Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate
Abstract
1. Introduction
2. Results
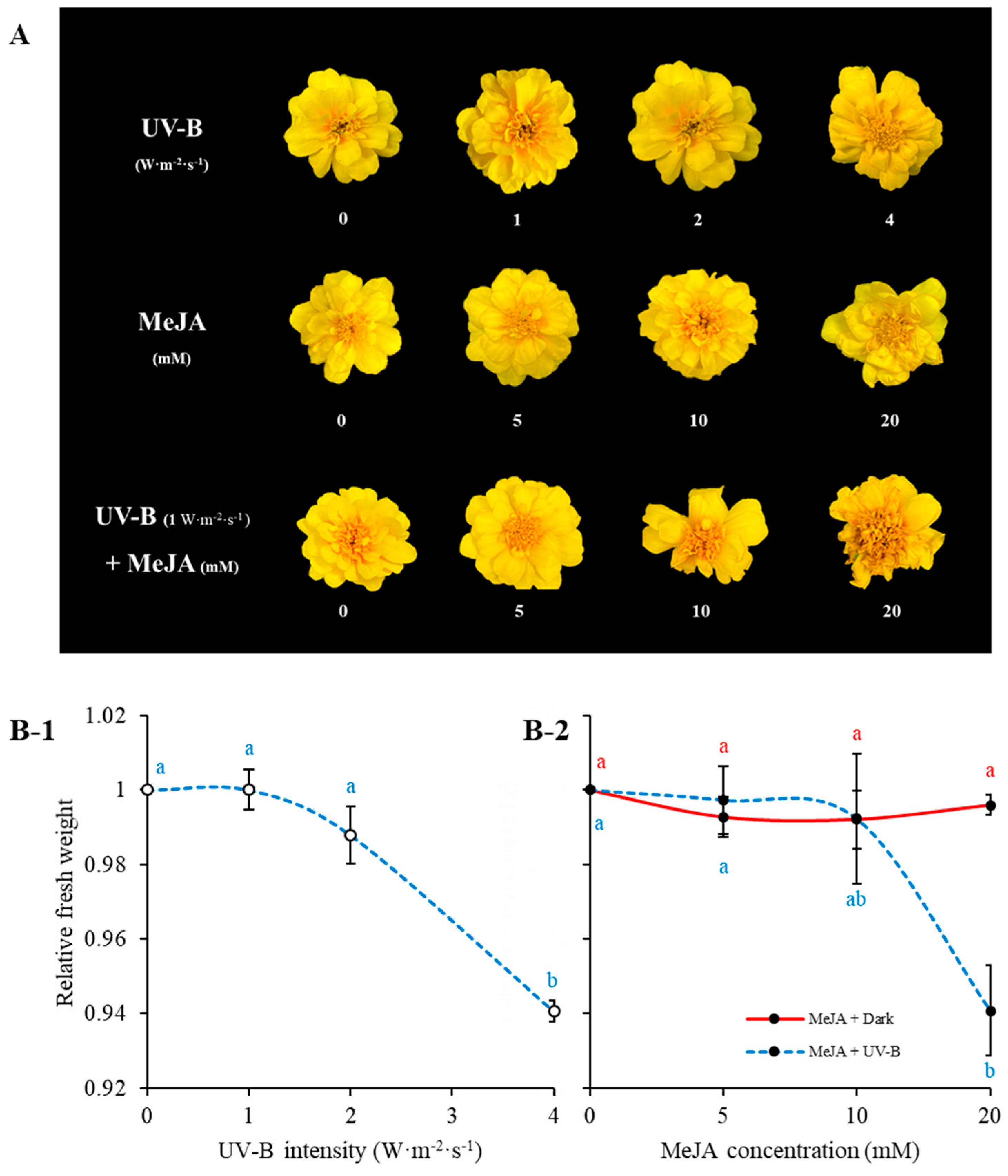
2.1. Morphology of Marigold Flowers Treated by UV-B Intensity and MeJA Concentration
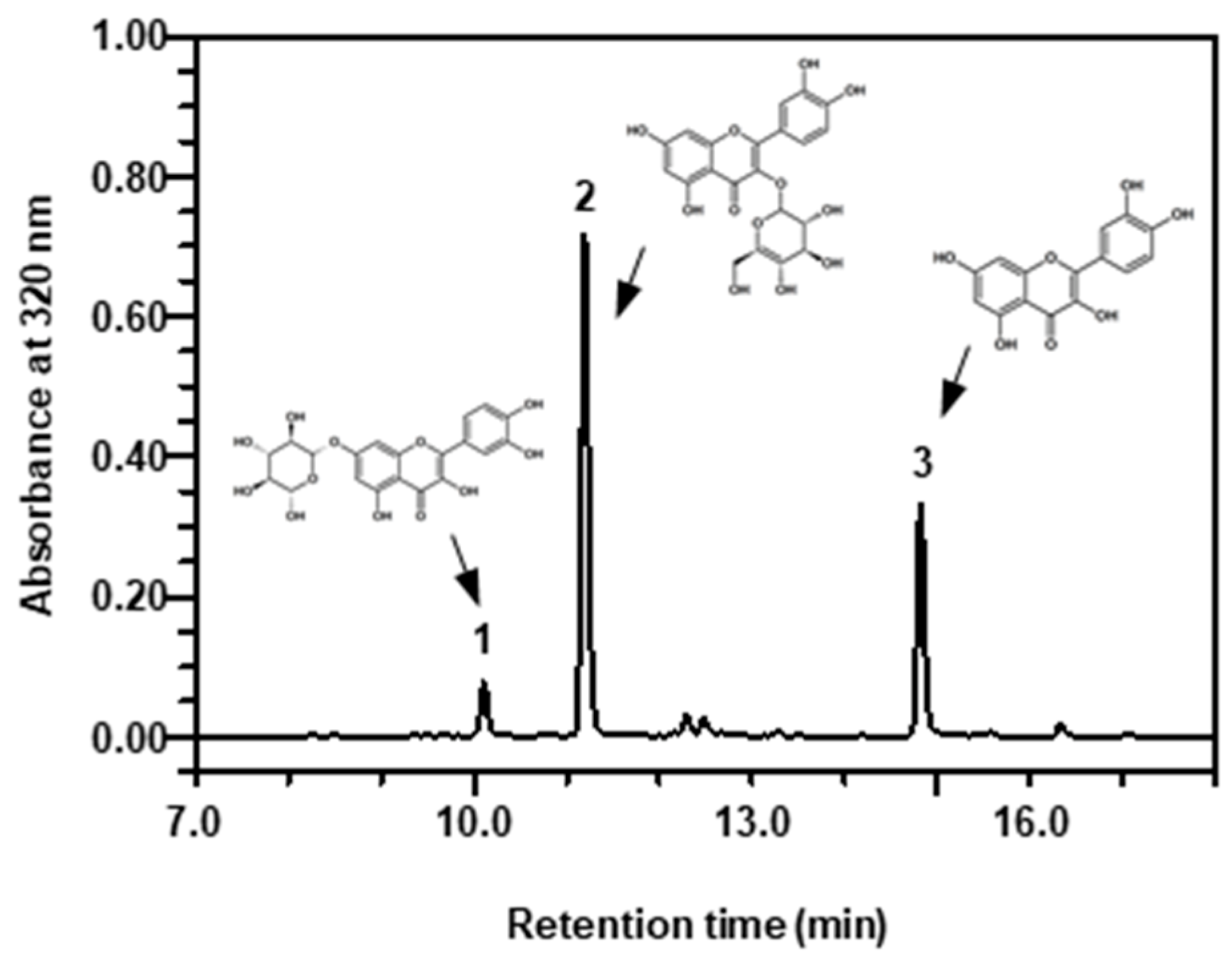
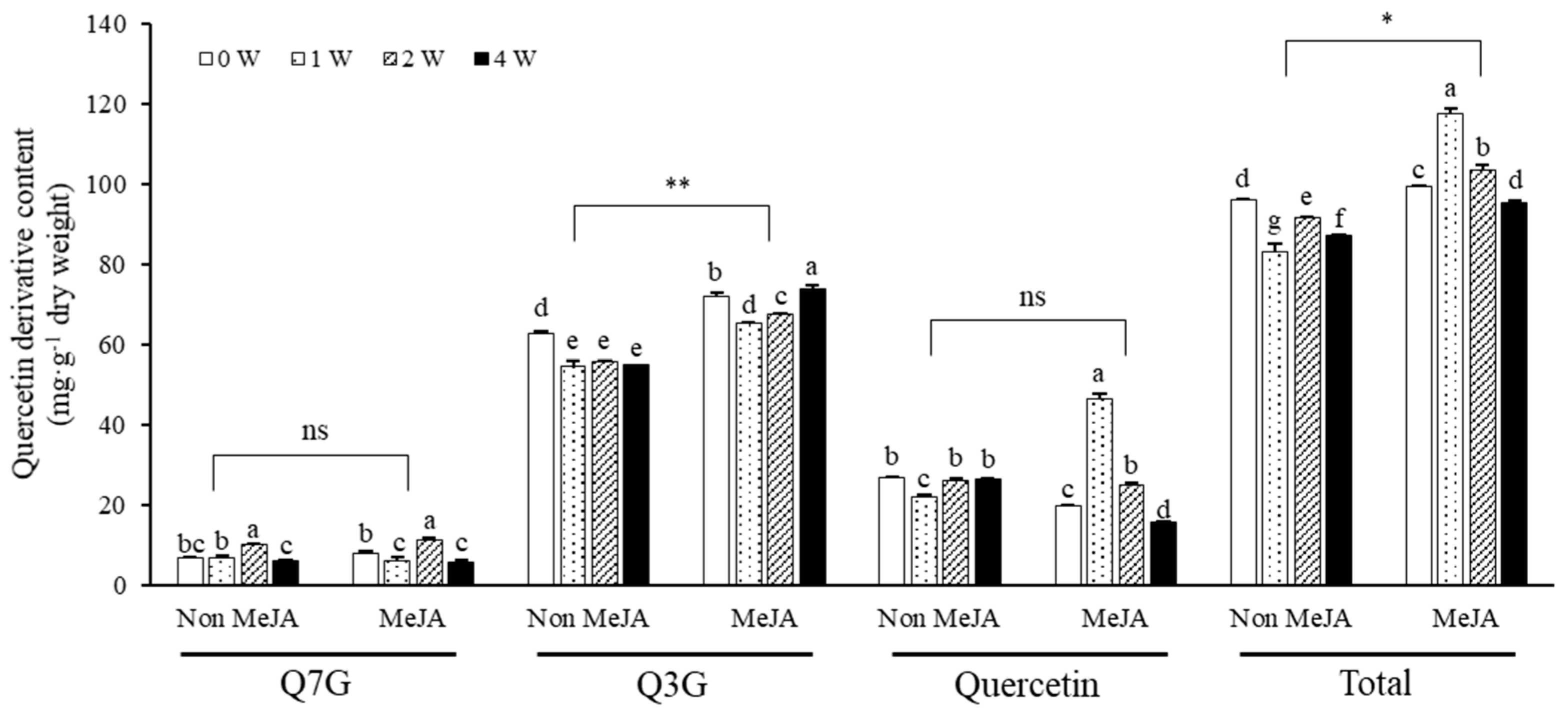
2.2. Changes in Quercetin Derivatives in Response to UV-B Irradiation with or without MeJA Treatment
2.3. Short- and Long-Term Effects of MeJA and UV-B Treatments on Quercetin Derivatives
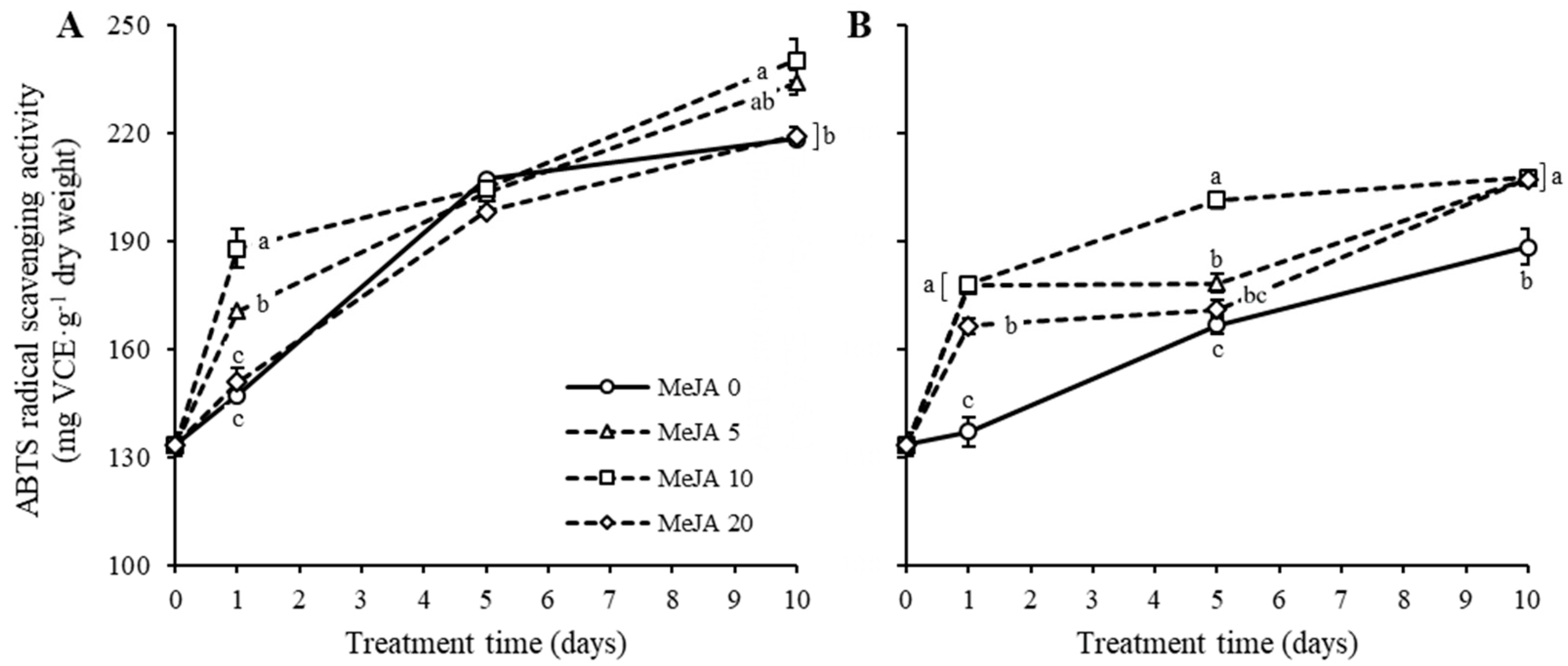
2.4. Antioxidant Activities in Response to UV-B Irradiation and MeJA Treatment Alone or in Combination
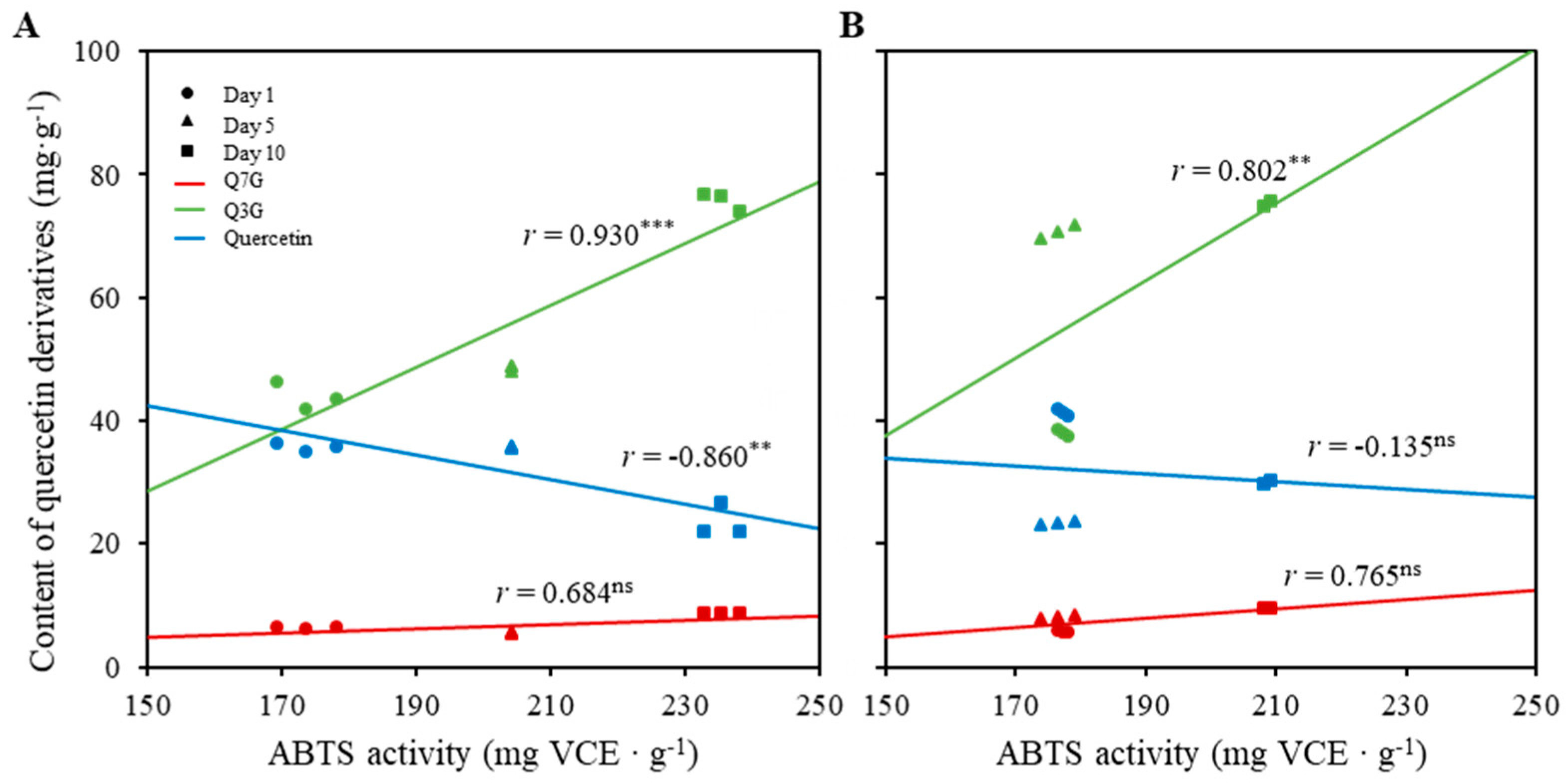
2.5. Correlation between Antioxidant Activity and Antioxidants
3. Discussion
4. Materials and Methods
4.1. Chemicals and Plant Materials
4.2. Treatment with UV-B Irradiation with or without MeJA for 5 Days
4.3. Treatment with MeJA under 1 W∙m−2∙s−1 UV-B Irradiation
4.4. Determination of Flavonoids by Reverse-Phase HPLC
4.5. Measurement of Antioxidant Activities
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vivaram, N.; Nandikatti, V.L.; Rani, V.; Namburi, R. Review on Tagetes patula plant and its pharmacological activities. IJPPR 2020, 17, 297–305. [Google Scholar]
- Anca, T.; Benedec, D.; Duda, M.; Hanganu, D.; Oniga, I. Determination of total carotenoid content in some Calendula officinalis and Tagetes patula varieties. Hop. Med. Plants 2014, 57, 1065. [Google Scholar]
