Phenotypic Diversity in Pre- and Post-Attachment Resistance to Striga hermonthica in a Core Collection of Rice Germplasms
Abstract
1. Introduction
2. Results
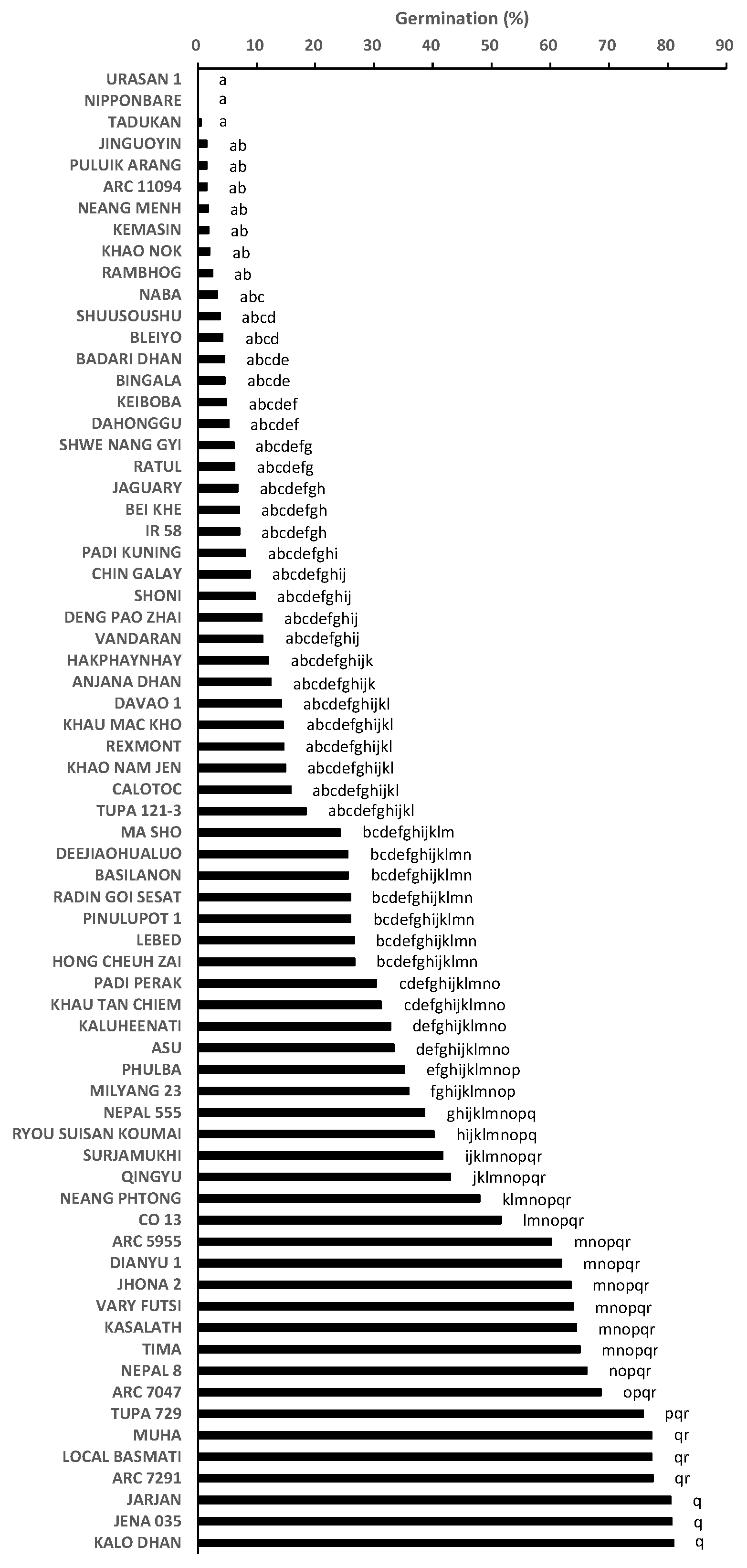
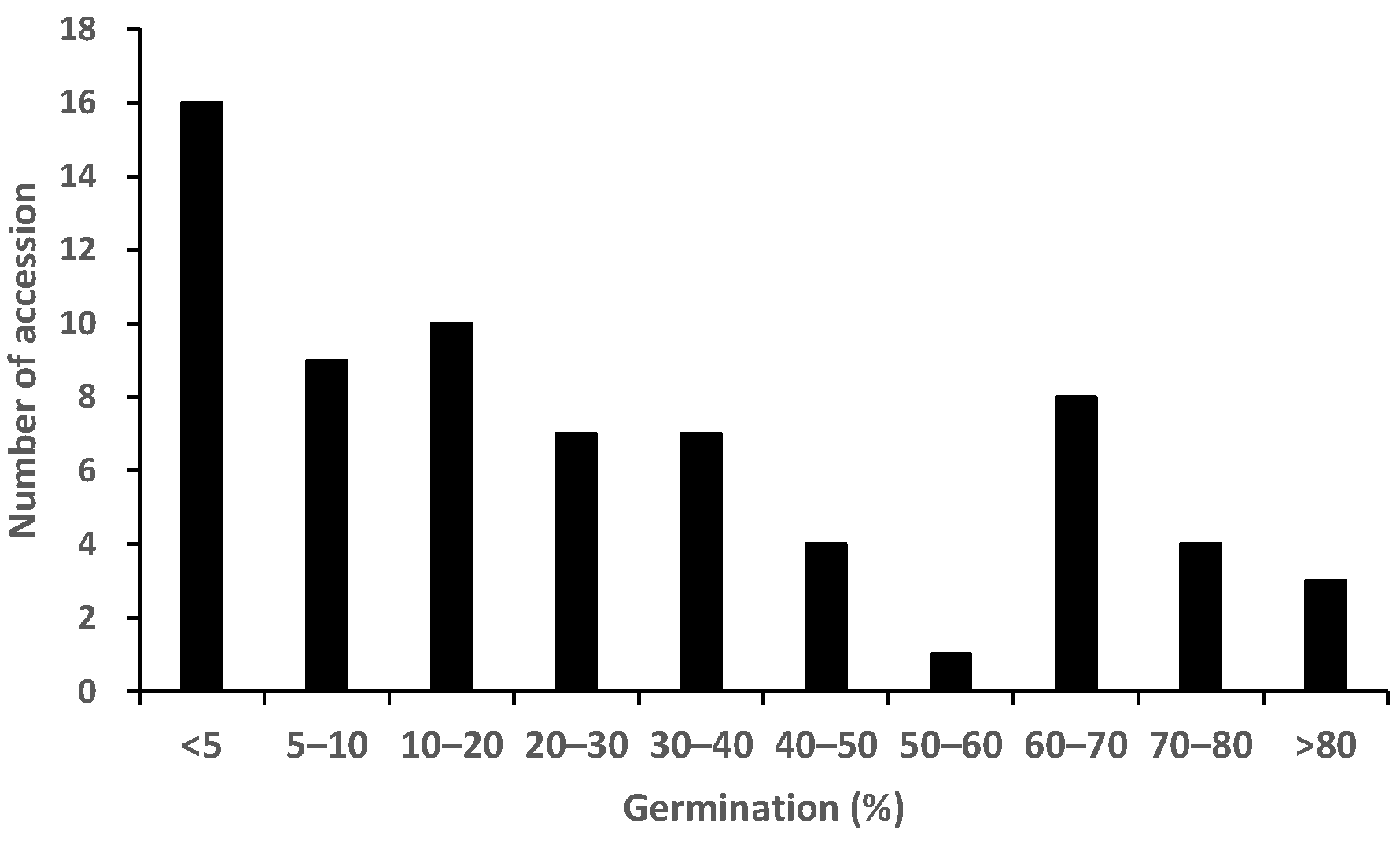
2.1. Germination Test to Phenotype for Pre-Attachment Resistance
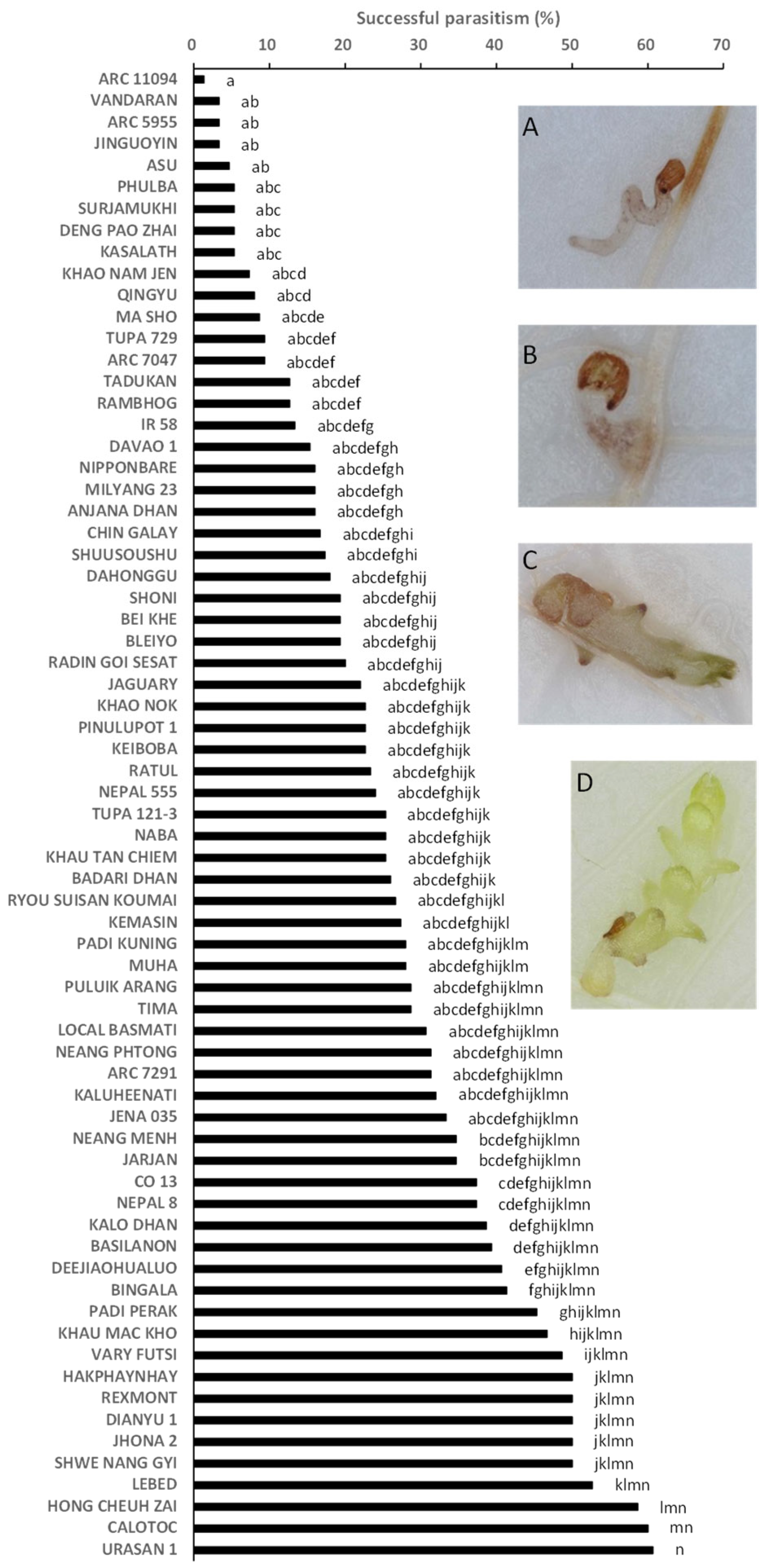
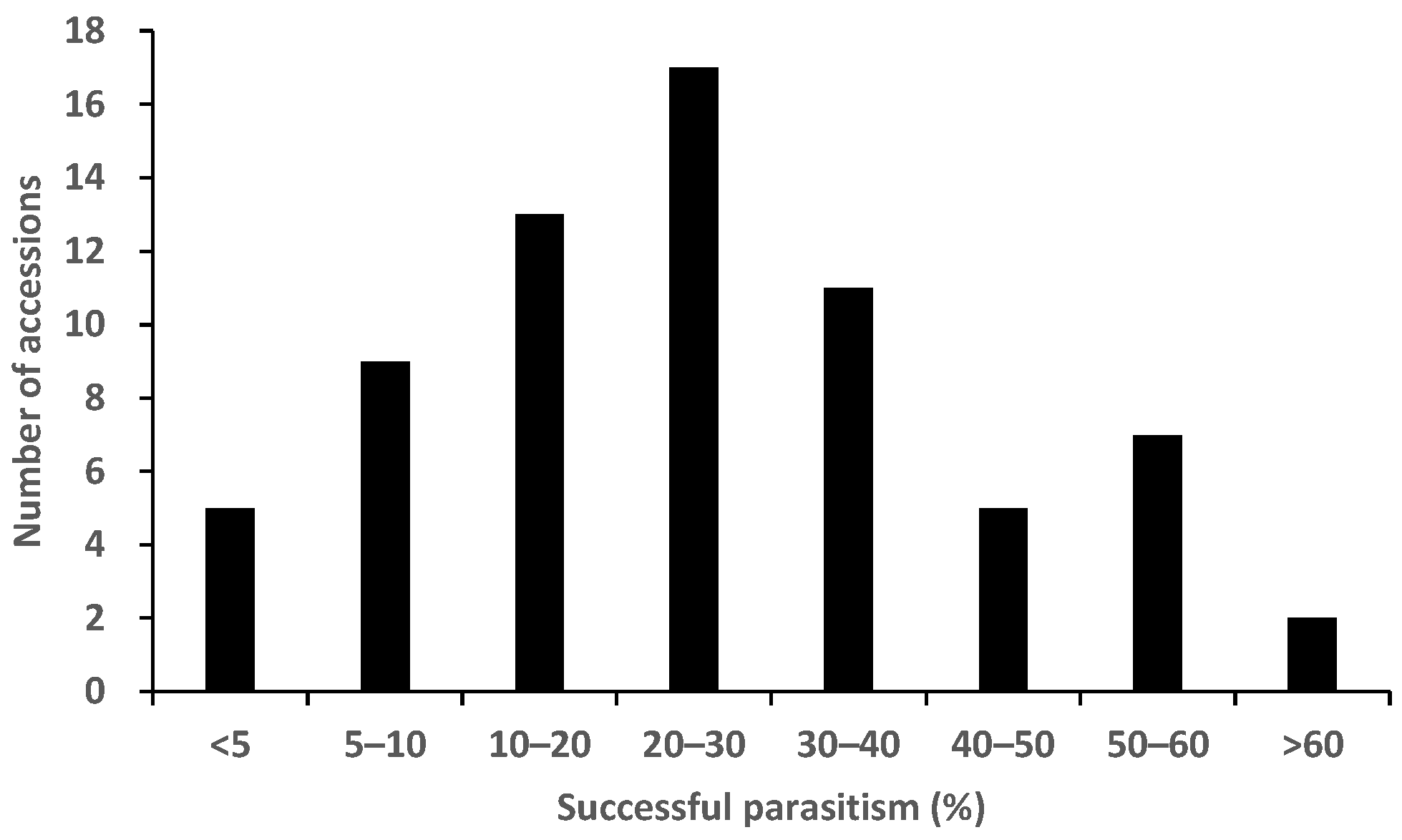
2.2. Rhizotron Evaluation to Phenotype for Post-Attachment Resistance
2.3. Clustering of the Accessions Based on Pre- and Post-Attachment Resistance
3. Discussion
3.1. Differences among Accessions in Pre- and Post-Attachment Resistance
3.2. Combination of Pre- and Post-Attachment Resistance in Each Accession
4. Materials and Methods
4.1. Plant Materials
4.2. Germination-Inducing Activity of Root Exudates
4.3. Rhizotron Experiment
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.G. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, M.C.S. Introduction. In Realizing Africa’s Rice Promise; Wopereis, M.C.S., Johnson, D.E., Ahmadi, N., Tollens, E., Jalloh, A., Eds.; CABI: Wallingford, UK, 2013; pp. xix–xxii. [Google Scholar]
