The Effect of Environmental Factors on the Nutrition of European Beech (Fagus sylvatica L.) Varies with Defoliation
Abstract
1. Introduction
2. Results
2.1. Foliar Nutrient Concentrations
2.2. Foliar Nutrient Ratios
2.3. Effects of Environmental Factors on Differences in Foliar Nutrition
3. Discussion
4. Materials and Methods
4.1. Study Area and Plot Design
4.2. Climate Data
4.3. Tree Selection Procedures, Foliar Sampling and Analysis
4.4. Soil Sampling and Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centritto, M.; Tognetti, R.; Leitgeb, E.; Střelcová, K.; Cohen, S. Above Ground Processes: Anticipating Climate Change Influences. In Forest Management and the Water Cycle: An Ecosystem-Based Approach; Bredemeier, M., Cohen, S., Godbold, D.L., Lode, E., Pichler, V., Schleppi, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 31–64. ISBN 978-90-481-9834-4. [Google Scholar]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest Health and Global Change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S.; et al. Technical Summary. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- de Vries, W.; Dobbertin, M.H.; Solberg, S.; van Dobben, H.F.; Schaub, M. Impacts of Acid Deposition, Ozone Exposure and Weather Conditions on Forest Ecosystems in Europe: An Overview. Plant Soil 2014, 380, 1–45. [Google Scholar] [CrossRef]
- Ferretti, M.; Waldner, P.; Verstraeten, A.; Schmitz, A.; Michel, A.; Žlindra, D.; Marchetto, A.; Hansen, K.; Pitar, D.; Gottardini, E.; et al. Criterion 2: Maintenance of Forest EcosystemHealth and Vitality. In Forest Europe, 2020: State of Europe’s Forests 2020; Ministerial Conference on the Protection of Forests in Europe—Liaison Unit Bratislava: Zvolen, Slovak Republic, 2020. [Google Scholar]
- Zierl, B. A Simulation Study to Analyse the Relations between Crown Condition and Drought in Switzerland. For. Ecol. Manag. 2004, 188, 25–38. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Beniston, M.; Stephenson, D.B.; Christensen, O.B.; Ferro, C.A.T.; Frei, C.; Goyette, S.; Halsnaes, K.; Holt, T.; Jylhä, K.; Koffi, B.; et al. Future Extreme Events in European Climate: An Exploration of Regional Climate Model Projections. Clim. Change 2007, 81, 71–95. [Google Scholar] [CrossRef]
- Marschner, H. 9—Functions of Mineral Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 313–404. ISBN 978-0-12-473543-9. [Google Scholar]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree Mineral Nutrition Is Deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Hallenbarter, D.; Landolt, W.; Bucher, J. Nutrition and Vitality: Phenological Investigation in Forest under Different Fertiliser Treatments. In Trends in European Forest Tree Physiology Research: Cost Action E6: EUROSILVA; Springer: Dordrecht, The Netherlands, 2001; pp. 9–12. [Google Scholar]
- Kreuzwieser, J.; Gessler, A. Global Climate Change and Tree Nutrition: Influence of Water Availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef]
- Talkner, U.; Meiwes, K.J.; Potočić, N.; Seletković, I.; Cools, N.; De Vos, B.; Rautio, P. Phosphorus Nutrition of Beech (Fagus sylvatica L.) Is Decreasing in Europe. Ann. For. Sci. 2015, 72, 919–928. [Google Scholar] [CrossRef]
- Potočić, N.; Timmermann, V.; Ognjenović, M.; Kirchner, T.; Prescher, A.K.; Ferretti, M. Tree Health Is Deteriorating in the European Forests; ICP Forests: 2021; Programme Coordinating Centre of ICP Forests, Thünen, Institute of Forest Ecosystems: Eberswalde, Germany, 2021. [Google Scholar] [CrossRef]
- Pernar, N.; Bakšić, D. The Soils of Beech Forests. In Common Beech (Fagus sylvatica L.) in Croatia; Matić, S., Ed.; Academy of Forestry Sciences: Zagreb, Croatia, 2003; ISBN 953-98571-1-2. [Google Scholar]
