Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales
Abstract
1. Introduction
2. Results
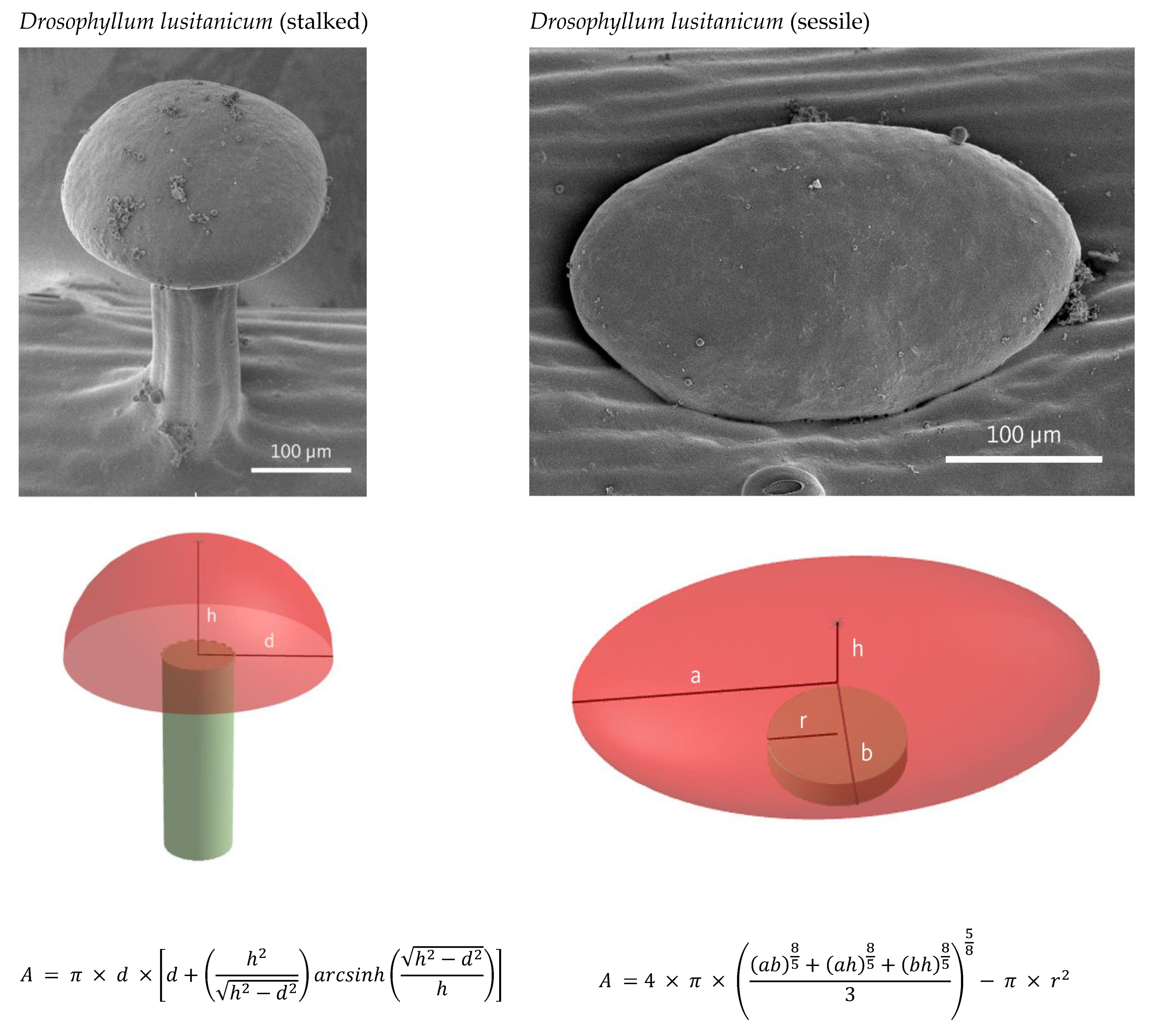
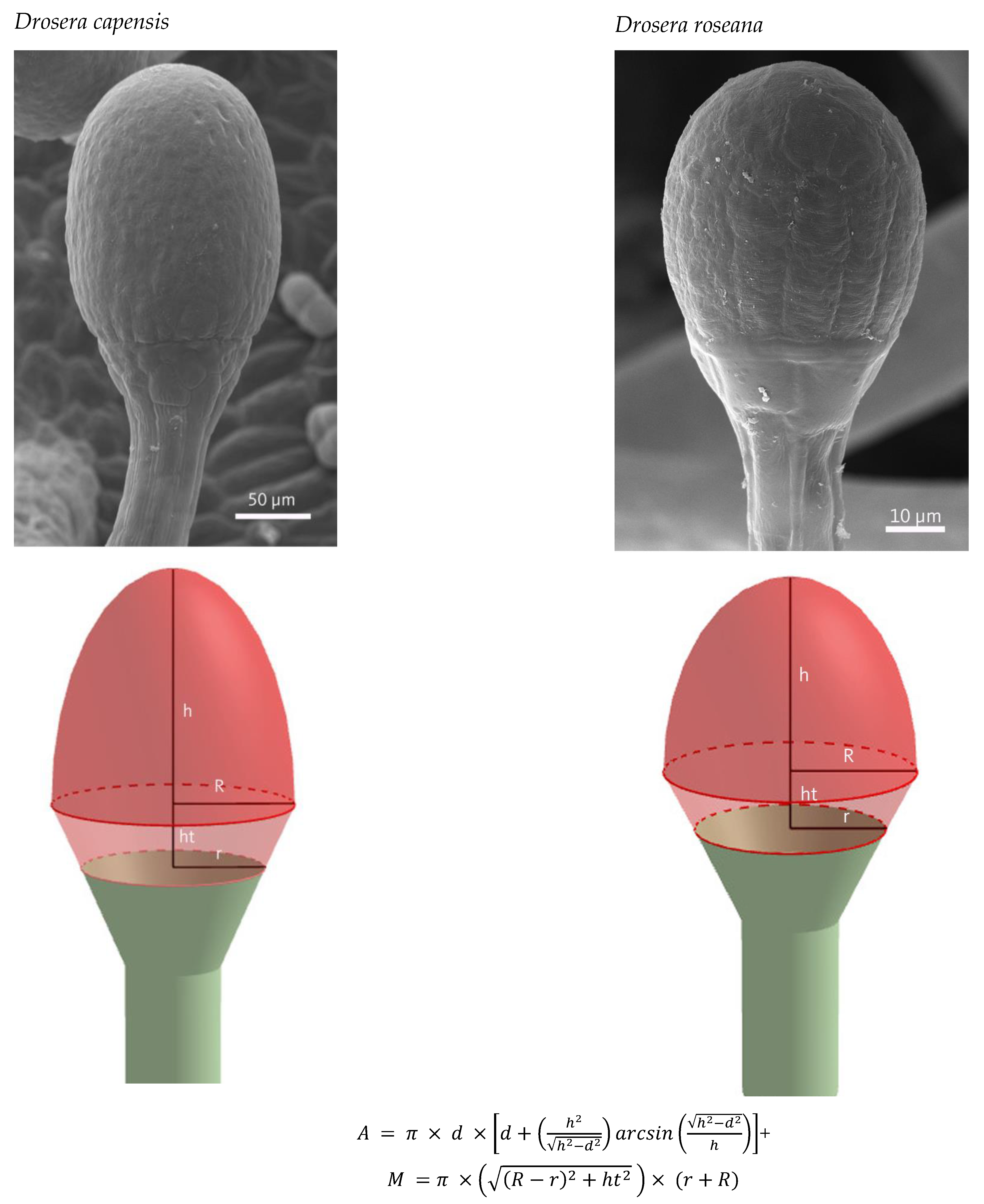
2.1. Quantification of Glandular Cells
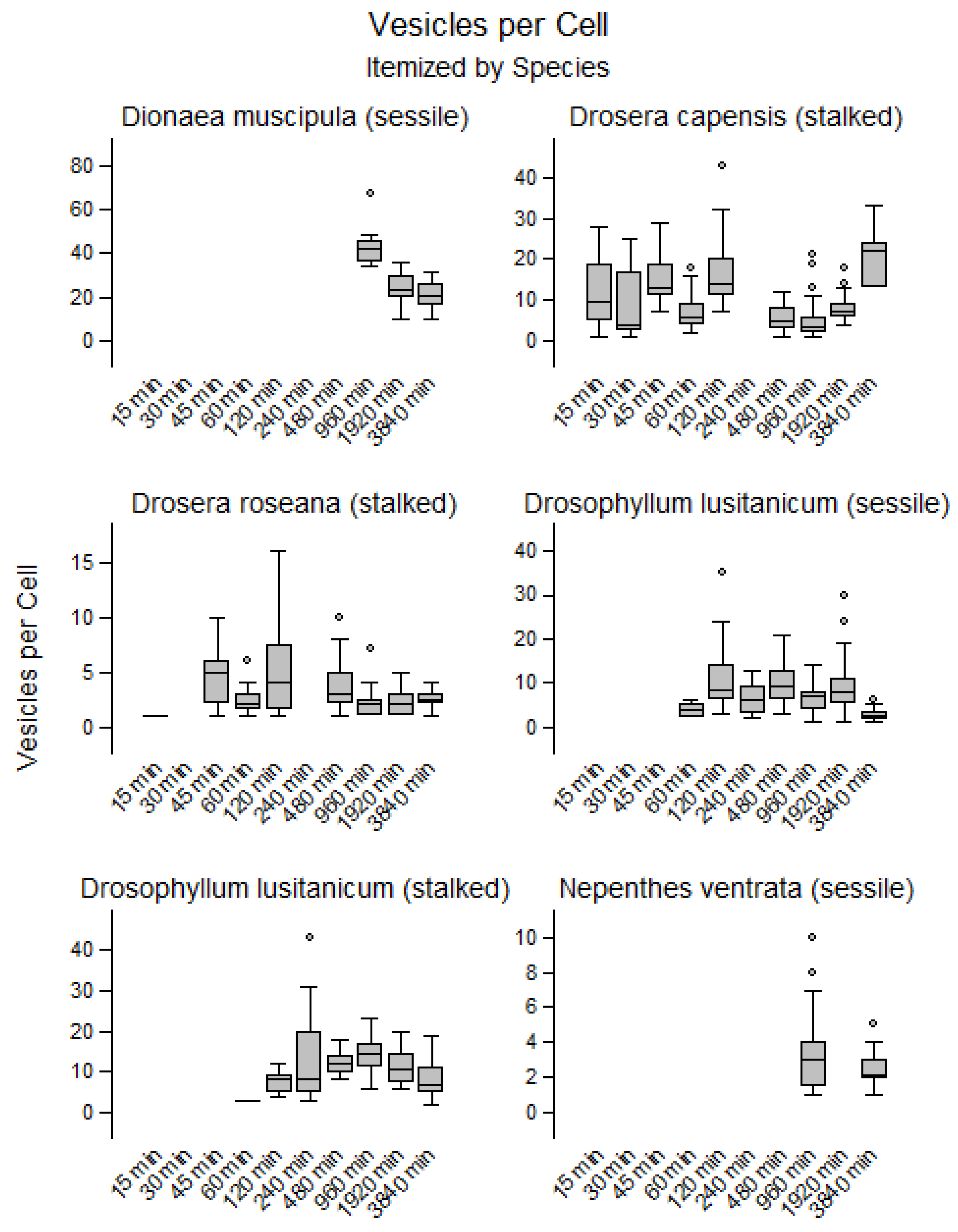
