Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Sea Fennel EOs
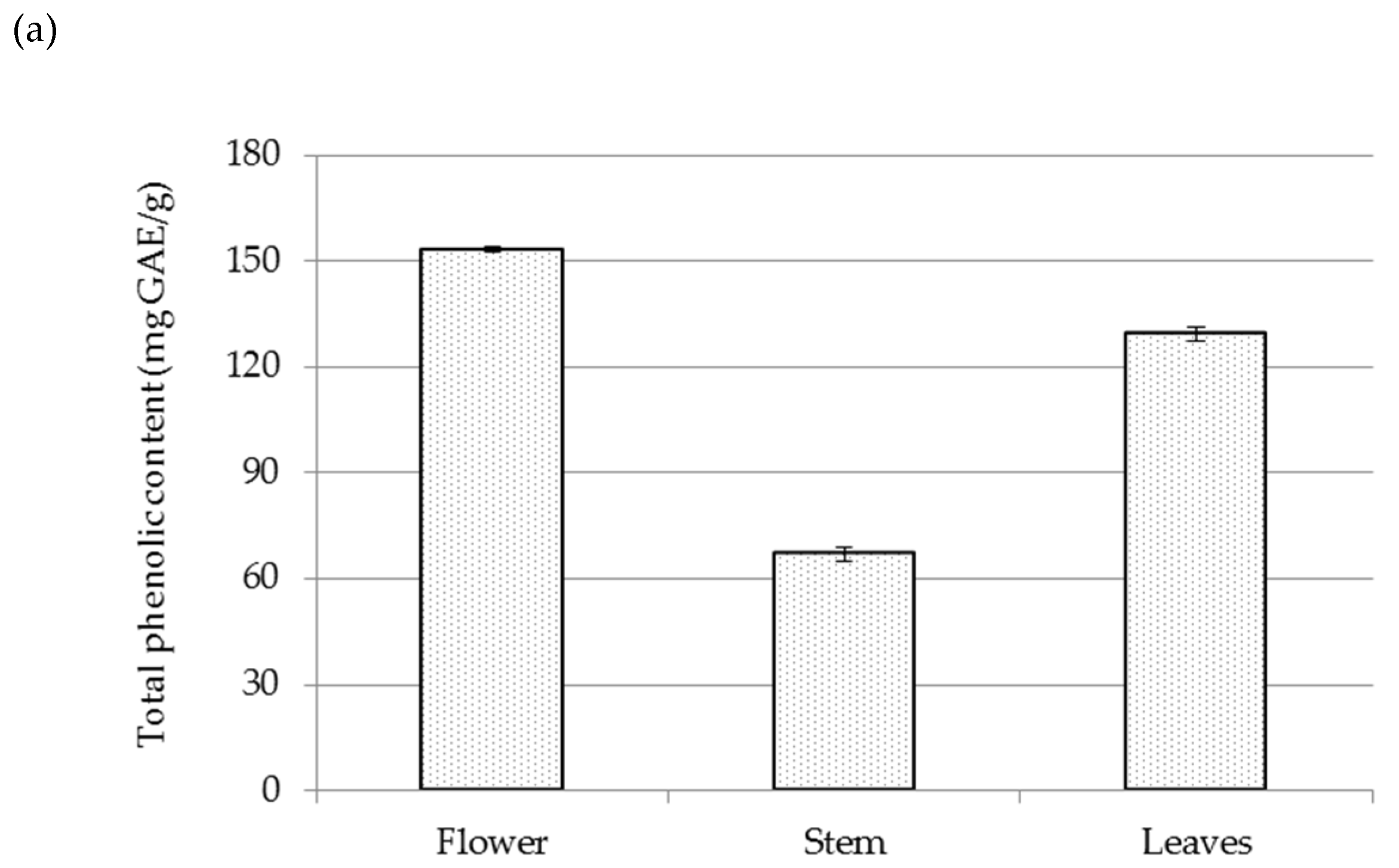
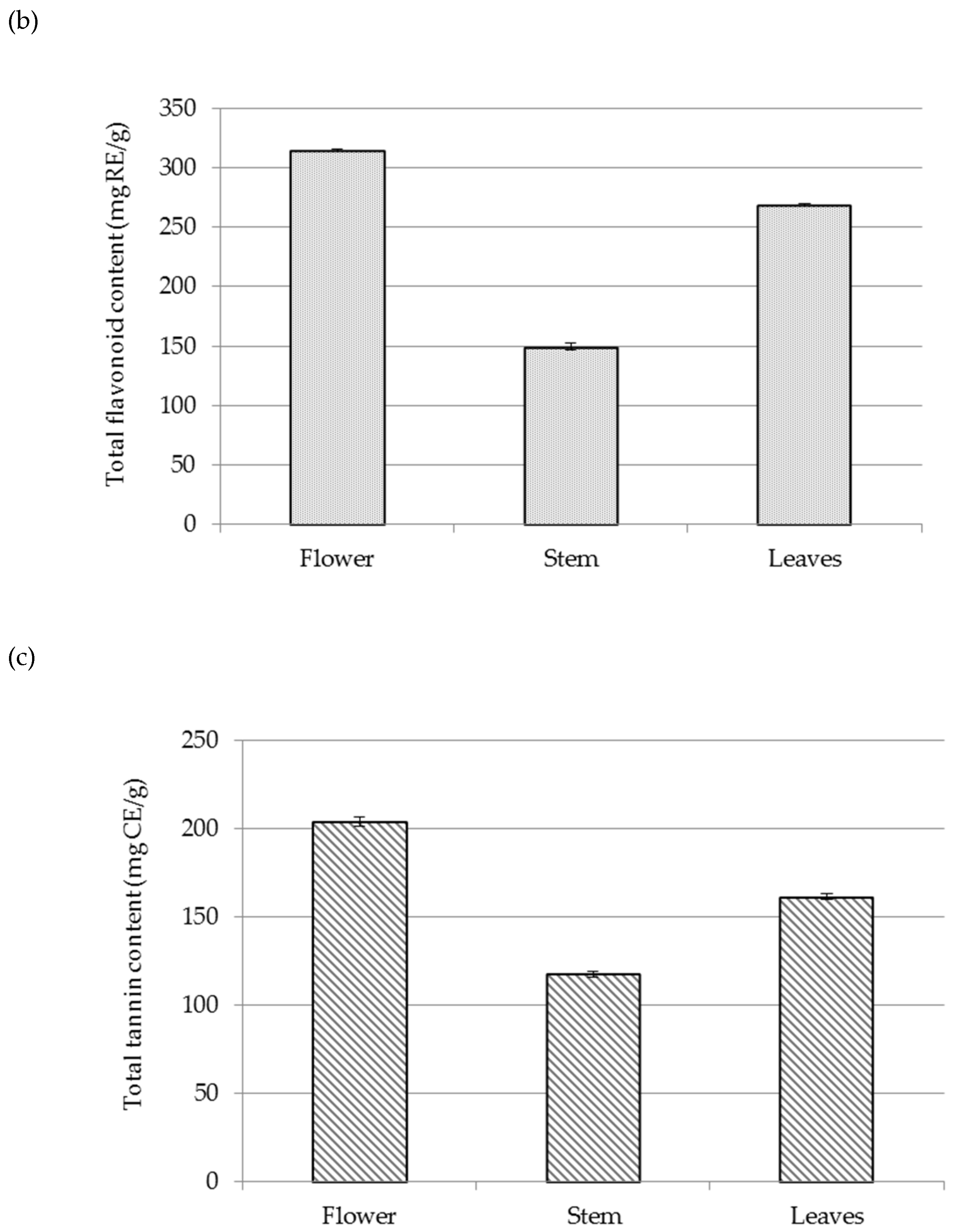
2.2. Chemical Composition of Residual Waste-Water from Sea Fennel EO Isolation by Hydrodistillation
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Isolation of Essential Oils and Preparation of Residual-Water Extracts
3.3. Gas Chromatography-Mass Spectrometry (GC-MS)
3.4. High Performance Liquid Chromatography (HPLC)
3.5. Spectrophotometric Measurements of Main Phenolic Groups
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Enviromental eco-physiology and economical potential of the halophtye Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Marín, D.; Taladrid, D.; Montero, P.; Gómez-Guillén, M.C. Encapsulation of antioxidant sea fennel (Crithmum maritimum) aqueous and ethanolic extracts in freeze-dried soy phosphatidylcholine liposomes. Food Res. Int. 2018, 119, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crop. Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Houta, O.; Akrout, A.; Neffati, M.; Amri, H. Phenolic Contents, Antioxidant and Antimicrobial Potentials of Crithmum maritimum Cultivated in Tunisia Arid Zones. J. Biol. Act. Prod. Nat. 2011, 1, 138–143. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Šimat, V.; Ljubenkov, I.; Burčul, F.; Grga, M.; Mihajlovski, M.; Lončar, R.; Katalinić, V.; Skroza, D. Influence of the vegetation period on sea fennel, Crithmum maritimum L. (Apiaceae), phenolic composition, antioxidant and anticholinesterase activities. Ind. Crop. Prod. 2018, 124, 947–953. [Google Scholar] [CrossRef]