- Dobbertin, M.; Brang, P. Crown Defoliation Improves Tree Mortality Models. For. Ecol. Manag. 2001, 141, 271–284. [Google Scholar] [CrossRef]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree Vitality and Forest Health: Can Tree-Ring Stable Isotopes Be Used as Indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- De Vries, W.; Klap, J.M.; Erisman, J.W. Effects of Environmental Stress on Forest Crown Condition in Europe. Part I: Hypotheses and Approach to the Study. Water. Air. Soil Pollut. 2000, 119, 317–333. [Google Scholar] [CrossRef]
- De Marco, A.; Proietti, C.; Cionni, I.; Fischer, R.; Screpanti, A.; Vitale, M. Future Impacts of Nitrogen Deposition and Climate Change Scenarios on Forest Crown Defoliation. Environ. Pollut. 2014, 194, 171–180. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A Multi-Proxy Approach Reveals Common and Species-Specific Features Associated with Tree Defoliation in Broadleaved Species. For. Ecol. Manag. 2020, 467, 118151. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schroeck, H.-W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. Version 2020-3. In Manual on Methods and Criteria For harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 49. ISBN 978-3-86576-162-0. [Google Scholar]
- Lorenz, M. International Co-Operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests-ICP Forests. Water. Air. Soil Pollut. 1995, 85, 1221–1226. [Google Scholar] [CrossRef]
- Timmermann, V.; Potočić, N.; Ognjenović, M.; Kirchner, T. Tree Crown Condition in 2021. Forest Condition in Europe: The 2022 Assessment. ICP Forests technical Report under the UNECE Convention on Long-Range Transboundary Air Pollution (Air Convention); Thünen Institute: Eberswalde, Germany, 2022; pp. 41–55. [Google Scholar]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Seidling, W. Signals of Summer Drought in Crown Condition Data from the German Level I Network. Eur. J. For. Res. 2007, 126, 529–544. [Google Scholar] [CrossRef]
- Seletković, I.; Potočić, N.; Ugarković, D.; Jazbec, A.; Pernar, R.; Seletković, A.; Benko, M. Climate and Relief Properties Influence Crown Condition of Common Beech (Fagus sylvatica L.) on the Medvednica Massif. Period. Biol. 2009, 111, 435–441. [Google Scholar]
- Ognjenović, M.; Seletković, I.; Potočić, N.; Marušić, M.; Tadić, M.P.; Jonard, M.; Rautio, P.; Timmermann, V.; Lovreškov, L.; Ugarković, D. Defoliation Change of European Beech (Fagus Sylvatica L.) Depends on Previous Year Drought. Plants 2022, 11, 730. [Google Scholar] [CrossRef]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus Sylvatica L.) in a Changing Climate. Trees-Struct. Funct. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Seletković, Z.; Tikvić, I.; Prpić, B. Ekološka Konstitucija Obične Bukve. In Obična Bukva (Fagus sylvatica L.) u Hrvatskoj; Matić, S., Ed.; Akademija Šumarskih Znanosti: Zagreb, Croatia, 2003. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef] [PubMed]
- Jonard, M.; André, F.; Giot, P.; Weissen, F.; Van der Perre, R.; Ponette, Q. Thirteen-Year Monitoring of Liming and PK Fertilization Effects on Tree Vitality in Norway Spruce and European Beech Stands. Eur. J. For. Res. 2010, 129, 1203–1211. [Google Scholar] [CrossRef]
- Thimonier, A.; Graf Pannatier, E.; Schmitt, M.; Waldner, P.; Walthert, L.; Schleppi, P.; Dobbertin, M.; Kräuchi, N. Does Exceeding the Critical Loads for Nitrogen Alter Nitrate Leaching, the Nutrient Status of Trees and Their Crown Condition at Swiss Long-Term Forest Ecosystem Research (LWF) Sites? Eur. J. For. Res. 2010, 129, 443–461. [Google Scholar] [CrossRef]
- Ferretti, M.; Calderisi, M.; Marchetto, A.; Waldner, P.; Thimonier, A.; Jonard, M.; Cools, N.; Rautio, P.; Clarke, N.; Hansen, K.; et al. Variables Related to Nitrogen Deposition Improve Defoliation Models for European Forests. Ann. For. Sci. 2015, 72, 897–906. [Google Scholar] [CrossRef]
- Toïgo, M.; Nicolas, M.; Jonard, M.; Croisé, L.; Nageleisen, L.M.; Jactel, H. Temporal Trends in Tree Defoliation and Response to Multiple Biotic and Abiotic Stresses. For. Ecol. Manag. 2020, 477, 118476. [Google Scholar] [CrossRef]