2.2. Endocytosis on the Cellular Level
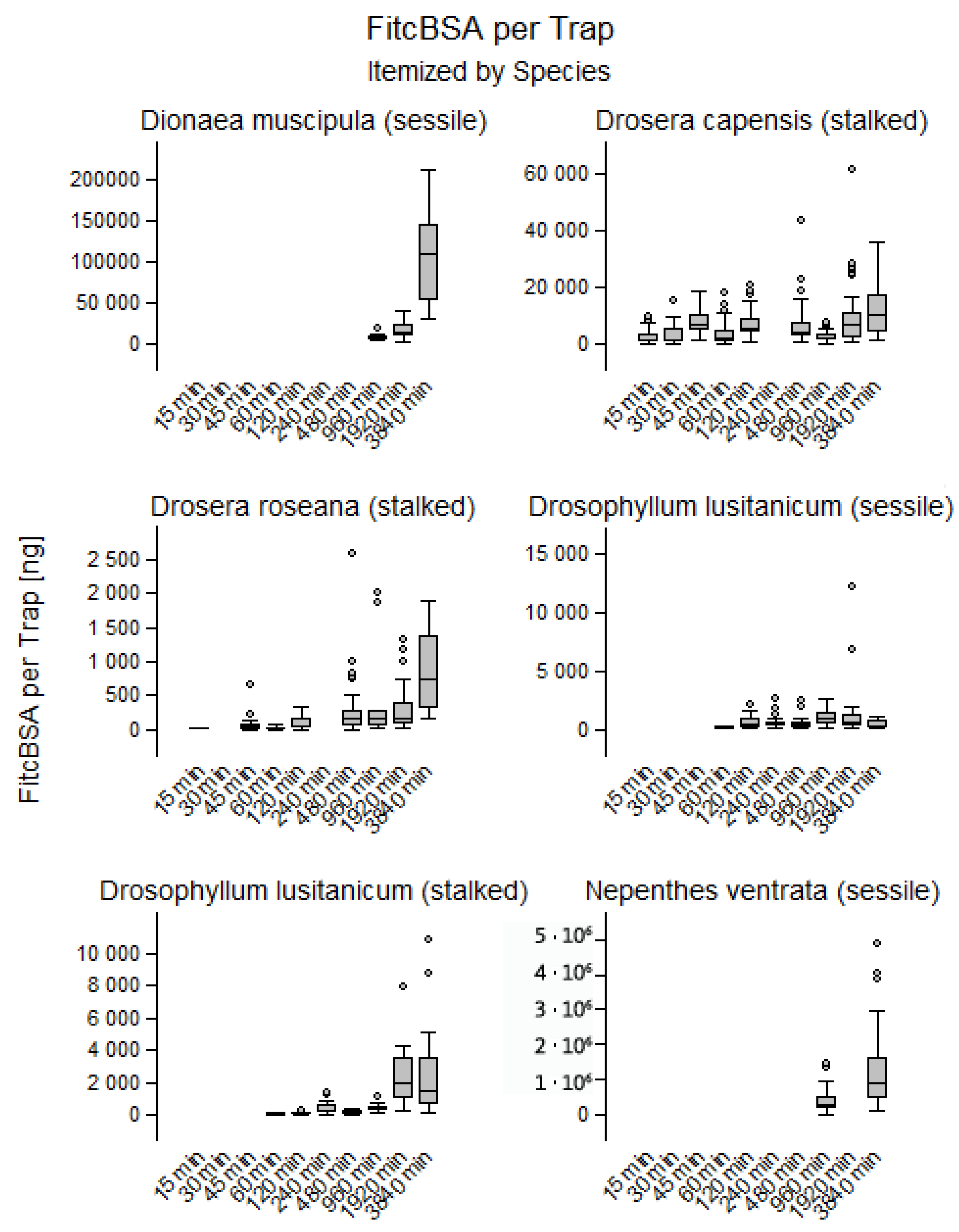
2.3. Quantification of Protein Uptake
3. Discussion
3.1. Endocytosis in Carnivorous Plants
3.2. Quantitative Assessment for Uptake via Endocytosis on a Cellular Level
3.3. Quantification of Endocytosis at Gland and Trap Level
3.4. Relevant N and S Nutrient Supply via Endocytosis
4. Materials and Methods
4.1. Experimental Setup
- Vesicle diameter
- Vesicle brightness on 0–256 greyscale values
- Number of vesicles per cell
4.2. Calculation of Cells per Trap Leaf/Digegestive Zone
4.3. Quantification of FITC-BSA Uptake
4.4. Statistics
5. Conclusions
- Endocytotic uptake of FITC-BSA starts almost immediately in adhesive traps but with a significant delay of up to 16 h in D. muscipula and N. × ventrata.