- Generalić Meknić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernández, F.; Carbonell-Barrachina, A.; Madani, K.; Larbat, R. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int. J. Food Prop. 2016, 20, 1843–1855. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step in Vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef] [PubMed]
- Kadoglidou, K.; Irakli, M.; Boutsika, A.; Mellidou, I.; Maninis, N.; Sarrou, E.; Georgiadou, V.; Tourvas, N.; Krigas, N.; Moysiadis, T.; et al. Metabolomic Fingerprinting and Molecular Characterization of the Rock Samphire Germplasm Collection from the Balkan Botanic Garden of Kroussia, Northern Greece. Plants 2022, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Amoruso, F.; Signore, A.; Gómez, P.A.; Martínez-Ballesta, M.D.C.; Giménez, A.; Franco, J.A.; Fernández, J.A.; Egea-Gilabert, C. Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants. Horticulturae 2022, 8, 127. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Ozcan, M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L. (Apiaceae) growing wild in Turkey. Flavour Fragr. J. 2000, 15, 186–189. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and Antimicrobial Activity of Foeniculum vulgare and Crithmum maritimum Essential Oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Souid, A.; Della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Ben Hamed, K.; Magné, C.; Longo, V. Nutraceutical Potential of Leaf Hydro-Ethanolic Extract of the Edible Halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, C.M.; Yeh, H.C.; Wu, H.M.; Li, W.J.; Li, H.T. Flavonoids of Crithmum maritimum. Chem. Nat. Compd. 2021, 57, 917–920. [Google Scholar] [CrossRef]
- Pereira, C.G.; Moraes, C.B.; Franco, C.H.; Feltrin, C.; Grougnet, R.; Barbosa, E.G.; Panciera, M.; Correia, C.R.D.; Rodrigues, M.J.; Custódio, L. In Vitro Anti-Trypanosoma cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmum maritimum L.). Plants 2021, 10, 2235. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the Prospects of Sea Fennel (Crithmum maritimum L.) as Emerging Vegetable Crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Piatti, D.; Angeloni, S.; Caprioli, G.; Maggi, F.; Ricciutelli, M.; Arnoldi, L.; Sagratini, G. Sea Fennel (Crithmum maritimum L.): A Promising Biosaline Crop. Extraction, Purification and Chemical Characterization of Polar Extracts. Biol. Life Sci. Forum 2021, 11, 61. [Google Scholar] [CrossRef]
- Karkanis, A.; Polyzos, N.; Kompocholi, M.; Petropoulos, S.A. Rock Samphire, a Candidate Crop for Saline Agriculture: Cropping Practices, Chemical Composition and Health Effects. Appl. Sci. 2022, 12, 737. [Google Scholar] [CrossRef]