- Ognjenović, M.; Levanič, T.; Potočić, N.; Ugarković, D.; Indir, K.; Seletković, I. Interrelations of Various Tree Vitality Indicators and Their Reaction to Climatic Conditions on a European Beech (Fagus sylvatica L.) Plot. Šumarski List 2020, 144, 351–365. [Google Scholar] [CrossRef]
- Mellert, K.H.; Göttlein, A. Comparison of New Foliar Nutrient Thresholds Derived from van Den Burg’s Literature Compilation with Established Central European References. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Seletković, I.; Potočić, N. Stanje Ishrane Obične Bukve (Fagus sylvatica L.) i Obične Jele (Abies alba Mill.) Na Području Medvednice. Rad. Šum. Inst. Izvanredno Izd. 2006, 9, 117–125. [Google Scholar]
- Raitio, H. Chemical Needle Analysis as a Diagnostic and Monitoring Method. In Nutrient Uptake and Cycling in Forest Ecosystems; Nilsson, L.O., Hüttl, R.F., Johansson, U.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; Volume 197–202, ISBN 0792330307. [Google Scholar]
- Komlenović, N.; Gračan, J.; Pezdric, N.; Rastovski, P. Utjecaj Polutanata Na Bukove Šume i Kulture Smreke u Sjeverozapadnoj Hrvatskoj. Šumarski List 1988, 112, 12. [Google Scholar]
- Komlenović, N.; Matković, N.; Moćan, D.; Rastovski, P. Unos Onečišćenja Iz Zraka u Šumu Bukve i Jele (Abieti-Fagetum “Dinaricum”) u Predjelu Lividrage u Zapadnoj Hrvatskoj. Šumarski List 1997, 121, 8. [Google Scholar]
- Sauter, U. Zeitliche Variationen Des Ernährungszustands Nordbayerischer Kiefernbestände. Forstwissenschaftliches Cent. Ver. mit Tharandter Forstl. Jahrb. 1991, 110, 13–33. [Google Scholar] [CrossRef]
- Prietzel, J.; Kolb, E.; Rehfuess, K.E. Langzeituntersuchung Ehemals Streugenutzter Kiefernökosysteme in der Oberpfalz: Veränderungen von Bodenchemischen Eigenschaften und der Nährelementversorgung der Bestände. Forstwissenschaftliches Cent. Ver. mit Tharandter Forstl. Jahrb. 1997, 116, 269–290. [Google Scholar] [CrossRef]
- Stefan, K.; Fürst, A.; Hacker, R.; Bartels, U. Forest Foliar Condition in Europe. Results of Large-Scale Foliar Chemistry Surveys.; European Commission—United Nations/Economic Commission for Europe: Brussels, Belgium, 1997. [Google Scholar]
- Duquesnay, A.; Dupouey, J.-L.; Clement, A.; Ulrich, E.; Le Tacon, F. Spatial and Temporal Variability of Foliar Mineral Concentration in Beech (Fagus Sylvatica) Stands in Northeastern France. Tree Physiol. 2000, 20, 13–22. [Google Scholar] [CrossRef]
- Jonard, M.; André, F.; Dambrine, E.; Ponette, Q.; Ulrich, E. Temporal Trends in the Foliar Nutritional Status of the French, Walloon and Luxembourg Broad-Leaved Plots of Forest Monitoring. Ann. For. Sci. 2009, 66, 412. [Google Scholar] [CrossRef][Green Version]
- Amores, G.; Santamaría, J.M. Beech Foliar Chemical Composition: A Bioindicator of Air Pollution Stress. In Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 301–313. ISBN 1474-8177. [Google Scholar]
- Amores, G.; Bermejo, R.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Nutritional Status of Northern Spain Beech Forests. Water Air Soil Pollut. 2006, 177, 227–238. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J.; Ogaya, R.; Estiarte, M. Nutrient Stoichiometric Relations and Biogeochemical Niche in Coexisting Plant Species: Effect of Simulated Climate Change. Polish J. Ecol. 2008, 56, 613–622. [Google Scholar]
- Sardans, J.; Peñuelas, J. Tree Growth Changes with Climate and Forest Type Are Associated with Relative Allocation of Nutrients, Especially Phosphorus, to Leaves and Wood. Glob. Ecol. Biogeogr. 2013, 22, 494–507. [Google Scholar] [CrossRef]
- Simon, A.; Wild, A. Mineral Nutrients in Leaves and Bast of Pedunculate Oak (Quercus robur L.) at Different States of Defoliation. Chemosphere 1998, 36, 955–959. [Google Scholar] [CrossRef]
- Ouimet, R.; Moore, J.-D. Effects of Fertilization and Liming on Tree Growth, Vitality and Nutrient Status in Boreal Balsam Fir Stands. For. Ecol. Manag. 2015, 345, 39–49. [Google Scholar] [CrossRef]
