Chemical Profiling of Hedyosmum cumbalense and Hedyosmum spectabile (Chloranthaceae) Essential Oils, and Their Antimicrobial, Antioxidant, and Anticholinesterase Properties
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of EO from Leaves of H. cumbalense
2.2. Chemical Composition of EO from Leaves of H. spectabile
2.3. Enantioselective GC Analysis of the Essential Oils
2.4. Antimicrobial Activity of H. cumbalense and H. spectabile
2.5. Antioxidant Capacity
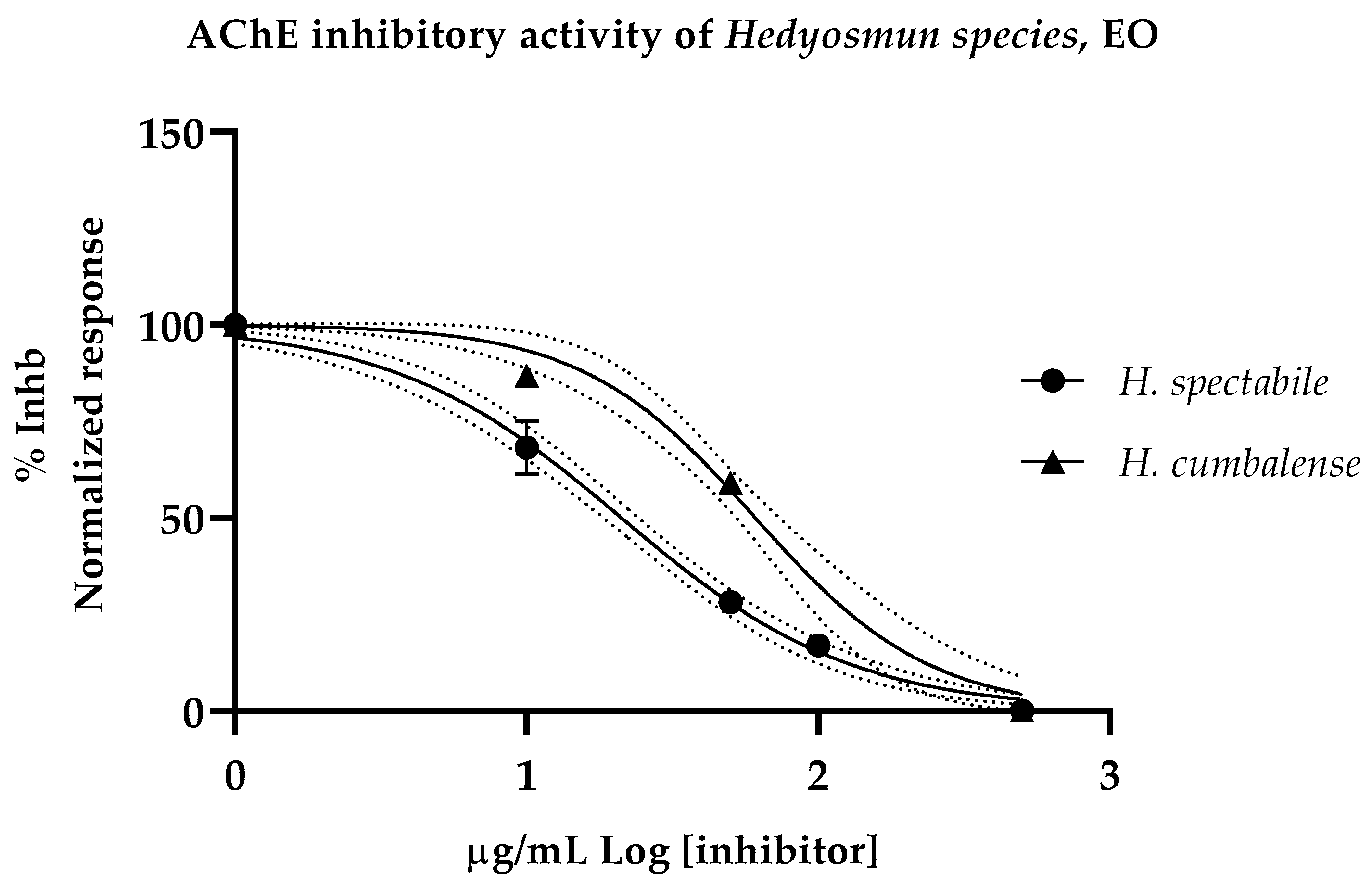
2.6. Anticholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Postharvest Treatments
4.3. Essential Oil Extraction
4.4. Identification of the Composition of the Essential Oils
4.4.1. Qualitative Analysis
4.4.2. Quantitative Analysis
4.4.3. Enantioselective Analysis
4.5. Antimicrobial Activity
4.6. Antioxidant Capacity
4.6.1. The 2,2-Diphenyl-1-picril hydrazyl (DPPH) Radical Scavenging Assay
4.6.2. The 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Assay
4.7. Anticholinesterase Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gentry, A. Field Guide to the Families and Genera of Woody Plants of Northwest South America (Colombia, Ecuador, Peru), with Supplementary Notes on Herbaceous Taxa, 1st ed.; The University of Chicago Press: Chicago, IL, USA, 1993; Volume 1, 330–331p. [Google Scholar]
- De Matos, L.P.; Giulietti, A.M.; De Oliveira, R.P. Flora Da Bahia: Chloranthaceae. Sitientibus Sér. Ci. Biol. 2016, 16, 858. [Google Scholar] [CrossRef]
- Plants of the World Online. Homepage. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:7058-1#source-KB (accessed on 9 November 2022).
- Palacios, W. Árboles del Ecuador: Familias y Géneros, 1st ed.; Editorial Universidad Técnica del Norte UTN: Ibarra, Ecuador, 2016; Volume 1. [Google Scholar]
- Lírio, E.J.D.; Freitas, J.; Alves-Araujo, A. Flora of Espírito Santo: Chloranthaceae. Rodriguésia 2022, 73, e00402021. [Google Scholar] [CrossRef]
- Verdcourt, B. Chloranthaceae. Flora Males. 1984, 10, 123–144. [Google Scholar]
- Radice, M.; Tasambay, A.; Pérez, A.; Diéguez-Santana, K.; Sacchetti, G.; Buso, P.; Buzzi, R.; Vertuani, S.; Manfredini, S.; Baldisserotto, A. Ethnopharmacology, Phytochemistry and Pharmacology of the Genus Hedyosmum (Chloranthaceae): A Review. J. Ethnopharmacol. 2019, 244, 111932. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, D.; Fan, G.; Wang, R.; Lu, X.; Gu, Y.; Shi, Q.-W. Constituents from Chloranthaceae Plants and Their Biological Activities. Heterocycl. Commun. 2016, 22, 175–220. [Google Scholar] [CrossRef]