- Incegül, Y.; Çam, M. Recovery of water-soluble materials after distillation of sage (Salvia officinalis L.) and the use of materials in the production of cake and ice cream. J. Food Meas. Charact. 2021, 15, 2688–2694. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Santiago, J.-L.; Álvarez-Acero, I.; Bartolomé, M.-A.M.; Martínez, M.-C. Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain). Horticulturae 2022, 8, 376. [Google Scholar] [CrossRef]
- Gerçek, Y.C.; Bayram, S.; Çelik, S.; Canlı, D.; Mavaldi, M.H.; Boztas, K.; Bastürk, F.N.; Kırkıncı, S.; Yesil, Y.; Kösesakal, T.; et al. Characterization of Essential Oil and Wastewater from Thymus nummularius M. Bieb. and Micromorphological Examination of Glandular Trichomes. J. Essent. Oil Bear. Plants 2022, 25, 690–706. [Google Scholar] [CrossRef]
- Rodrigues, L.; Coelho, E.; Madeira, R.; Teixeira, P.; Henriques, I.; Coimbra, M.A. Food Ingredients Derived from Lemongrass Byproduct Hydrodistillation: Essential Oil, Hydrolate, and Decoction. Molecules 2022, 27, 2493. [Google Scholar] [CrossRef]
- Pateira, L.; Nogueira, T.; Antunes, A.; Venâncio, F.; Tavares, R.; Capelo, J. Two chemotypes of Crithmum maritimum L. from Portugal. Flavour Fragr. J. 1999, 14, 333–343. [Google Scholar] [CrossRef]
- Baser, K.H.; Özek, T.; Demirci, B.; Saritas, Y. Essential Oil of Crithmum maritimum L. from Turkey. J. Essent. Oil Res. 2000, 12, 424–426. [Google Scholar] [CrossRef]
- Katsouri, E.; Demetzos, C.; Perdetzoglou, D.; Loukis, A. An Interpopulation Study of the Essential Oils of Various Parts of Crithmum maritimum L. Growing in Amorgos Island, Greece. J. Essent. Oil Res. 2001, 13, 303–308. [Google Scholar] [CrossRef]