- Ewald, J. Ecological Background of Crown Condition, Growth and Nutritional Status of Picea Abies (L.) Karst. in the Bavarian Alps. Eur. J. For. Res. 2005, 124, 9–18. [Google Scholar] [CrossRef]
- Potočić, N.; Ćosić, T.; Pilaš, I. The Influence of Climate and Soil Properties on Calcium Nutrition and Vitality of Silver Fir (Abies Alba Mill.). Environ. Pollut. 2005, 137, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Schindler, C.; Rihm, B. Foliar Nutrient Concentrations of European Beech in Switzerland: Relations with Nitrogen Deposition, Ozone, Climate and Soil Chemistry. Front. For. Glob. Chang. 2020, 3, 33. [Google Scholar] [CrossRef]
- Bergmann, W. (Ed.) Nutritional Disorders of Plants; Gustav Fischer Verlag: Jena, Germany; Stuttgart, Germany; New York, NY, USA, 1992. [Google Scholar]
- Lukac, M.; Calfapietra, C.; Lagomarsino, A.; Loreto, F. Global Climate Change and Tree Nutrition: Effects of Elevated CO2 and Temperature. Tree Physiol. 2010, 30, 1209–1220. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Nutrient Dynamics along a Precipitation Gradient in European Beech Forests. Biogeochemistry 2014, 120, 51–69. [Google Scholar] [CrossRef]
- Ferretti, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Croisé, L.; Frati, L.; Nicolas, M. Tree Canopy Defoliation Can Reveal Growth Decline in Mid-Latitude Temperate Forests. Ecol. Indic. 2021, 127, 107749. [Google Scholar] [CrossRef]
- Tallieu, C.; Badeau, V.; Allard, D.; Nageleisen, L.M.; Bréda, N. Year-to-Year Crown Condition Poorly Contributes to Ring Width Variations of Beech Trees in French ICP Level I Network. For. Ecol. Manag. 2020, 465, 118071. [Google Scholar] [CrossRef]
- Ferretti, M.; Fischer, R.; Mues, V.; Granke, O.; Lorenz, M.; Seidling, W.; Nicolas, M. Part II:Basic Design principlesfor the ICP Forests Monitoring Networks. Version 2020-2. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution On forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 33. ISBN 978-3-86576-162-0. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological Maps for the Main European Woody Species. Data Br. 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Pravilnik o Vrstama Stanišnih Tipova, Karti Staništa, Ugroženim i Rijetkim Stanišnim Tipovima Te o Mjerama Za Očuvanje Stanišnih Tipova NN 7/2006. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2006_01_7_156.html (accessed on 14 July 2022).
- Cervellini, M.; Zannini, P.; Di Musciano, M.; Fattorini, S.; Jiménez-Alfaro, B.; Rocchini, D.; Field, R.; Vetaas, O.R.; Irl, S.D.H.; Beierkuhnlein, C. A Grid-Based Map for the Biogeographical Regions of Europe. Biodivers. Data J. 2020, 8, e53720. [Google Scholar] [CrossRef]
- Perčec Tadić, M. Gridded Croatian Climatology for 1961–1990. Theor. Appl. Climatol. 2010, 102, 87–103. [Google Scholar] [CrossRef]
- Perčec Tadić, M.; Pasarić, Z.; Guijarro, J.A. Croatian High-Resolution Monthly Gridded Data Set of Homogenised Surface Air Temperature. Theor. Appl. Climatol. 2022. [Google Scholar] [CrossRef]
- Palmer, W.C. Meteorological Drought; US Department of Commerce, Weather Bureau: Washington, DC, USA, 1965; Volume 45. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Dial, R.; Tobin, S.C. Description of Arborist Methods for Forest Canopy Access and Movement. Selbyana 1994, 15, 24–37. [Google Scholar]
- Jepson, J. The Tree Climber’s Companion; Beaver Tree Publishing: Longville, MI, USA, 2000. [Google Scholar]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE, ICP Forests Programme Co-Ordinating Centre: Hamburg, Germany, 2016. [Google Scholar]
- ISO 11277; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material. ISO: Geneva, Switzerland, 2020.
- ISO-10390; Soil Quality—Determination of PH. ISO: Geneva, Switzerland, 2005.
- ISO-11260; Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution4. ISO: Geneva, Switzerland, 1994.
- ISO 14254; Soil Quality—Determination of Exchangeable Acidity Using Barium Chloride Solution as Extractant 2018. ISO: Geneva, Switzerland, 2018.
