Abstract
Drought stress severely affects plant growth and development, causing significant yield loss in rice. This study demonstrates the relevance of water use efficiency with deeper rooting along with other root traits and gas exchange parameters. Forty-nine rice genotypes were evaluated in the basket method to examine leaf-level water use efficiency (WUEi) variation and its relation to root traits. Significant variation in WUEi was observed (from 2.29 to 7.39 µmol CO2 mmol−1 H2O) under drought stress. Regression analysis revealed that high WUEi was associated with higher biomass accumulation, low transpiration rate, and deep rooting ratio. The ratio of deep rooting was also associated with low internal CO2 concentration. The association of deep rooting with lower root number and root dry weight suggests that an ideal drought-tolerant genotype with higher water use efficiency should have deeper rooting (>30% RDR) with moderate root number and root dry weight to be sustained under drought for a longer period. The study also revealed that, under drought stress conditions, landraces are more water-use efficient with superior root traits than improved genotypes.
1. Introduction
The impact of climate change and rapid human population growth has imposed a strong challenge for sustainable agriculture [1,2]. Climate change, increasing water shortages, more frequent drought, and high temperatures cause significant loss in grain yield [3,4]. Agriculture consumes approximately 70% of freshwater annually [5]. The major crop rice requires a large amount of water [6] and is highly susceptible to drought stress. It is estimated that approximately 2500 L of water are required to produce 1 kg of rice [7]. Rice production is particularly affected in rainfed ecosystems where drought is not uncommon due to erratic rainfall caused by climate change [4,8]. However, different strategies such as the use of sprinklers and drip irrigation to minimize excessive water use [9,10], antitranspirants [11,12], and variety development through conventional breeding [13] were explored to improve water use efficiency (WUE). The most plausible way is the genetic approach, which would be easy to adapt to minimize water use [14]. Therefore, identifying genotypes with an inherent ability to use water efficiently is a promising approach.
Plants exchange gases and water vapor through stomata, thereby regulating canopy temperature by controlling transpiration. When water is limited, transpiration is restricted by the closure of stomata, which affects carbon assimilation and increases canopy temperature [15,16]. The transpiration rate is dependent on the vapor pressure deficit (VPD). Genotypes tend to transpire more when the VPD is higher. Drought-tolerant genotypes can restrict their transpiration rate after a threshold VPD value by the partial closing of stomata, but susceptible genotypes continue to transpire even at a high VPD, resulting in severe water loss and wilting [17]. Severe water loss leads to a higher canopy temperature in susceptible genotypes than in tolerant ones under drought. In addition to severe water loss, the complete closure of stomata in susceptible genotypes inhibits carbon assimilation. Therefore, stomata play an important role in regulating water use efficiency in rice [14].
In the post-Green Revolution period, more emphasis was given to increasing grain yield. With the gradual rise in problems caused by global climate change, loss in grain yield was observed due to adverse environmental factors, particularly drought, for which the genotypes released were not adaptable to drought stress conditions. To mitigate this, plant breeders began to identify genotypes with an inherent capacity to tolerate drought, along with the putative linked QTLs. Since drought tolerance is a complex trait, no candidate genes have been identified to date for drought tolerance; rather, QTLs have been identified for traits associated with drought tolerance. In recent decades, the characterization of root architecture traits has become the target for breeders to enhance tolerance of drought and nutrient deficiency [18]. Several root-attributed QTLs have been identified in breeding lines and diverse rice germplasm [19,20,21,22,23]. These QTLs explained the role of root traits in drought tolerance. For the past two decades, efforts have been made to develop drought-tolerant rice varieties through the characterization and manipulation of root traits using breeding approaches [23,24,25,26,27,28,29,30]. The QTL Dro1 is one of them. It controls root growth angle and is responsible for deeper rooting in rice [31]. Dro1 is reported to minimize water loss and maintain a lower canopy temperature in rice by maintaining the water status of the plant under drought. However, the effect of rooting patterns on water use efficiency is a little explored area. Dharmappa et al. [13] reported a 50% increase in root dry weight and a 23.5% increase in root length, resulting in a significant increase in WUE. Similarly, Zhou et al. [32] reported that deeper root distribution resulted in increased WUE in super rice. However, the relevance of deeper rooting to water use efficiency is debatable. Our study demonstrates the relevance of WUE to deeper rooting along with other root traits and gas exchange parameters.
2. Results
2.1. Genotype Performance and Significance of ANOVA for Gas Exchange Measurements and Root Traits
A total of 49 rice genotypes that included 20 landraces and 29 improved varieties were evaluated for their deeper rooting using the basket method. Root samplings were taken from a depth of 50 cm from the soil surface for both well-watered and drought stress conditions. Highly significant differences (p < 0.0001) in various trait values such as photosynthesis rate, transpiration rate, leaf-level water use efficiency, internal CO2 concentration, shoot dry weight, root dry weight, ratio of deep rooting, root number, and root diameter were observed between the two treatments. Significant differences were also observed between the improved genotypes and landraces in all traits under well-watered (WW) and drought stress (DS) conditions (Table 1). The genotypes were exposed to drought stress for 20 days, during which the soil moisture content dropped to 11.20% at 30 cm and 16.39% at 45 cm of soil depth. As a result, the soil moisture tension dropped to −62.9 kPa and −51.9 kPa at 30 cm and 45 cm of soil depth, respectively, which was sufficient to cause moderate to severe drought stress that can affect plant growth significantly (Figure 1A,B). Drought stress diminished the average shoot biomass up to 51.6%. The decrease in shoot dry weight (SDW) was 56.1% in improved genotypes, whereas landraces registered a decrease of 45.7% under drought stress. Among all the genotypes, AC 35717 (12.1%), AC 36762 (12.6%), Moroberekan (19.8%), AC 36735 (23.8%), AC 36734 (26.9%), AC 35729 (28.1%), and AC 36738 (30%) had a minimal decrease in SDW and can thus be considered as drought tolerant (Table 2).

