Google Scholar]
- Wilcoxson, R.D.; Saari, E.E. Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management; Hettel, G., McNab, A., Mexico, D.F., Eds.; CIMMYT: Veracruz, Mexico, 1996; ISBN 9686923373. [Google Scholar]
- Mathre, D.E. Stinking Smut of Wheat. Available online: http://www.apsnet.org/edcenter/intropp/lessons/fungi/Basidiomycetes/Pages/StinkingSmut.aspx (accessed on 23 January 2017).
- De Wolf, E. Common Bunt of Wheat; Kansas State University: Manhattan, KS, USA, 2010. [Google Scholar]
- Mourad, A.M.I.; Mahdy, E.; Bakheit, B.R.; Abo-elwafaa, A.; Baenziger, P.S. Effect of common bunt infection on agronomic traits in wheat (Triticum aestivum L.). J. Plant Genet. Breed. 2018, 2, 1–7. [Google Scholar]
- Huber, K.; Bürstmayr, H. Development of methods for bunt resistance breeding for organic farming. Czech J. Genet. Plant Breed. 2012, 42, 66–71. [Google Scholar] [CrossRef]
- Andrews, J.A. The bread wheat races of bunt represented in the Australian bunt collection. Euphytica 1987, 36, 577–580. [Google Scholar] [CrossRef]
- Darchau, N.; Waldow, F.; Heyden, B.; Furth, U.; Weng, W.; Miedaner, T.; Stephan, D. Charakterisierung der Resistenz von Winterweizensorten und -zuchtlinien gegenüber Steinbrand (Tilletia tritici) und Zwergsteinbrand (T. controversa). Nachr. Des Dtsch. Pflanzenschutzd. 2007, 59, 30–39. [Google Scholar]
- Liatukas, Ž.; Ruzgas, V. Resistance genes and sources for the control of wheat common bunt (Tilletia tritici (DC.) Tul.). Biologija 2008, 54, 274–278. [Google Scholar] [CrossRef]
- Ganeva, G.; Landjeva, S.; Belchev, I.; Koleva, L. Characterization of two wheat doubled haploid populations for resistance to common bunt and its association with agronomic traits. Cereal Res. Commun. 2014, 42, 484–494. [Google Scholar] [CrossRef]
- Ehn, M.; Michel, S.; Morales, L.; Gordon, T.; Dallinger, H.; Buerstmay, H. Genome-wide association mapping identifies common bunt (Tilletia caries) resistance loci in bread wheat (Triticum aestivum) accessions of the USDA National Small Grains Collection. Theor. Appl. Genet. 2022, 135, 3103–3115. [Google Scholar] [CrossRef]
| Wheat Lines | Resistance Gene | CI or PI Number | % of Infected Heads with Common Bunt |
|---|---|---|---|
| Red Bobs | None | CI 6255 | 73.40 |
| Heines VII | None | PI 209794 | 14.40 |
| Sel 2092 | Bt1 | PI 554101 | 10.90 |
| Sel 1102 | Bt2 | PI 554097 | 16.67 |
| Ridit | Bt3 | CI 6703 | 10.00 |
| Rio | Bt6 | CI 10061 | 0.00 |
| Sel 50077 | Bt7 | PI 554100 | 3.80 |
| PI 173438/Elgin | Bt8 | PI 554120 | 12.50 |
| Elgin/PI 178383 | Bt9 | PI 554099 | 0.00 |
| Elgin/PI 178383 | Bt10 | PI 554118 | 1.20 |
| Elgin/PI 166910 | Bt11 | PI 554119 | 0.00 |
| PI 119333 | Bt12 | PI 119333 | 0.00 |
| Thule III | Bt13 | PI 181463 | 0.00 |
| Doubbi | Bt14 | CI 13711 | 33.3 |
| Carleton | Bt15 | CI 12064 | 0.00 |
| PI 173437 | Btp | PI 173437 | 0.00 |
| Genotype Number | Variety | Cross ID |
|---|---|---|
| Checks | ||
| Gen. 1 | KARAHAN | |
| Gen. 2 | GEREK | |
| Synthetics genotypes | ||
| Gen. 3 and 4 | AISBERG/AE.SQUARROSA(369) | C04GH3 |
| Gen. 5 and 6 | AISBERG/AE.SQUARROSA(511) | C04GH5 |
| Gen. 7 | UKR-OD 1530.94/AE.SQUARROSA(310) | C04GH68 |
| Gen. 8 and 9 | UKR-OD952.92/AE.SQUARROSA(1031) | C04GH61 |
| Gen. 10 and 11 | UKR-OD 1530.94/AE.SQUARROSA(458) | C04GH74 |
| CYMMIT winter synthetics × modern varieties | ||
| Gen. 12 and 13 | AISBERG/AE.SQUARROSA(369)//DEMIR | TCI091254 |
| Gen. 14 and 15 | LEUC 84693/AE.SQUARROSA(310)//ADYR | TCI091259 |
| Gen. 16 | LEUC 84693/AE.SQUARROSA(1026)//GEREK79 | |
| Gen. 17 and 18 | UKR-OD 952.92/AE.SQUARROSA(409)//SONMEZ | TCI091266 |
| Gen. 19 and 20 | UKR-OD 1530.94/AE.SQUARROSA(446)//KATIA1 | TCI091274 |
| Gen. 21, 22 and 23 | UKR-OD 1530.94/AE.SQUARROSA(311)//EKIZ | |
| Gen. 24 and 25 | UKR-OD 1530.94/AE.SQUARROSA(312)//BAGCI2002 | TCI091272 |
| Source of Variance | Common Bunt Resistance | Seedling Vigor | Plant Height | |||
|---|---|---|---|---|---|---|
| d.f. | Mean Squares | d.f | Mean Squares | d.f | Mean Squares | |
| Location (L) Replicate (R) Genotypes (G) G×L Error | 1 1 24 24 40 | 109.80 141.23 394.87 ** 110.76 * 54.78 | 1 1 24 24 44 | 220.09 ** 3.16 2.76 ** 1.71 * 0.87 | 1 1 24 24 47 | 18.65 728.33 ** 280.17 ** 34.24 101.08 |
| Broad-sense Heritability | 0.86 | 0.64 | 0.69 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourad, A.M.I.; Morgounov, A.; Baenziger, P.S.; Esmail, S.M. Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat. Plants 2023, 12, 2. https://doi.org/10.3390/plants12010002
Mourad AMI, Morgounov A, Baenziger PS, Esmail SM. Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat. Plants. 2023; 12(1):2. https://doi.org/10.3390/plants12010002
Chicago/Turabian StyleMourad, Amira M. I., Alexey Morgounov, P. Stephen Baenziger, and Samar M. Esmail. 2023. "Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat" Plants 12, no. 1: 2. https://doi.org/10.3390/plants12010002
APA StyleMourad, A. M. I., Morgounov, A., Baenziger, P. S., & Esmail, S. M. (2023). Genetic Variation in Common Bunt Resistance in Synthetic Hexaploid Wheat. Plants, 12(1), 2. https://doi.org/10.3390/plants12010002