- Over time, FITC-BSA accumulates in glandular cells, either by fusion of small endocytotic vesicles to larger compartments, or, in D. capensis by increasing FITC-BSA concentration within the vesicles. However, the vesicular concentration of FITC-BSA does not exceed that in the external medium.
- After 64 h, estimated uptake per trap ranges from 263 ng in D. roseana to 915 µg in N. × ventrata, whereby most of the difference is explained by trap size.
- In D. lusitanicum sessile glands contribute very little to endocytotic nutrient uptake compared to the tentacles.
- Estimated uptake rates per trap range from 36 ng/d N in D. roseana to 63 µg/d in N. × ventrata. Differences between species are in accordance with the available information on prey dependency for nutrient supply.
- Endocytotic nutrient uptake seems to play an equally important role as uptake via carrier proteins in the observed species. Furthermore, endocytotic nutrient uptake requires a far less efficient digestion since whole protein molecules can be absorbed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellison, A.M.; Adamec, L. Carnivorous Plants: Physiology, Ecology, and Evolution; Oxford University Press: Oxford, UK, 2018; 510p. [Google Scholar]
- Juniper, B.E.; Robins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989; 353p. [Google Scholar]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The digestive systems of carnivorous plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A. Carnivorous plants and their biotic interactions. J. Plant Interact. 2022, 17, 333–343. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lendl, T. Traps of carnivorous pitcher plants as a habitat: Composition of the fluid, biodiversity and mutualistic activities. Ann. Bot. 2011, 107, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Adlassnig, W.; Steinhauser, G.; Peroutka, M.; Musilek, A.; Sterba, J.H.; Lichtscheidl, I.K.; Bichler, M. Expanding the menu for carnivorous plants: Uptake of Potassium, Iron and Manganese by carnivorous pitcher plants. Appl. Radiat. Isot. 2009, 67, 2117–2122. [Google Scholar] [CrossRef]
- Fabian-Galan, G.; Salageanu, N. Considerations on the nutrition of certain carnivorous plants (Drosera capensis and Aldrovanda vesiculosa). Rev. Romaine Biol. 1968, 13, 275–280. [Google Scholar]
- Adamec, L. Mineral nutrition of carnivorous plants: A Review. Bot. Rev. 1997, 63, 273–299. [Google Scholar] [CrossRef]
- Friday, L.; Quarmby, C. Uptake and translocation of prey-derived 15N and 32P in Utricularia vulgaris L. New Phytol. 1994, 126, 273–281. [Google Scholar] [CrossRef]
- Peroutka, M.; Adlassnig, W.; Lendl, T.; Pranjić, K.; Lichtscheidl, I.K. Functional Biology of Carnivorous Plants. In Floriculture Ornamental and Plant Biotechnology Advances and Topical Issues; Texeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume 5, pp. 266–286. [Google Scholar]
- Lloyd, F.E. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942; 352p. [Google Scholar]