- Breithaupt, D.E.; Wirt, U.; Bamedi, A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta L.) and several fruits by liquid chromatography−Mass spectrometry. J. Agric. Food Chem. 2002, 50, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Bano, S.; Naqvi, S.; Faizi, S.; Lubna; Ahmed Mesaik, M.; Azeemi, K.S.; Farooq, A.D. Cytotoxic and antioxidant properties of phenolic compounds from Tagetes patula flower. Pharm. Biol. 2015, 53, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Bruni, R.; Andreotti, E.; Rai, M.K.; Vicentini, C.B.; Mares, D. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma 2005, 225, 57–65. [Google Scholar] [CrossRef]
- Riaz, M.; Ahmad, R.; Rahman, N.U.; Khan, Z.; Dou, D.; Sechel, G.; Manea, R. Traditional uses, Phyto-chemistry and pharmacological activities of Tagetes Patula L. J. Ethnopharmacol. 2020, 255, 112718. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Gallori, S.; Mazzi, G.; Vincieri, F.F. Stability of the constituents of calendula, milk-thistle and passionflower tinctures by LC-DAD and LC-MS. J. Pharm. Biomed. Anal. 2002, 30, 613–624. [Google Scholar] [CrossRef]
- Toker, G.; Aslan, M.; Yeşilada, E.; Memişoğlu, M.; Ito, S. Comparative evaluation of the flavonoid content in officinal Tiliae flos and Turkish lime species for quality assessment. J. Pharm. Biomed. Anal. 2001, 26, 111–121. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharm. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Šamec, D.; Linić, I.; Salopek-Sondi, B. Salinity stress as an elicitor for phytochemicals and minerals accumulation in selected leafy vegetables of Brassicaceae. Agronomy 2021, 11, 361. [Google Scholar] [CrossRef]
- Pandey, N.; Pandey-Rai, S. Short term UV-B radiation-mediated transcriptional responses and altered secondary metabolism of in vitro propagated plantlets of Artemisia annua L. PCTOC 2014, 116, 371–385. [Google Scholar] [CrossRef]
- Lim, Y.J.; Jeong, H.Y.; Gil, C.S.; Kwon, S.J.; Na, J.K.; Lee, C.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food chem. 2020, 303, 125376. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]
- Si, C.; Yao, X.Q.; He, X.L.; Chu, J.Z.; Ma, C.H.; Shi, X.F. Effects of enhanced UV-B radiation on biochemical traits in postharvest flowers of medicinal chrysanthemum. Photochem. Photobiol. 2015, 91, 845–850. [Google Scholar] [CrossRef]
- Ma, C.H.; Chu, J.Z.; Shi, X.F.; Liu, C.Q.; Yao, X.Q. Effects of enhanced UV-B radiation on the nutritional and active ingredient contents during the floral development of medicinal chrysanthemum. J. Photochem. Photobiol. B Biol. 2016, 158, 228–234. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.K.; Lee, I.J. Methyl jasmonate alleviated salinity stress in soybean. JCSB 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Rakkammal, K.; Largia, M.J.V.; Thamilarasan, S.K.; Balaji, S.; Zoclanclounon, Y.A.B.; Shilpha, J.; Ramesh, M. Jasmonates