- Rodenburg, J.; Tippe, D.E.; Touré, A.; Irakiza, R.; Kayeke, J.; Bastiaans, L. From rice-like plants to plants liking rice: A review of research on weeds and their management in African rice systems. Field Crop. Res. 2022, 276, 108397. [Google Scholar] [CrossRef]
- Ejeta, G. The Striga scourge in Africa: A growing pandemic. In Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt; Ejeta, G., Gressel, J., Eds.; World Scientific Publishing: Singapore, 2007; pp. 3–16. [Google Scholar] [CrossRef]
- Raturi, D.; Chaudhary, M.; Bhat, V.; Goel, S.; Raina, S.N.; Rajpal, V.R.; Singh, A. Overview of developed core and mini core collections and their effective utilization in cultivated rice and its related species (Oryza sp.)—A review. Plant Breed. 2022, 141, 501–512. [Google Scholar] [CrossRef]
- Tanaka, N.; Shenton, M.; Kawahara, K.; Kumagai, M.; Sakai, H.; Kanamori, H.; Yonemaru, J.; Fukuoka, S.; Sugimoto, K.; Ishimoto, M.; et al. Whole-genome sequencing of the NARO World Rice Core Collection (WRC) as the basis for diversity and association studies. Plant Cell Physiol. 2020, 61, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.R.; Shirasu, K. How to resist parasitic plants: Pre- and post-attachment strategies. Curr. Opin. Plant Biol. 2021, 62, 102004. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Matusova, R.; Cardoso, C.; Jamil, M.; Charnikhova, T.; Kohlen, W.; Ruyter-Spira, C.; Verstappen, F.; Bouwmeester, H. Strigolactones: Ecological significance and use as a target for parasitic plant control. Pest Manag. Sci. 2009, 65, 471–477. [Google Scholar] [CrossRef]
- Jamil, M.; Rodenburg, J.; Charnikhova, T.; Bouwmeester, H.J. Pre-attachment Striga hermonthica resistance of new rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol. 2011, 192, 964–975. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 4471–4476. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gurney, A.L.; Slate, J.; Press, M.C.; Scholes, J.D. A novel form of resistance in rice to the angiosperm parasite Striga hermonthica. New Phytol. 2006, 169, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, P.J.; Huang, K.; Liu, G.; Slate, J.; Press, M.C.; Scholes, J.D. Global patterns of gene expression in rice cultivars undergoing a susceptible or resistant interaction with the parasitic plant Striga hermonthica. New Phytol. 2008, 179, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, J.M.; Cui, S.; Hori, C.; Takeda, Y.; Tobimatsu, Y.; Nakabayashi, R.; Mori, T.; Saito, K.; Demura, T.; Umezawa, T.; et al. The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiol. 2019, 179, 1796–1809. [Google Scholar] [CrossRef]
- Samejima, H.; Babiker, A.G.; Mustafa, A.; Sugimoto, Y. Identification of Striga hermonthica-resistant upland rice varieties in Sudan and their resistance phenotypes. Front. Plant Sci. 2016, 7, 634. [Google Scholar] [CrossRef]
- Kojima, Y.; Ebana, K.; Fukuoka, S.; Nagamine, T.; Kawase, M. Development of an RFLP-based rice diversity set of germplasm. Breed. Sci. 2005, 55, 431–440. [Google Scholar] [CrossRef]
- Huang, K.; Whitlock, R.; Press, M.C.; Scholes, J.K. Variation for host range within and among populations of the parasitic plant Striga hermonthica. Heredity 2012, 108, 96–104. [Google Scholar] [CrossRef]
- Bebawi, F.F. Intraspecific physiological variants of Striga hermonthica. Exp. Agric. 1981, 17, 419–423. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Ota, T.; Berqdar, L.; Traore, H.; Margueritte, O.; Zwanenburg, B.; Asami, T.; Al-Babili, S. Striga hermonthica suicidal germination activity of potent strigolactone analogs: Evaluation from laboratory bioassays to field trials. Plants 2022, 11, 1045. [Google Scholar] [CrossRef]