- ISO-13878; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“elemental Analysis”) 1998. ISO: Geneva, Switzerland, 1998.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen Über Die Chemische Bodenanalyse Als Grundlage Für Die Beurteilung Des Nährstoffzustandes Der Böden. II. Chemische Extraktionsmethoden Zur Phosphor-Und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Škorić, A. Priručnik Za Pedološka Istraživanja; Fakultet Poljoprivrednih Znanosti: Zagreb, Croatia, 1985. [Google Scholar]
- Lix, L.M.; Keselman, J.C.; Keselman, H.J. Consequences of Assumption Violations Revisited: A Quantitative Review of Alternatives to the One-Way Analysis of Variance F Test. Rev. Educ. Res. 1996, 66, 579–619. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Ross, A.; Willson, V.L. Independent Samples T-Test. In Basic and Advanced Statistical Tests; Springer: Rotterdam, The Netherlands, 2017; pp. 13–16. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Johnson, J.B.; Omland, K.S. Model Selection in Ecology and Evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
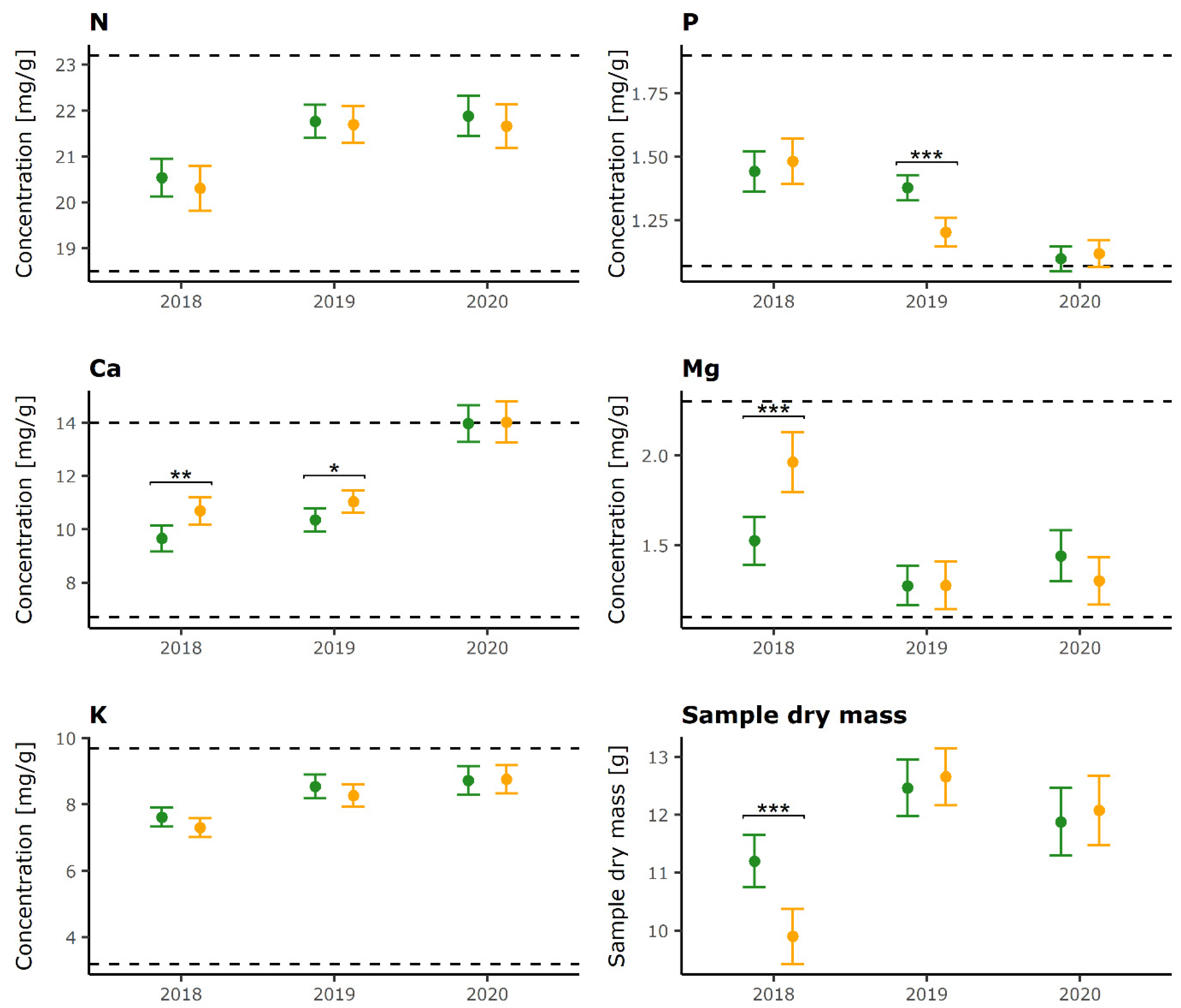
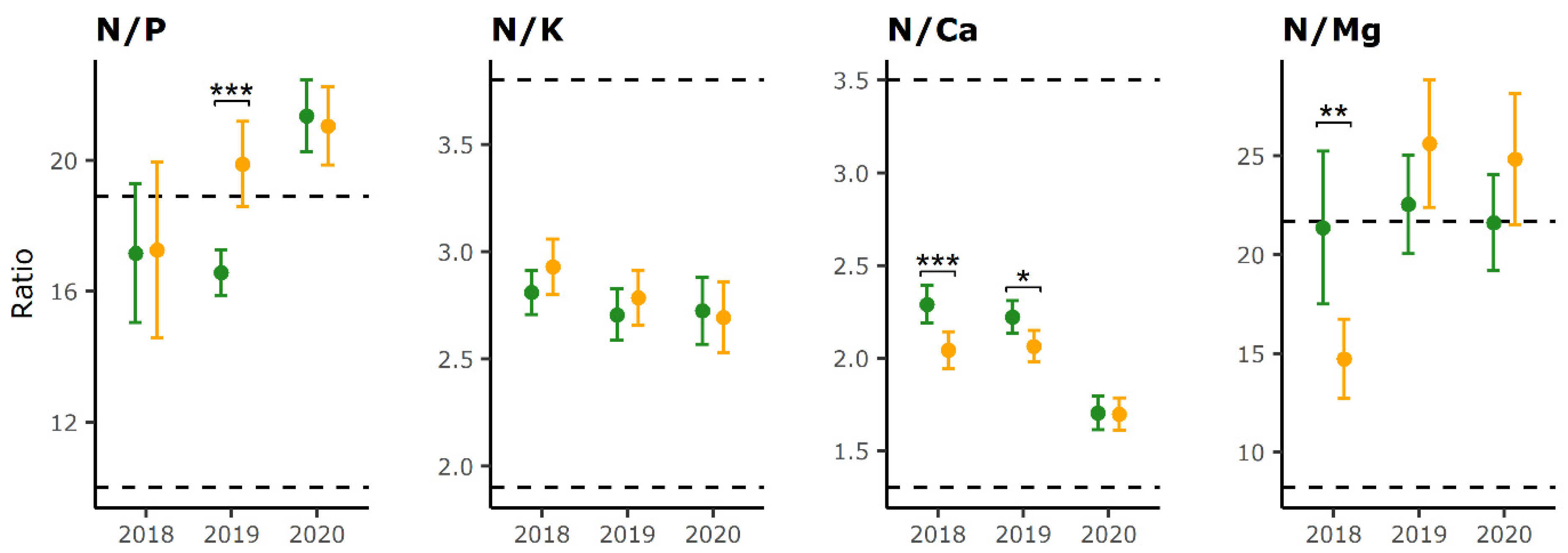
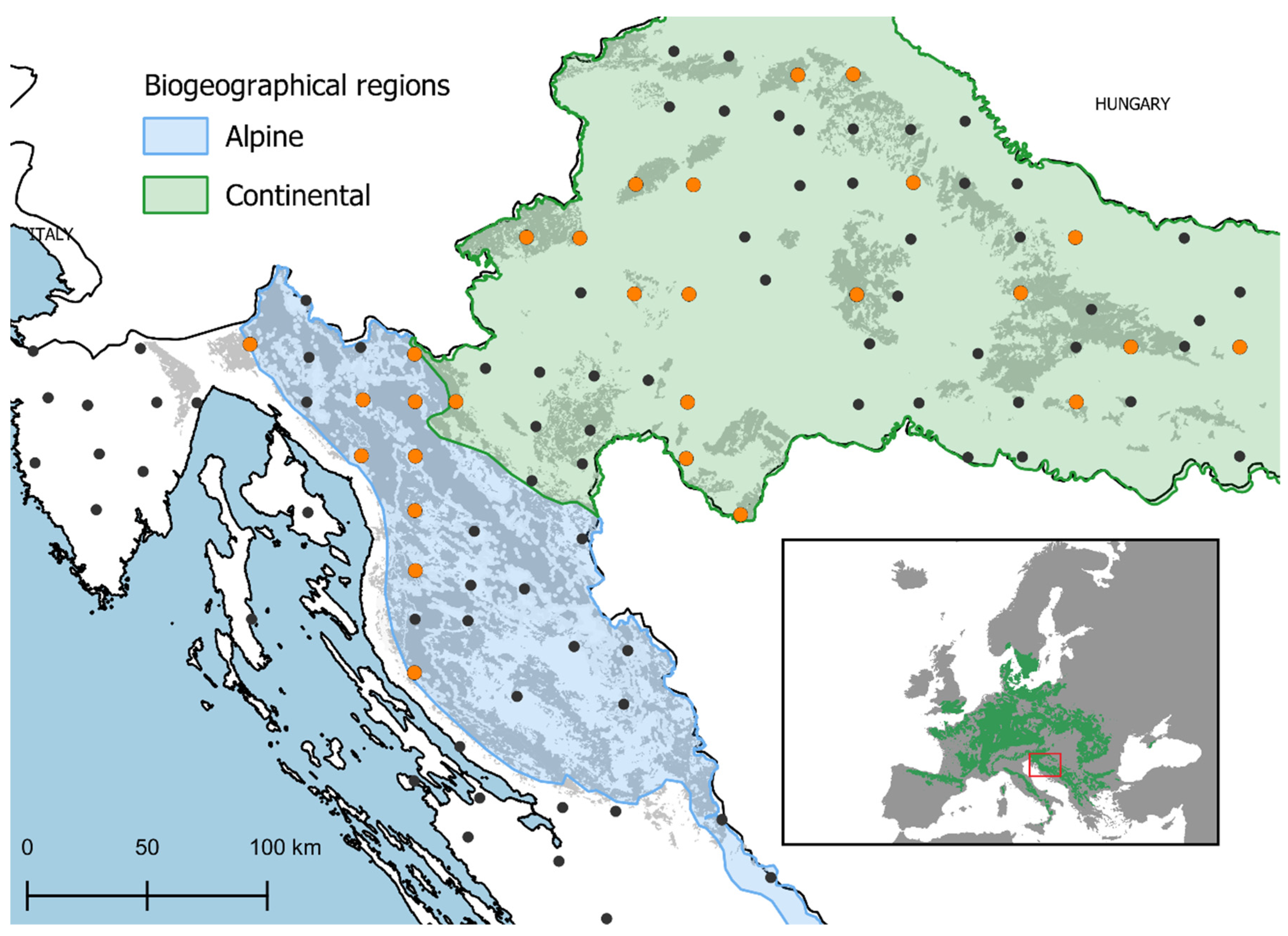
| Defoliation Category | Year 1 | Year 2 | N | P | K | Ca | Mg | Sample Dry Mass |
|---|---|---|---|---|---|---|---|---|
| LD | 2018 | 2019 | *** | ns | *** | ** | *** | *** |
| LD | 2018 | 2020 | *** | *** | *** | *** | ns | * |
| LD | 2019 | 2020 | ns | *** | ns | *** | * | * |