in plant growth and development and elicitation of secondary metabolites: An updated overview. Front. Plant Sci. 2022, 13, 942789. [Google Scholar] [CrossRef]
- Fedina, I.; Nedeva, D.; Georgieva, K.; Velitchkova, M. Methyl jasmonate counteract UV-B Stress in barley seedlings. J. Agron. Crop. Sci. 2009, 195, 204–212. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of anthocyanin and associated gene expression in radish sprouts exposed to light and methyl jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef]
- Kianersi, F.; Pour-Aboughadareh, A.; Majdi, M.; Poczai, P. Effect of methyl jasmonate on thymol, carvacrol, phytochemical accumulation, and expression of key genes involved in thymol/carvacrol biosynthetic pathway in some Iranian Thyme species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q.; Pei, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.A.; Duan, S.; Jeong, H.Y.; Lee, C.; Kang, I.K.; Eom, S.H. Pigmentation and Flavonoid Metabolite Diversity in Immature ‘Fuji’ Apple Fruits in Response to Lights and Methyl Jasmonate. Int. J. Mol. Sci. 2022, 23, 1722. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.A.; Duan, S.; Gil, C.S.; Jeong, H.Y.; Lee, C.; Kang, I.K.; Eom, S.H. Combined UV-B and methyl jasmonate treatments enhance postharvest pigmentation of “Fuji” apples. Postharvest Biol. Technol. 