- Kavuluko, J.; Kibe, M.; Sugut, I.; Kibet, W.; Masanga, J.; Mutinda, S.; Wamalwa, M.; Magomere, T.; Odeny, D.; Runo, S. GWAS provides biological insights into mechanisms of the parasitic plant (Striga) resistance in sorghum. BMC Plant Biol. 2021, 21, 392. [Google Scholar] [CrossRef]
- Mallu, T.S.; Irafasha, G.; Mutinda, S.; Owuor, E.; Githiri, S.M.; Odeny, D.A.; Runo, S. Mechanisms of pre-attachment Striga resistance in sorghum through genome-wide association studies. Mol. Genet. Genom. 2022, 297, 751–762. [Google Scholar] [CrossRef]
- Ebana, K.; Yonemaru, J.; Fukuoka, S.; Iwata, H.; Kanamori, H.; Namiki, N.; Nagasaki, H.; Yano, M. Genetic structure revealed by a whole-genome single-nucleotide polymorphism survey of diverse accessions of cultivated Asian rice (Oryza sativa L.). Breed Sci. 2010, 60, 390–397. [Google Scholar] [CrossRef]
- Timko, M.P.; Huang, K.; Lis, K.E. Host resistance and parasite virulence in Striga-host plant interaction: A Shifting balance of power. Weed Sci. 2012, 60, 307–315. [Google Scholar] [CrossRef]