- Özcan, M.; Akgül, A.; Bas¸cr, K.H.C.; Özck, T.; Tabanca, N. Essential oil composition of sea fennel (Crithmum maritimum) form Turkey. Nahrung 2001, 45, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Zafeiropoulou, V.; Tomou, E.M.; Ioannidou, O.; Karioti, A.; Skaltsa, H. Sea fennel: Phytochemical analysis of Greek wild and cultivated Crithmum maritimum L. populations, based on HPLC-PDA-MS and NMR methods. J. Pharmacogn. Phytochem. 2020, 9, 998–1004. [Google Scholar]
- Kulisic-Bilusic, T.; Blažević, I.; Dejanović, B.; Miloš, M.; Pifat, G. Evaluation of the antioxidant activity of essential oils from caper (Capparis Spinosa) and sea fennel (Crithmum maritimum) by different methods. J. Food Biochem. 2010, 34, 286–302. [Google Scholar] [CrossRef]
- Maleš, Ž.; Žuntar, I.; Nigović, B.; Plazibat, M.; Bilušić Vundač, V. Quantitative analysis of the polyphenols of the aerial parts of rock samphire—Crithmum Maritimum L. Acta Pharm. 2003, 53, 139–144. [Google Scholar]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.d.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Ben Amor, N.; Ben Hamed, K.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J. Colorimetry of total phenolics with phospho-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Yang, J.; Meyers, K.J.; van der Heide, A.J.; Liu, R.H. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
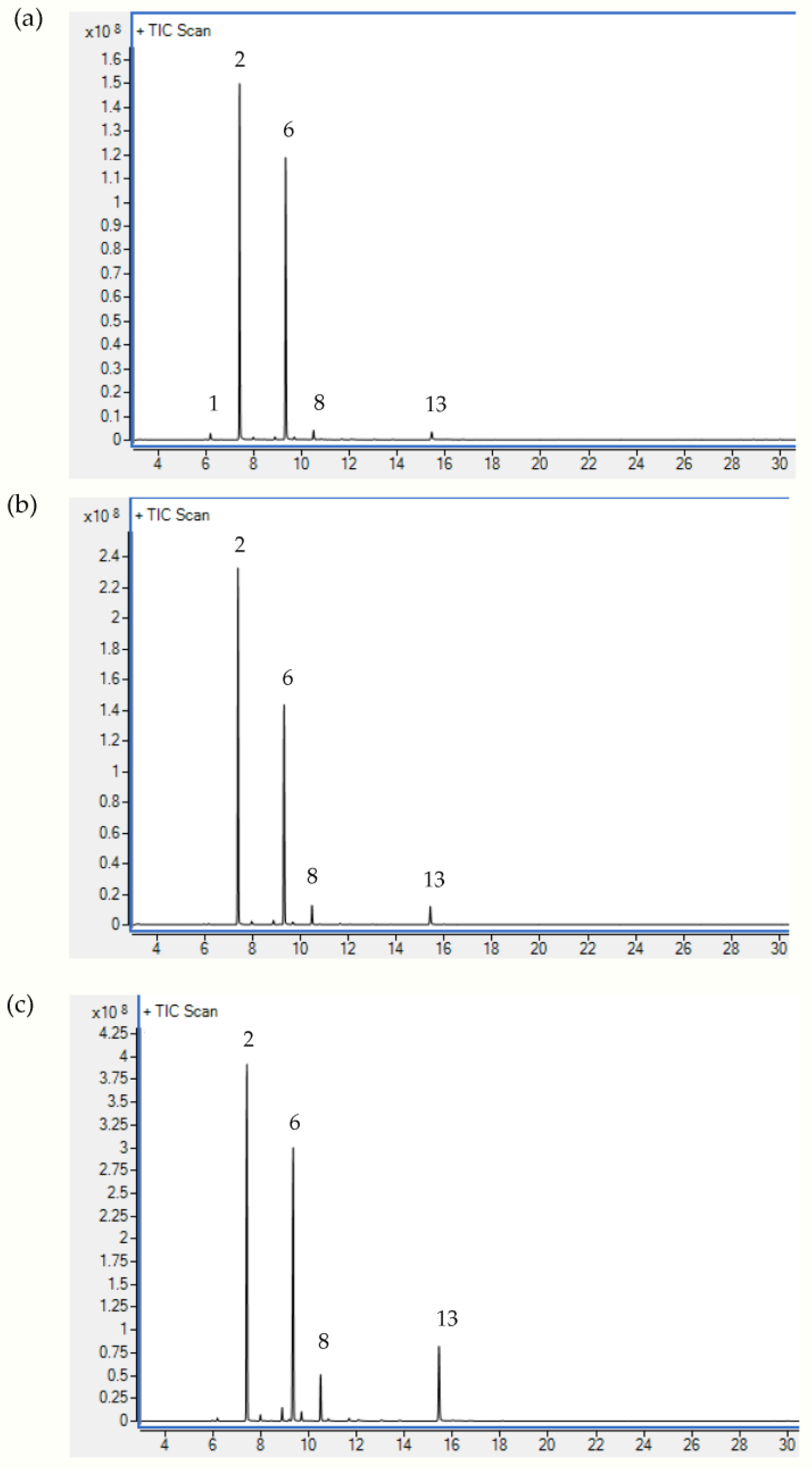
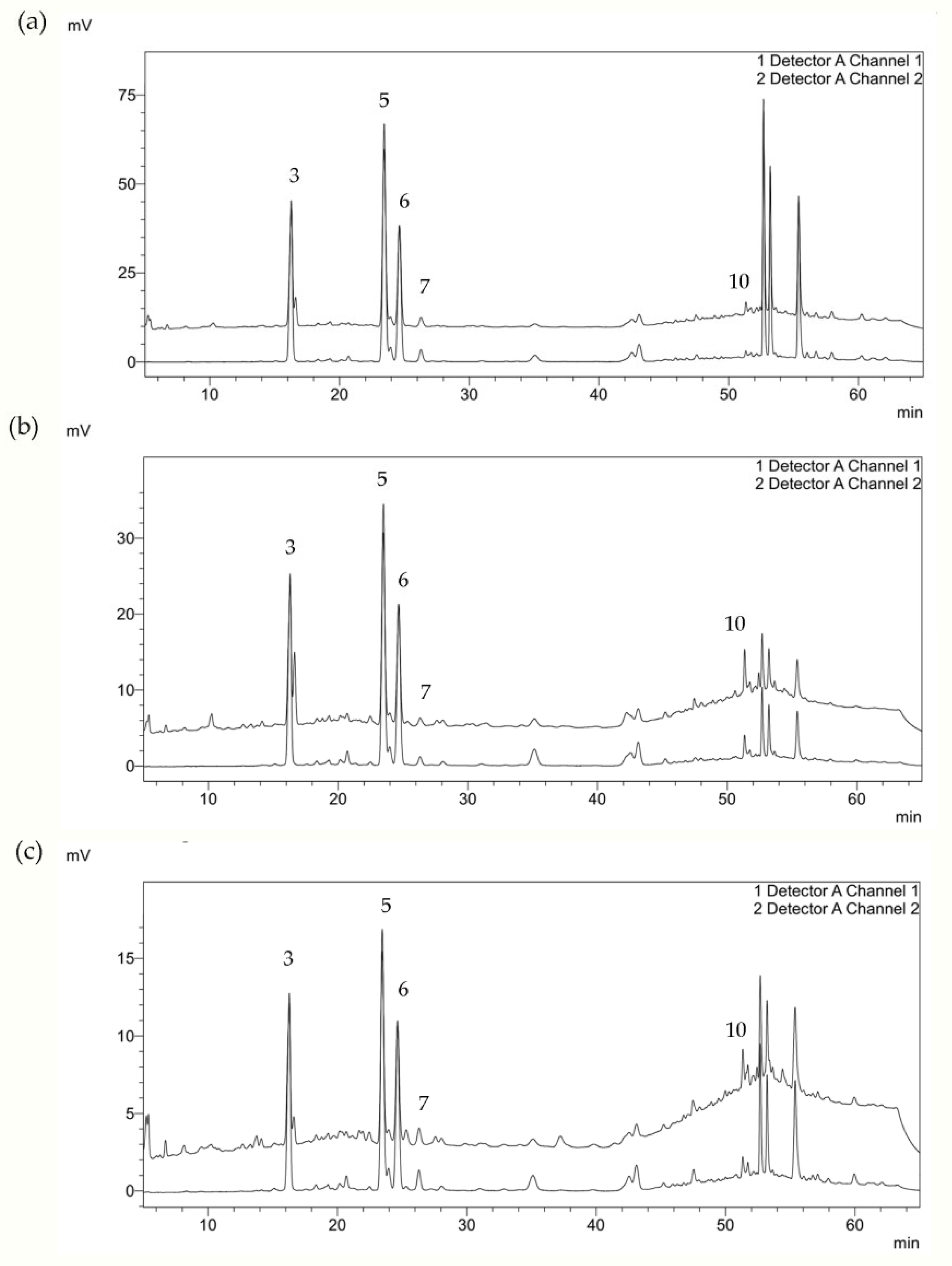
| No. | Component | RI | Flower | Leaf | Stem | Mode of Identification |