| HD | 2018 | 2019 | *** | *** | *** | ns | *** | *** |
| HD | 2018 | 2020 | *** | *** | *** | *** | *** | *** |
| HD | 2019 | 2020 | ns | * | ** | *** | ns | * |
| N | P | K | Ca | Mg | ||
|---|---|---|---|---|---|---|
| 2018 | HD higher | 1 | - | - | 6 | 4 |
| LD higher | 1 | - | 2 | - | 1 | |
| 2019 | HD higher | - | - | 1 | - | 2 |
| LD higher | 1 | 4 | 1 | 1 | 1 | |
| 2020 | HD higher | - | 2 | 1 | 1 | 1 |
| LD higher | 2 | - | - | - | 1 |
| Defoliation Category | Year 1 | Year 2 | N/P | N/K | N/Ca | N/Mg |
|---|---|---|---|---|---|---|
| LD | 2018 | 2019 | ns | ns | ns | ns |
| LD | 2018 | 2020 | *** | ns | *** | ns |
| LD | 2019 | 2020 | *** | ns | *** | ns |
| HD | 2018 | 2019 | ns | ns | ns | *** |
| HD | 2018 | 2020 | * | * | *** | *** |
| HD | 2019 | 2020 | ns | ns | *** | ns |
| N/P | N/Ca | N/Mg | N/K | ||
|---|---|---|---|---|---|
| 2018 | HD higher | - | - | 1 | 2 |
| LD higher | - | 5 | 2 | - | |
| 2019 | HD higher | 5 | 1 | 1 | 2 |
| LD higher | - | 2 | 1 | 1 | |
| 2020 | HD higher | 1 | - | - | - |
| LD higher | 1 | 2 | 1 | - |
| DFC | |||
|---|---|---|---|
| Element | Environmental Factor 1 | HD Higher | LD Higher |
| K | Alt | 1.000 | 1.005 *** |
| (0.996, 1.003) | (1.002, 1.008) | ||
| MAT | 0.644 *** | 0.884 | |
| (0.480, 0.809) | (0.659, 1.108) | ||
| AIC | 48.042 | 48.042 | |
| Ca | Tmax | 1.298 *** | 0.00002 *** |
| (1.112, 1.485) | (0.00002, 0.00002) | ||
| sand | 0.926 | 0.048 *** | |
| (0.809, 1.043) | (0.048, 0.048) | ||