2022, 190, 111938. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Xu, M.; Chen, G.; Tan, M.; Xiang, Z. Light and Potassium Improve the Quality of Dendrobium officinale through Optimizing Transcriptomic and Metabolomic Alteration. Molecules 2022, 27, 4866. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lyu, J.I.; Kwon, S.J.; Eom, S.H. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chem. 2021, 339, 128080. [Google Scholar] [CrossRef]
- Nam, T.G.; Lim, Y.J.; Eom, S.H. Flavonoid accumulation in common buckwheat (Fagopyrum esculentum) sprout tissues in response to light. HEB 2018, 59, 19–27. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible flowers with the common name “marigold”: Their therapeutic values and processing. Trends Food Sci. Technol. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Munhoz, V.M.; Longhini, R.; Souza, J.R.; Zequi, J.A.; Mello, E.V.; Lopes, G.C.; Mello, J.C. Extraction of flavonoids from Tagetes patula: Process optimization and screening for biological activity. Rev. Bras. Farmacogn. 2014, 24, 576–583. [Google Scholar] [CrossRef]
- Poorter, H.; Van Berkel, Y.; Baxter, R.; Den Hertog, J.; Dijkstra, P.; Gifford, R.M.; Wong, S.C. The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ. 1997, 20, 472–482. [Google Scholar] [CrossRef]
- Nithia, S.M.J.; Shanthi, N.; Kulandaivelu, G. Different responses to UV-B enhanced solar radiation in radish and carrot. Photosynthetica 2005, 43, 307–311. [Google Scholar] [CrossRef]