- Todzia, C.A. Four New Species of Hedyosmum (Chloranthaceae) from South America. Syst. Bot. 1988, 13, 21. [Google Scholar] [CrossRef]
- Marín, C.; Parra, S. Bitácora de Flora. Guía Visual de Plantas de Páramos de Colombia., 1st ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogota, Colombia, 2015; ISBN 9789588889344. [Google Scholar]
- Torre, L.D.L. Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Denmark, 2008; ISBN 9789978771358. [Google Scholar]
- Kirchner, K.; Wisniewski Jr, A.; Cruz, A.B.; Biavatti, M.W.; Netz, D.J.A. Chemical Composition and Antimicrobial Activity of Hedyosmum brasiliense Miq., Chloranthaceae, Essential Oil. Rev. Bras. Farmacogn. 2010, 20, 692–699. [Google Scholar] [CrossRef]
- Lorenzo, D.; Loayza, I.; Dellacassa, E. Composition of the Essential Oils from Leaves of TwoHedyosmum spp. from Bolivia. Flavour Fragr. J. 2003, 18, 32–35. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Holl, D. Antimicrobial Natural Product Research: A Review from a South African Perspective for the Years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- Murakami, C.; Cordeiro, I.; Scotti, M.T.; Moreno, P.R.H.; Young, M.C.M. Chemical Composition, Antifungal and Antioxidant Activities of Hedyosmum Brasiliense Mart. Ex Miq. (Chloranthaceae) Essential Oils. Medicines 2017, 4, 55. [Google Scholar] [CrossRef]
- Valarezo, E.; Morocho, V.; Cartuche, L.; Chamba-Granda, F.; Correa-Conza, M.; Jaramillo-Fierro, X.; Meneses, M.A. Variability of the Chemical Composition and Bioactivity between the Essential Oils Isolated from Male and Female Specimens of Hedyosmum racemosum (Ruiz & Pav.) G. Don. Molecules 2021, 26, 4613. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Grandini, A.; Spagnoletti, A.; Asanza, M.; Scalvenzi, L. Cytotoxic Effect and TLC Bioautography-Guided Approach to Detect Health Properties of Amazonian Hedyosmum sprucei Essential Oil. Evid. Based Complement. Altern. Med. 2016, 2016, 1638342. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.B.L.; Martins, R.L.; Rabelo, É.D.M.; Matos, J.L.D.; Santos, L.L.; Brandão, L.B.; Chaves, R.D.S.B.; Costa, A.L.P.D.; Faustino, C.G.; Sá, D.M.D.C.; et al. In Silico and in Vivo Study of Adulticidal Activity from Ayapana Triplinervis Essential Oils Nano-Emulsion against Aedes Aegypti. Arab. J. Chem. 2022, 15, 104033. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Calva, J.; Valarezo, E.; Chuchuca, N.; Morocho, V. Chemical and Biological Activity Profiling of Hedyosmum strigosum Todzia Essential Oil, an Aromatic Native Shrub from Southern Ecuador. Plants 2022, 11, 2832. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Valarezo, E.; MeriNo, G.; Cruz-Erazo, C.; Cartuche, L. Study of the Essential Oil from Native Amazonian Species of Ecuador Piper Lineatum, Presence of Apiole and Safrole. Nat. Volatiles Essent. Oils 2020, 7, 14–25. [Google Scholar] [CrossRef]
- Salinas, M.; Calva, J.; Cartuche, L.; Valarezo, E.; Armijos, C. Chemical Composition, Enantiomeric Distribution and Anticholinesterase and Antioxidant Activity of the Essential Oil of Diplosthephium Juniperinum. Plants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Andrade, J.M.; Pachar, P.; Trujillo, L.; Cartuche, L. Suillin: A Mixed-Type Acetylcholinesterase Inhibitor from Suillus Luteus Which Is Used by Saraguros Indigenous, Southern Ecuador. PLoS ONE 2022, 17, e0268292. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for Acetylcholinesterase Inhibitors from Amaryllidaceae Using Silica Gel Thin-Layer Chromatography in Combination with Bioactivity Staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef] [PubMed]
| Nº | Compounds | LRIa | LRIb | % | CF |
|---|---|---|---|---|---|
| 1 | α-Thujene | 920 | 924 | 0.32 ± 0.08 | C10H16 |
| 2 | α-Pinene | 927 | 932 | 7.91 ± 2.10 | C10H16 |
| 3 | Camphene | 944 | 946 | 3.01 ± 0.72 | C10H16 |
| 4 | Sabinene | 968 | 969 | 14.37 ± 1.64 | C10H16 |
| 5 | β-Pinene | 973 | 974 | 7.41 ± 1.46 | C10H16 |
| 6 | Myrcene | 985 | 988 | 0.56 ± 0.07 | C10H16 |
| 7 | α-Phellandrene | 1004 | 1002 | 0.52 ± 0.04 | C10H16 |
| 8 | α-Terpinene | 1014 | 1014 | 0.65 ± 0.06 | C10H16 |
| 9 | ρ-Cymene | 1023 | 1020 | 0.93 ± 0.02 | C10H14 |
| 10 | Sylvestrene | 1026 | 1025 | 2.07 ± 0.17 | C10H16 |
| 11 | β-Phellandrene | 1028 | 1025 | 0.18 ± 0.01 | C10H16 |
| 12 | 1,8-Cineole | 1029 | 1026 | 1.89 ± 0.48 | C10H18O |
| 13 | (Z)-β-Ocimene | 1032 | 1032 | 1.30 ± 0.08 | C10H16 |
| 14 | (E)-β-Ocimene | 1042 | 1044 | 0.64 ± 0.05 | C10H16 |
| 15 | γ-Terpinene | 1054 | 1054 | 1.23 ± 0.12 | C10H16 |
| 16 | cis-Sabinene hydrate (IPP vs. OH) | 1069 | 1065 | 0.59 ± 0.15 | C10H18O |