| MAP | 0.988 ** | 1.900 *** | |
| (0.977, 0.999) | (1.900, 1.900) | ||
| Alt | 1.005 ** | 0.372 *** | |
| (1.001, 1.009) | (0.372, 0.372) | ||
| AIC | 56.908 | 56.908 | |
| Mg | MAT | 6.578 *** | 1.671 * |
| (6.330, 6.826) | (1.149, 2.194) | ||
| MAP | 1.002 | 1.006 | |
| (0.996, 1.007) | (0.998, 1.015) | ||
| Alt | 1.009 *** | 1.001 | |
| (1.005, 1.013) | (0.992, 1.009) | ||
| sand | 0.991 | 1.121 ** | |
| (0.885, 1.097) | (1.020, 1.222) | ||
| pH | 2.600 ** | 1.015 | |
| (1.708, 3.492) | (−0.815, 2.846) | ||
| AIC | 80.311 | 80.311 | |
| DFC | |||
|---|---|---|---|
| Environmental Factor 1 | HD Higher | LD Higher | |
| N/P | MAP | 1.006 * | 0.989 |
| (1.000, 1.012) | (0.967, 1.011) | ||
| Tmax | 0.820 *** | 0.006 *** | |
| (0.696, 0.943) | (−0.438, 0.449) | ||
| Alt | 0.992 ** | 0.960 *** | |
| (0.986, 0.999) | (0.937, 0.983) | ||
| AIC | 59.378 | 59.378 | |
| N/Ca | Tmax | 0.070 *** | 0.402 *** |
| (−1.553, 1.693) | (0.029, 0.775) | ||
| PDSI | 1.401 | 1.835 ** | |
| (−0.956, 3.758) | (1.251, 2.419) | ||
| Ca2+ | 0.534 | 0.909 ** | |
| (−0.314, 1.381) | (0.829, 0.988) | ||
| MAP | 0.996 | 0.993 *** | |
| (0.957, 1.036) | (0.989, 0.998) | ||
| silt | 0.741 | 1.158 * | |
| (−0.353, 1.835) | (0.996, 1.319) | ||
| AIC | 72.131 | 72.131 | |
| N/Mg | MAP | 0.999 | 1.008 *** |
| (0.989, 1.009) | (1.002, 1.014) | ||
| MAT | 1.070 | 2.502 *** | |
| (0.648, 1.493) | (2.198, 2.805) | ||
| pH | 0.631 | 3.708 *** | |
| (−1.419, 2.681) | (2.770, 4.646) | ||