|---|---|---|---|---|---|---|
| Monoterpene Hydrocarbons | ||||||
| 1. | α-Pinene | 938 | 1.76 | 0.08 | 0.35 | RI, MS |
| 2. | Sabinene | 977 | 44.94 | 51.47 | 42.55 | RI, MS |
| 3. | β-Pinene | 993 | 0.07 | 0.89 | 0.80 | RI, MS |
| 4. | α-Terpinene | 1019 | 0.86 | 0.98 | 1.47 | RI, MS |
| 5. | p-Cymene | 1028 | n.d. | 0.09 | 0.27 | RI, MS |
| 6. | Limonene | 1032 | 43.58 | 36.28 | 36.45 | RI, MS |
| 7. | (E)-β-Ocimene | 1042 | 0.77 | 0.07 | 1.15 | RI, MS |
| 8. | γ-Terpinene | 1062 | 2.79 | 3.49 | 5.28 | RI, MS |
| 10. | Terpinolene | 1090 | n.d. | 0.37 | 0.47 | RI, MS |
| Oxygenated Monoterpenes | ||||||
| 9. | cis-Sabinene hydrate | 1070 | 0.11 | 0.10 | 0.38 | RI, MS |
| 11. | trans-Sabinene hydrate | 1098 | 0.08 | n.d. | 0.15 | RI, MS |
| 12. | trans-p-Menth-2-en-1-ol | 1122 | n.d. | 0.10 | 0.13 | RI, MS |
| 13. | Terpinen-4-ol | 1179 | 3.53 | 5.35 | 10.35 | RI, MS |
| 14. | α-Terpineol | 1190 | n.d. | n.d. | 0.07 | RI, MS |
| Total identified (%) | 98.49 | 99.27 | 99.87 | |||
| Oil yield (%, w/w) | 1.35 | 0.63 | 0.62 |
| No. | Component | Flower | Leaf | Stem |
|---|---|---|---|---|
| Phenolic acids | ||||
| 1. | Gallic acid | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.03 ± 0.03 |
| 2. | Protocatechuic acid | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.06 ± 0.00 |
| 3 | Neochlorogenic acid (5-O-caffeoylquinic acid) | 11.47 ± 0.09 | 6.56 ± 0.05 | 3.27 ± 0.02 |
| 4. | p-Hydroxybenzoic acid | 0.11 ± 0.01 | 0.39 ± 0.16 | 0.23 ± 0.02 |