| 17 | Terpinolene | 1082 | 1086 | 0.37 ± 0.04 | C10H16 |
| 18 | 6-Camphenone | 1091 | 1095 | 0.91 ± 0.17 | C10H14O |
| 19 | Linalool | 1101 | 1095 | 4.94 ± 0.93 | C10H18O |
| 20 | trans-Sabinene hydrate (IPP vs. OH) | 1103 | 1098 | 0.31 ± 0.11 | C10H18O |
| 21 | Chrysanthenone | 1124 | 1124 | 0.22 ± 0.05 | C10H14O |
| 22 | cis-ρ-Menth-2-en-1-ol | 1125 | 1118 | 0.32 ± 0.11 | C10H18O |
| 23 | α-Campholenal | 1128 | 1122 | 0.42 ± 0.05 | C10H16O |
| 24 | trans-Pinocarveol | 1141 | 1135 | 0.30 ± 0.05 | C10H16O |
| 25 | trans-Verbenol | 1147 | 1140 | 1.18 ± 0.31 | C10H16O |
| 26 | Camphor | 1149 | 1141 | 1.17 ± 0.39 | C10H16O |
| 27 | Citronellal | 1153 | 1148 | 3.51 ± 0.37 | C10H18O |
| 28 | Eucarvone | 1157 | 1146 | 0.17 ± 0.04 | C10H14O |
| 29 | cis-Chrysanthenol | 1160 | 1160 | 0.43 ± 0.11 | C10H16O |
| 30 | Pinocarvone | 1164 | 1160 | 1.28 ± 0.32 | C10H14O |
| 31 | Borneol | 1173 | 1165 | 0.93 ± 0.26 | C10H18O |
| 32 | cis-Pinocamphone | 1178 | 1172 | 0.56 ± 0.20 | C10H16O |
| 33 | Terpinen-4-ol | 1181 | 1174 | 2.43 ± 0.86 | C10H18O |
| 34 | cis-Dihydro carvone | 1186 | 1191 | 0.33 ± 0.07 | C10H16O |
| 35 | neo-Dihydro carveol | 1198 | 1193 | 1.50 ± 0.38 | C10H18O |
| 36 | trans-Piperitol | 1212 | 1207 | 0.32 ± 0.17 | C10H18O |
| 37 | Thymol, methyl ether | 1231 | 1232 | 4.75 ± 0.66 | C11H16O |
| 38 | Neral | 1243 | 1235 | 0.99 ± 0.20 | C10H16O |
| 39 | Geraniol | 1255 | 1249 | 0.23 ± 0.07 | C10H18O |
| 40 | Geranial | 1273 | 1264 | 1.75 ± 0.24 | C10H16O |
| 41 | Isobornyl acetate | 1283 | 1283 | 9.12 ± 1.11 | C12H20O2 |
| 42 | ρ-Cymen-7-ol | 1297 | 1289 | 0.15 ± 0.09 | C10H14O |
| 43 | α-Terpinyl acetate | 1346 | 1346 | 0.67 ± 0.09 | C12H20O2 |
| 44 | Citronellyl acetate | 1350 | 1350 | 0.68 ± 0.08 | C12H22O2 |
| 45 | Eugenol | 1357 | 1356 | 2.55 ± 0.99 | C10H12O2 |
| 46 | Neryl acetate | 1359 | 1359 | 0.16 ± 0.04 | C12H20O2 |
| 47 | Geranyl acetate | 1379 | 1379 | 1.95 ± 0.17 | C12H20O2 |
| 48 | Cyperene | 1394 | 1398 | 0.20 ± 0.08 | C15H24 |
| 49 | Methyl eugenol | 1406 | 1403 | 0.50 ± 0.14 | C11H14O2 |
| 50 | (E)-Caryophyllene | 1412 | 1417 | 0.19 ± 0.04 | C15H24 |
| 51 | β-Copaene | 1422 | 1430 | 0.23 ± 0.05 | C15H24 |
| 52 | Rotundene | 1455 | 1457 | 0.15 ± 0.05 | C15H24 |
| 53 | allo-Aromadendrene | 1466 | 1458 | 0.23 ± 0.07 | C15H24 |
| 54 | Germacrene D | 1475 | 1480 | 0.70 ± 0.28 | C15H24 |