- Schnepf, E. Zur Feinstruktur der Drüsen von Drosophyllum lusitanicum. Planta 1960, 54, 641–674. [Google Scholar] [CrossRef]
- Robins, R.J.; Juniper, B.E. The secretory cycle of Dionaea muscipula Ellis. V. The absorption of nutrients. New Phytol. 1980, 86, 413–422. [Google Scholar] [CrossRef]
- Schulze, W.; Frommer, W.B.; Ward, J.M. Transporters for Ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. Plant J. 1999, 17, 637–646. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Creelman, R.A. Mutualism between the carnivorous purple pitcher plant and its inhabitants. Am. Midl. Nat. 1984, 112, 294–304. [Google Scholar] [CrossRef]
- Plummer, G.L.; Kethley, J.B. Foliar absorption of amino acids, peptides, and other nutrients by the pitcher plant, Sarracenia flava. Bot. Gaz. 1964, 125, 245–260. [Google Scholar] [CrossRef]
- McWilliams, E.L. Evolutionary Ecology. In Bromeliaceae (Pitcairnioideae); Smith, L.B., Downs, R.J., Eds.; Hafner Press: New York, NY, USA, 1974; pp. 40–55. [Google Scholar]
- Juniper, B.E.; Gilchrist, A.J.; Robins, R.J. Some features of secretory systems in plants. Histochem. J. 1977, 9, 659–680. [Google Scholar] [CrossRef]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Koller-Peroutka, M.; Krammer, S.; Pavlik, A.; Edlinger, M.; Lang, I.; Adlassnig, W. Endocytosis and digestion in carnivorous pitcher plants of the Family Sarraceniaceae. Plants 2019, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of Carnivory in Angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 1, pp. 22–42. [Google Scholar]
- Wu, L.-G.; Hamid, E.; Shin, W.; Chiang, H.-C. Exocytosis and endocytosis: Modes, functions, and coupling mechanisms. Annu. Rev. Physiol. 2014, 76, 301–331. [Google Scholar] [CrossRef]
- Paez Valencia, J.; Goodman, K.; Otegui, M.S. Endocytosis and endosomal trafficking in plants. Annu. Rev. Plant Biol. 2016, 67, 309–335. [Google Scholar] [CrossRef]
- Battey, N.H.; James, N.C.; Greenland, A.J.; Brownlee, C. Exocytosis and endocytosis. Plant Cell 1999, 11, 643–659. [Google Scholar] [CrossRef]
- Dong-Hui, W.; Dong-Qi, W.; Yi-Wei, C.; Lu, Y.; Xiao-Di, G.; Wen-Fei, S.; Feng, L. Photoperiod regulates cape sundew (Drosera capensis) gland secretion and leaf development. Carniv. Plant Newsl. 2015, 44, 197–203. [Google Scholar] [CrossRef]
- Pavlovič, A.; Krausko, M.; Libiaková, M.; Adamec, L. Feeding on prey increases photosynthetic efficiency in the carnivorous sundew Drosera capensis. Ann. Bot. 2014, 113, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Adamec, L. Ecophysiological investigation on Drosophyllum lusitanicum: Why doesn’t the plant dry out? Carniv. Plant Newsl. 2009, 38, 71–74. [Google Scholar] [CrossRef]
- Darwin, C. Insectivorous Plants; John Murray: London, UK, 1875; 462p. [Google Scholar]
- Escalante-Pérez, M.; Krol, E.; Stange, A.; Geiger, D.; Al-Rasheid, K.A.S.; Hause, B.; Neher, E.; Hedrich, R. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus Flytrap. Proc. Natl. Acad. Sci. USA 2011, 108, 15492–15497. [Google Scholar] [CrossRef] [PubMed]