- Matsunami, M.; Matsunami, T.; Kon, K.; Ogawa, A.; Kodama, I.; Kokubun, M. Genotypic variation in nitrogen uptake during early growth among rice cultivars under different soil moisture regimes. Plant Prod. Sci. 2013, 16, 238–246. [Google Scholar] [CrossRef][Green Version]
- Fukui, K.; Ito, S.; Asami, T. Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol. Plant 2013, 6, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kgosi, R.L.; Zwanenburg, B.; Mwakaboko, A.S.; Murdoch, A.J. Strigolactone analogues induce suicidal seed germination of Striga spp. in soil. Weed Res. 2012, 52, 197–203. [Google Scholar] [CrossRef]
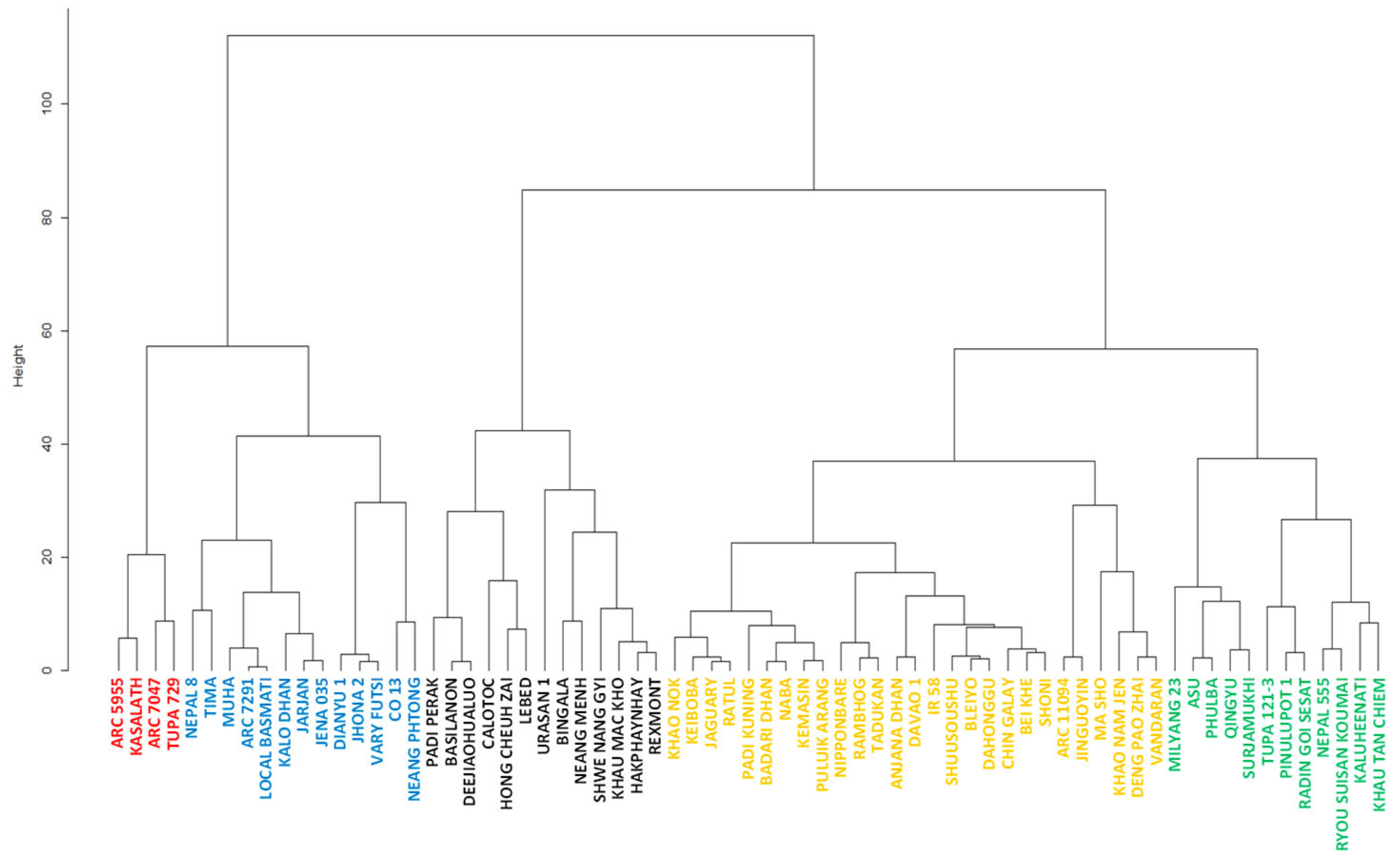
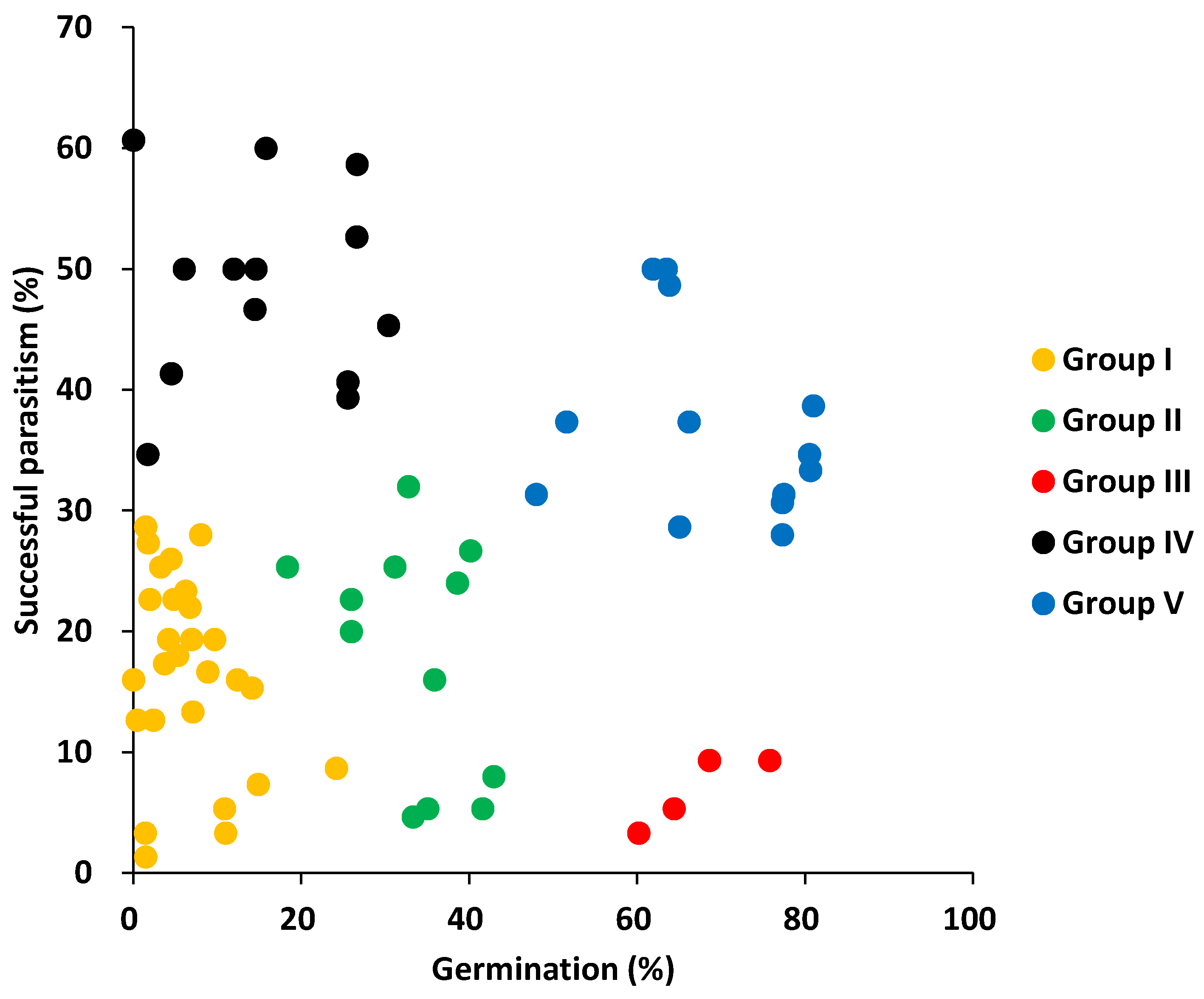
| Type | Group I | Group II | Group III | Group IV | Group V | Sum |
|---|---|---|---|---|---|---|
| japonica | 5 (35.7) | 2 (14.3) | 1 (7.1) | 4 (28.6) | 2 (14.3) | 14 (100) |
| indica I | 5 (22.7) | 4 (18.2) | 3 (13.6) | 2 (9.1) | 8 (36.4) | 22 (100) |
| indica II | 17 (51.5) | 6 (18.2) | 0 (0.0) | 7 (21.2) | 3 (9.1) | 33 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samejima, H.; Sugimoto, Y. Phenotypic Diversity in Pre- and Post-Attachment Resistance to Striga hermonthica in a Core Collection of Rice Germplasms. Plants 2023, 12, 19. https://doi.org/10.3390/plants12010019
Samejima H, Sugimoto Y. Phenotypic Diversity in Pre- and Post-Attachment Resistance to Striga hermonthica in a Core Collection of Rice Germplasms. Plants. 2023; 12(1):19. https://doi.org/10.3390/plants12010019
Chicago/Turabian StyleSamejima, Hiroaki, and Yukihiro Sugimoto. 2023. "Phenotypic Diversity in Pre- and Post-Attachment Resistance to Striga hermonthica in a Core Collection of Rice Germplasms" Plants 12, no. 1: 19. https://doi.org/10.3390/plants12010019
APA StyleSamejima, H., & Sugimoto, Y. (2023). Phenotypic Diversity in Pre- and Post-Attachment Resistance to Striga hermonthica in a Core Collection of Rice Germplasms. Plants, 12(1), 19. https://doi.org/10.3390/plants12010019