| 55 | α-Selinene | 1494 | 1498 | 0.19 ± 0.06 | C15H24 |
| 56 | δ-Amorphene | 1512 | 1511 | 0.25 ± 0.15 | C15H24 |
| 57 | Eugenol acetate | 1520 | 1521 | 1.60 ± 0.33 | C12H14O3 |
| 58 | Germacrene B | 1554 | 1559 | 0.23 ± 0.04 | C15H24 |
| 59 | Spathulenol | 1574 | 1577 | 0.71 ± 0.19 | C15H24O |
| 60 | Caryophyllene oxide | 1580 | 1582 | 0.67 ± 0.22 | C15H24O |
| 61 | Globulol | 1584 | 1590 | 0.12 ± 0.02 | C15H26O |
| 62 | Carotol | 1600 | 1594 | 0.81 ± 0.21 | C15H26O |
| 63 | cis-Isolongifolanone | 1611 | 1612 | 0.33 ± 0.09 | C15H24O |
| 64 | Silphiperfol-6-en-5-one | 1623 | 1624 | 0.15 ± 0.04 | C15H22O |
| 65 | Daucol | 1646 | 1641 | 0.31 ± 0.14 | C15H26O2 |
| 66 | Elemol acetate | 1679 | 1680 | 0.16 ± 0.07 | C17H28O2 |
| HM | 21.80 | ||||
| OM | 37.79 | ||||
| HS | 13.08 | ||||
| OS | 10.18 | ||||
| Others | 13.08 | ||||
| Total | 95.94 |
| Nº | Compounds | LRIa | LRIb | % | CF |
|---|---|---|---|---|---|
| 1 | α-Pinene | 928 | 932 | 1.64 ± 0.51 | C10H16 |
| 2 | Sabinene | 969 | 969 | 0.41 ± 0.20 | C10H16 |
| 3 | β-Pinene | 975 | 974 | 0.25 ± 0.12 | C10H16 |
| 4 | Myrcene | 987 | 988 | 0.24 ± 0.04 | C10H16 |
| 5 | Sylvestrene | 1027 | 1025 | 0.29 ± 0.15 | C10H16 |
| 6 | (Z)-β-Ocimene | 1034 | 1032 | 1.84 ± 0.53 | C10H16 |
| 7 | (E)-β-Ocimene | 1044 | 1044 | 5.35 ± 1.01 | C10H16 |
| 8 | Linalool | 1102 | 1095 | 0.99 ± 0.53 | C10H18O |
| 9 | Chrysanthenone | 1123 | 1124 | 0.68 ± 0.35 | C10H14O |
| 10 | 1,3,8-ρ-Menthatriene | 1131 | 1108 | 4.99 ± 1.15 | C10H18O |
| 11 | Verbenone | 1200 | 1204 | 0.31 ± 0.23 | C10H14O |
| 12 | Bornyl acetate | 1285 | 1284 | 0.25 ± 0.16 | C12H20O2 |
| 13 | Myrtenyl acetate | 1326 | 1324 | 0.84 ± 0.28 | C12H18O2 |
| 14 | δ-Elemene | 1331 | 1335 | 0.88 ± 0.14 | C15H24 |
| 15 | α-Cubebene | 1343 | 1348 | 0.35 ± 0.26 | C15H24 |
| 16 | α-Copaene | 1371 | 1374 | 2.48 ± 0.46 | C15H24 |
| 17 | β-Cubebene | 1384 | 1387 | 0.4 ± 0.17 | C15H24 |
| 18 | β-Elemene | 1386 | 1389 | 3.69 ± 0.87 | C15H24 |
| 19 | (E)-Caryophyllene | 1414 | 1417 | 1.04 ± 0.26 | C15H24 |
| 20 | β-Gurjunene | 1426 | 1431 | 0.15 ± 0.03 | C15H24 |
| 21 | 6,9-Guaiadiene | 1438 | 1442 | 0.17 ± 0.06 | C15H24 |
| 22 | α-Humulene | 1452 | 1452 | 0.74 ± 0.23 | C15H24 |
| 23 | α-neo-Clovene | 1456 | 1452 | 0.53 ± 0.10 | C15H24 |
| 24 | Dauca-5,8-diene | 1469 | 1471 | 0.35 ± 0.12 | C15H24 |
| 25 | γ-Muurolene | 1473 | 1478 | 1.94 ± 0.49 | C15H24 |