- Devecka, A. Diversität der Nepenthes-Falle unter Besonderer Berücksichtigung der Oberflächenstrukturen und Ihrer Rolle beim Beutefang. Diploma Thesis, Technical University Zvolen, Zvolen, Slovakia, 2007. [Google Scholar]
- Heslop-Harrison, Y. Enzyme Secretion and Uigest Uptake in Carnivorous Plants; Sunderland, N., Ed.; Pergamon Press: Oxford, UK, 1976; Volume 2, pp. 463–476. [Google Scholar]
- Błedzki, L.; Ellison, A. Population growth and production of Habrotrocha rosa Donner (Rotifera: Bdelloidea) and its contribution to the nutrient supply of its host, the Northern pitcher plant, Sarracenia purpurea L. (Sarraceniaceae). Hydrobiologia 1998, 1, 193–200. [Google Scholar] [CrossRef]
- Schulze, W.; Schulze, E.D.; Schulze, I.; Oren, R. Quantification of insect Nitrogen utilization by the Venus Fly Trap Dionaea muscipula catching prey with highly variable isotope signatures. J. Exp. Bot. 2001, 52, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.; Schulze, E.D.; Pate, J.S.; Gillison, A.N. The Nitrogen Supply from Soils and Insects during Growth of the Pitcher Plants Nepenthes Mirabilis, Cephalotus Follicularis and Darlingtonia Californica. Oecologia 1997, 112, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E. Karnivore Kesselfallenpflanzen—Untersuchungen zur Verdauung, Nährstoffaufnahme und Mikroflora. Diploma Thesis, University of Vienna, Vienna, Austria, 2005. [Google Scholar]
- Kresta, S.M.; Etchells, A.W.; Dickey, D.S.; Atiemo-Obeng, V.A. Advances in Industrial Mixing: A Companion to the Handbook of Industrial Mixing; John Wiley & Sons: Hoboke, NJ, USA, 2016; 524p. [Google Scholar]
- IonSource. Available online: https://www.ionsource.com/Card/protein/BovineSerumAlbumin.htm (accessed on 26 November 2022).
- Hirayama, K.; Akashi, S.; Furuya, M.; Fukuhara, K. Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun. 1990, 173, 639–646. [Google Scholar] [CrossRef]
- Merck. Available online: http://www.sigmaaldrich.com/AT/de/product/sigma/a9771 (accessed on 26 November 2022).


| Drosophyllum lusitanicum | Drosera capensis | Drosera roseana | Dionaea muscipula | Nepenthes × ventrata | ||
|---|---|---|---|---|---|---|
| Gland types | sessile | stalked | stalked | stalked | sessile | sessile |
| Gland Area [µm2] | 26,294 ± 14,708 | 94,534 ± 57,135 | 46,576 ± 11,955 | 10,232 ± 9108 | ||
| Cell Area [µm2] | 570 ± 106 | 545 ± 100 | 223 ± 61 | 288 ± 71 | ||
| Cells per Gland | 46 ± 65 | 174 ± 9 | 208 ± 47 | 36 ± 17 | 24 ± 6 | 164 ± 53 |
| Area DZ [mm2] | 12 ± 2 | 27 ± 2 | 427 ± 70 | 4161 ± 831 | ||
| Glands/DZ 1 | 41 ± 5 | 21 ± 2 | 225 ± 19 | 107 ± 36 | 5213 ± 1101 | 19,139 ± 3128 |
| Cells/DZ 1 | 1891 ± 1149 | 3711 ± 265 | 46,864 ± 6103 | 3817 ± 1319 | 126,675 ± 25,277 | 3,130,519 ± 635,483 |
| Drosophyllum lusitanicum | Drosera capensis2 | Drosera roseana2 | Dionaea muscipula1 | Nepenthes × ventrata 1 | ||