| SPEI | 2.613 | 0.071 ** | |
| (−1.249, 6.474) | (−2.192, 2.333) | ||
| AIC | 65.799 | 65.799 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognjenović, M.; Seletković, I.; Marušić, M.; Jonard, M.; Rautio, P.; Timmermann, V.; Tadić, M.P.; Lanšćak, M.; Ugarković, D.; Potočić, N. The Effect of Environmental Factors on the Nutrition of European Beech (Fagus sylvatica L.) Varies with Defoliation. Plants 2023, 12, 168. https://doi.org/10.3390/plants12010168
Ognjenović M, Seletković I, Marušić M, Jonard M, Rautio P, Timmermann V, Tadić MP, Lanšćak M, Ugarković D, Potočić N. The Effect of Environmental Factors on the Nutrition of European Beech (Fagus sylvatica L.) Varies with Defoliation. Plants. 2023; 12(1):168. https://doi.org/10.3390/plants12010168
Chicago/Turabian StyleOgnjenović, Mladen, Ivan Seletković, Mia Marušić, Mathieu Jonard, Pasi Rautio, Volkmar Timmermann, Melita Perčec Tadić, Miran Lanšćak, Damir Ugarković, and Nenad Potočić. 2023. "The Effect of Environmental Factors on the Nutrition of European Beech (Fagus sylvatica L.) Varies with Defoliation" Plants 12, no. 1: 168. https://doi.org/10.3390/plants12010168
APA StyleOgnjenović, M., Seletković, I., Marušić, M., Jonard, M., Rautio, P., Timmermann, V., Tadić, M. P., Lanšćak, M., Ugarković, D., & Potočić, N. (2023). The Effect of Environmental Factors on the Nutrition of European Beech (Fagus sylvatica L.) Varies with Defoliation. Plants, 12(1), 168. https://doi.org/10.3390/plants12010168