| 26 | cis-Muurola-4(14),5-diene | 1479 | 1479 | 17.87 ± 2.79 | C15H24O |
| 27 | δ-Selinene | 1484 | 1492 | 0.98 ± 0.25 | C15H24 |
| 28 | β-Selinene | 1486 | 1489 | 1.69 ± 0.95 | C15H24 |
| 29 | trans-Muurola-4(14),5-diene | 1489 | 1493 | 0.61 ± 0.14 | C15H24 |
| 30 | α-Zingiberene | 1493 | 1493 | 1.84 ± 0.35 | C15H24 |
| 31 | cis-β-Guaiene | 1496 | 1492 | 0.54 ± 0.10 | C15H24 |
| 32 | Valencene | 1499 | 1496 | 1.54 ± 0.37 | C15H24 |
| 33 | Aciphyllene | 1504 | 1501 | 5.37 ± 0.27 | C15H24 |
| 34 | (Z)-α-Bisabolene | 1508 | 1506 | 0.98 ± 0.44 | C15H24 |
| 35 | Cubebol | 1514 | 1514 | 0.7 ± 0.04 | C15H26O |
| 36 | δ-Amorphene | 1516 | 1511 | 2.44 ± 0.28 | C15H24 |
| 37 | γ-Patchoulene | 1521 | 1502 | 1.02 ± 0.46 | C15H24 |
| 38 | trans-Cadina-1,4-diene | 1531 | 1533 | 0.19 ± 0.10 | C15H24 |
| 39 | α-Cadinene | 1536 | 1537 | 0.24 ± 0.11 | C15H24 |
| 40 | α-Copaen-11-ol | 1544 | 1539 | 1.39 ± 0.14 | C15H24O |
| 41 | Germacrene B | 1558 | 1559 | 0.94 ± 0.07 | C15H24 |
| 42 | (E)-Nerolidol | 1561 | 1561 | 1.18 ± 0.28 | C15H26O |
| 43 | Spathulenol | 1579 | 1577 | 1.34 ± 0.28 | C15H24O |
| 44 | Globulol | 1596 | 1590 | 2.03 ± 0.57 | C15H26O |
| 45 | β-Oplopenone | 1606 | 1607 | 0.19 ± 0.10 | C15H24O |
| 46 | Guaiol | 1610 | 1600 | 0.20 ± 0.09 | C15H26O |
| 47 | 1,10-di-epi-Cubenol | 1613 | 1618 | 0.65 ± 0.14 | C15H26O |
| 48 | Isolongifolan-7-α-ol | 1621 | 1618 | 0.2 ± 0.07 | C15H26O |
| 49 | γ-Eudesmol | 1626 | 1630 | 1.44 ± 0.33 | C15H26O |
| 50 | Muurola-4,10(14)-dien-1-β-ol | 1635 | 1630 | 6.55 ± 0.58 | C15H24O |
| 51 | Cubenol | 1646 | 1645 | 2.77 ± 0.22 | C15H26O |
| 52 | Valerianol | 1654 | 1656 | 0.42 ± 0.08 | C15H26O |
| 53 | α-Cadinol | 1662 | 1652 | 2.12 ± 0.24 | C15H26O |
| 54 | neo-Intermedeol | 1666 | 1658 | 0.79 ± 0.34 | C15H26O |
| 55 | Germacra-4(15),5,10(14)-trien-1-α-ol | 1691 | 1685 | 2.09 ± 0.50 | C15H24O |
| 56 | Eudesma-4(15),7-dien-1-β-ol (impure) | 1693 | 1687 | 0.34 ± 0.19 | C15H24O |
| 57 | Amorpha-4,9-dien-2-ol | 1697 | 1700 | 0.90 ± 0.23 | C15H24O |
| HM | 11.34 | ||||
| OM | 6.48 | ||||
| HS | 40.51 | ||||
| OS | 30.79 | ||||
| Others | 3.24 | ||||
| Total | 92.37 |
| Enantiomers | LRI | Enantiomeric Ratio (%) | Enantiomeric Excess (%) |
|---|---|---|---|
| H. cumbalense | |||
| (1R,5R)-(+)-sabinene | 999 | 34.51 | 30.98 |
| (1S,5S)-(–)-sabinene | 1000 | 65.49 | |
| (S)-(+)-terpinen-4-ol | 1270 | 21.27 | 57.47 |