|---|---|---|---|---|---|---|
| Gland types | sessile | stalked | stalked | stalked | sessile | sessile |
| Sample size | 56 | 242 | 209 | 24 | 420 | 121 |
| Volume per Cell [µm3] | 11.2 ± 20.1 | 119.6 ± 240.1 | 18.6 ± 24.5 | 21.1 ± 21.9 | 115 ± 67.3 | 53.5 ± 43.6 |
| FITC-BSA per Cell [ng] | 0.1 ± 0.1 | 0.7 ± 1.2 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.7 ± 0.4 | 0.3 ± 0.3 |
| Volume per Gland [µm3] | 1940± 3485 | 5517 ± 11075 | 3879 ± 5099 | 748 ± 777 | 2810 ± 1644 | 8747 ± 7135 |
| FITC-BSA per Gland [ng] | 12.3 ± 23.1 | 30.1 ± 55.1 | 39.9 ± 55.2 | 5.9 ± 6.3 | 17.5 ± 10.7 | 47.8 ± 44.3 |
| Volume per DZ [µm3] | 41,489 ± 74,525 | 226,159 ± 454,008 | 871,926 ± 1146,124 | 80,352 ± 83,406 | 14,648,188 ± 8,570,270 | 167,406,304 ± 136,562,528 |
| FITC-BSA per DZ [ng] | 263 ± 495 | 1235 ± 2257 | 8969± 12,396 | 632 ± 682 | 91,260 ± 55,964 | 915,266 ± 847,190 |
| Nitrogen 1 per DZ [ng/day] | 12 (−21–44) | 135 (94–176) | 475 (295–654) | 36 (24–48) | 8338 (6468–10,170) | 63,561 (35,893–91,603) |
| Sulfur 2 per DZ [ng/day] | 1 (−2–5) | 15 (11–20) | 54 (34–75) | 4 (3–6) | 954 (740–1164) | 7274 (4108–10,483) |
| Trap Type | 15 min–32 h | 64 h |
|---|---|---|
| Adhesive traps (Drosera capensis, Drosera roseana and Drosophyllum lusitanicum) | Staining | |
|
| |
| Preparation for microscopy | ||
| ||
| Pitcher traps (Nepenthes × ventrata) | Staining | |
|
| |
| Preparation for microscopy | ||
| ||
| Snap traps (Dionaea muscipula) | Staining | |
|
| |
| Preparation for microscopy | ||
| ||
| Species | 15 min | 30 min | 45 min | 1 h | 2 h | 4 h | 8 h | 16 h | 32 h | 64 h |
|---|---|---|---|---|---|---|---|---|---|---|
| Drosera capensis | X | X | X | X | X | X | X | X | X | X |
| Drosera roseana | X | X | X | X | X | X | X | X | X | X |
| Drosophyllum lusitanicum | X | X | X | X | X | X | X | X | X | X |
| Nepenthes × ventrata | X | X | X | X | X | X | ||||
| Dionaea muscipula | X | X | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivesic, C.; Krammer, S.; Koller-Peroutka, M.; Laarouchi, A.; Gruber, D.; Lang, I.; Lichtscheidl, I.K.; Adlassnig, W. Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants 2023, 12, 341. https://doi.org/10.3390/plants12020341
Ivesic C, Krammer S, Koller-Peroutka M, Laarouchi A, Gruber D, Lang I, Lichtscheidl IK, Adlassnig W. Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants. 2023; 12(2):341. https://doi.org/10.3390/plants12020341
Chicago/Turabian StyleIvesic, Caroline, Stefanie Krammer, Marianne Koller-Peroutka, Aicha Laarouchi, Daniela Gruber, Ingeborg Lang, Irene K. Lichtscheidl, and Wolfram Adlassnig. 2023. "Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales" Plants 12, no. 2: 341. https://doi.org/10.3390/plants12020341
APA StyleIvesic, C., Krammer, S., Koller-Peroutka, M., Laarouchi, A., Gruber, D., Lang, I., Lichtscheidl, I. K., & Adlassnig, W. (2023). Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants, 12(2), 341. https://doi.org/10.3390/plants12020341