- Tong, H.; Leasure, C.D.; Hou, X.; Yuen, G.; Briggs, W.; He, Z.H. Role of root UV-B sensing in Arabidopsis early seedling development. Proc. Natl. Acad. Sci. USA 2008, 105, 21039–21044. [Google Scholar] [CrossRef]
- Li, B.; Krumbein, A.; Neugart, S.; Li, L.; Schreiner, M. Mixed cropping with maize combined with moderate UV-B radiations lead to enhanced flavonoid production and root growth in faba bean. J. Plant Interact. 2012, 7, 333–340. [Google Scholar] [CrossRef]
- Moreira, X.; Sampedro, L.; Zas, R. Defensive responses of Pinus pinaster seedlings to exogenous application of methyl jasmonate: Concentration effect and systemic response. Environ. Exp. Bot. 2009, 67, 94–100. [Google Scholar] [CrossRef]
- Kianersi, F.; Amin Azarm, D.; Pour-Aboughadareh, A.; Poczai, P. Change in Secondary Metabolites and Expression Pattern of Key Rosmarinic Acid Related Genes in Iranian Lemon Balm (Melissa officinalis L.) Ecotypes Using Methyl Jasmonate Treatments. Molecules 2022, 27, 1715. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wel, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Huang, X.; Gao, W.; Yun, X.; Qing, Z.; Zeng, J. Effect of Natural Antioxidants from Marigolds (Tagetes erecta L.) on the Oxidative Stability of Soybean Oil. Molecules 2022, 27, 2865. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids-Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef]