| (R)-(−)-terpinen-4-ol | 1275 | 78.73 | |
| H. spectabile | |||
| (1R,5R)-(+)-sabinene | 999 | 47.87 | 4.25 |
| (1S,5S)-(–)-sabinene | 1001 | 52.13 | |
| (S)-(+)-linalool | 1198 | 0.13 | 99.74 |
| (R)-(-)-linalool | 1202 | 99.87 | |
| Microorganism | H. cumbalense | H. spectabile | Positive Control * |
|---|---|---|---|
| (µg/mL) | (µg/mL) | (µg/mL) | |
| Gram-positive cocci | |||
| Enterococcus faecalis ATCC ® 19433 | - | - | 0.78 |
| Enterococcus faecium ATCC ® 27270 | 4000 | - | <0.39 |
| Staphylococcus aureus ATCC ® 25923 | 4000 | - | <0.39 |
| Gram-negative bacilli | |||
| Escherichia coli (O157:H7) ATCC ® 43888 | - | - | 1.56 |
| Pseudomonas aeruginosa ATCC ® 10145 | - | - | <0.39 |
| Yeasts and sporulated fungi | |||
| Candida albicans ATTC ® 10231 | 1000 | 2000 | <0.09 |
| Aspergillus niger ATCC ® 6275 | 1000 | 1000 | <0.09 |
| Sample | DPPH | ABTS |
|---|---|---|
| SC50 (µg/mL—µM *) ± SD | ||
| H. spectabile | 2366.6 ± 2.99 | 214. 41 ± 4.03 |
| H. cumbalense | 209.99 ± 1.33 | 96.02 ± 0.33 |
| Trolox | 29.99 ± 1.04 | 23.27 ± 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, A.; Guerrero, E.; Cartuche, L.; Cumbicus, N.; Morocho, V. Chemical Profiling of Hedyosmum cumbalense and Hedyosmum spectabile (Chloranthaceae) Essential Oils, and Their Antimicrobial, Antioxidant, and Anticholinesterase Properties. Plants 2023, 12, 39. https://doi.org/10.3390/plants12010039
Guerrero A, Guerrero E, Cartuche L, Cumbicus N, Morocho V. Chemical Profiling of Hedyosmum cumbalense and Hedyosmum spectabile (Chloranthaceae) Essential Oils, and Their Antimicrobial, Antioxidant, and Anticholinesterase Properties. Plants. 2023; 12(1):39. https://doi.org/10.3390/plants12010039
Chicago/Turabian StyleGuerrero, Alisson, Emilye Guerrero, Luis Cartuche, Nixon Cumbicus, and Vladimir Morocho. 2023. "Chemical Profiling of Hedyosmum cumbalense and Hedyosmum spectabile (Chloranthaceae) Essential Oils, and Their Antimicrobial, Antioxidant, and Anticholinesterase Properties" Plants 12, no. 1: 39. https://doi.org/10.3390/plants12010039
APA StyleGuerrero, A., Guerrero, E., Cartuche, L., Cumbicus, N., & Morocho, V. (2023). Chemical Profiling of Hedyosmum cumbalense and Hedyosmum spectabile (Chloranthaceae) Essential Oils, and Their Antimicrobial, Antioxidant, and Anticholinesterase Properties. Plants, 12(1), 39. https://doi.org/10.3390/plants12010039