| 5. | Chlorogenic acid (3-O-caffeoylquinic acid) | 17.69 ± 0.00 | 9.17 ± 0.05 | 4.48 ± 0.02 |
| 6. | Cryptochlorogenic acid (4-O-caffeoylquinic acid) | 11.04 ± 0.01 | 6.16 ± 0.03 | 3.10 ± 0.01 |
| 7. | Caffeic acid | 0.52 ± 0.00 | 0.18 ± 0.00 | 0.20 ± 0.00 |
| 8. | Ferulic acid | 0.30 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.00 |
| 9. | Sinapic acid | 0.08 ± 0.01 | tr | 0.02 ± 0.00 |
| Flavonoids | ||||
| 10. | Quercetin | 1.55 ± 0.06 | 2.01 ± 0.03 | 0.97 ± 0.14 |
| 11. | Rutin | 0.53 ± 0.01 | 0.47 ± 0.01 | 0.37 ± 0.02 |
| 12. | Myricetin | 0.44 ± 0.02 | 0.09 ± 0.02 | 0.14 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Kovačević, K.; Urlić, B.; Generalić Mekinić, I. Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters. Plants 2023, 12, 214. https://doi.org/10.3390/plants12010214
Politeo O, Popović M, Veršić Bratinčević M, Kovačević K, Urlić B, Generalić Mekinić I. Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters. Plants. 2023; 12(1):214. https://doi.org/10.3390/plants12010214
Chicago/Turabian StylePoliteo, Olivera, Marijana Popović, Maja Veršić Bratinčević, Kristina Kovačević, Branimir Urlić, and Ivana Generalić Mekinić. 2023. "Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters" Plants 12, no. 1: 214. https://doi.org/10.3390/plants12010214
APA StylePoliteo, O., Popović, M., Veršić Bratinčević, M., Kovačević, K., Urlić, B., & Generalić Mekinić, I. (2023). Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters. Plants, 12(1), 214. https://doi.org/10.3390/plants12010214