- Liu, L.; Gregan, S.; Winefield, C.; Jordan, B. From UVR 8 to flavonol synthase: UV-B-induced gene expression in S auvignon blanc grape berry. Plant Cell Environ. 2015, 38, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kwon, S.J.; Qu, S.; Kim, D.G.; Eom, S.H. Antioxidant contributors in seed, seed coat, and cotyledon of γ-ray-induced soybean mutant lines with different seed coat colors. Antioxidants 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.C.; Kwon, S.J.; Eom, S.H. Effect of thermal processing on color, phenolic compounds, and antioxidant activity of faba bean (Vicia faba L.) leaves and seeds. Antioxidants 2021, 10, 1207. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Duan, S.; Lim, Y.J.; Eom, S.H. Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate. Plants 2022, 11, 2947. https://doi.org/10.3390/plants11212947
Kim JH, Duan S, Lim YJ, Eom SH. Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate. Plants. 2022; 11(21):2947. https://doi.org/10.3390/plants11212947
Chicago/Turabian StyleKim, Ji Hye, Shucheng Duan, You Jin Lim, and Seok Hyun Eom. 2022. "Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate" Plants 11, no. 21: 2947. https://doi.org/10.3390/plants11212947
APA StyleKim, J. H., Duan, S., Lim, Y. J., & Eom, S. H. (2022). Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate. Plants, 11(21), 2947. https://doi.org/10.3390/plants11212947