Table 1.
Overall significant difference level (t-test) between treatments and improved genotypes vs landraces for the studied traits.
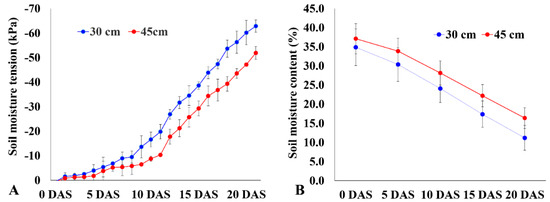
Figure 1.
Soil moisture tension (A) and soil moisture content (B) measured by tensiometer and time-domain reflectometer, respectively, at soil depths of 30 and 45 cm. Values shown are mean ± SE, n = 3. DAS = days after stress.

Table 2.
Variation in root and shoot parameters under well-watered and drought stress conditions in 49 rice genotypes.
2.2. Variation in Instantaneous WUE (WUEi) and Biomass among Rice Accessions under Varying Moisture Levels
The WUEi values varied significantly (p < 0.0001) among genotypes (Table 1) and a significant increase in WUEi (p < 0.0001) was observed under drought conditions. During the entire investigation period, WUEi varied from 2.02 to 4.54 µmol CO2 mmol−1 H2O under well-watered conditions and from 2.29 to 7.39 µmol CO2 mmol−1 H2O under drought stress conditions. No significant variation in WUEi was observed between the improved lines (2.95 µmol CO2 mmol−1 H2O) and the landraces (3.01 µmol CO2 mmol−1 H2O) under WW conditions. However, under drought conditions, the landraces (4.70 µmol CO2 mmol−1 H2O) showed significantly higher WUEi than the improved genotypes (3.54 µmol CO2 mmol−1 H2O). Among the improved genotypes, Ranbir Basmati 2 alone registered >5 µmol CO2 mmol−1 H2O, while five landraces (AC 36762, AC 36734, AC 35717, AC 36735, and AC 36722) had higher WUEi under DS, from 5.42 to 7.39 µmol CO2 mmol−1 H2O (Table 3).

Table 3.
Variation in photosynthetic gas exchange traits under well-watered and drought stress conditions in 49 rice genotypes.
Similarly, a significant variation was observed in shoot biomass among the genotypes. Biomass accumulation varied significantly between the improved genotypes and the landraces. The improved genotypes had a significantly higher shoot biomass, ranging from 28.05 g to 58.68 g with an average value of 41.92 g under WW conditions, whereas the landraces had a shoot biomass ranging from 14.23 g to 55.92 g, with an average value of 37.96 g. In contrast to WW conditions, landraces had a much higher value for shoot biomass under DS conditions, ranging from 5.16 g to 42.31 g, with an average value of 21.06 g compared with the average value of 18.38 g in improved genotypes. Among the landraces, AC 35717 (42.31 g) and AC 36762 (38.8 g) had the highest SDW. CR Dhan 207 (26.6 g) and Sattari (25.3 g) had the highest SDW among the improved genotypes.
2.3. Differences in Root Traits among Genotypes under Varying Moisture Levels
Variation was much wider for the ratio of deep rooting (RDR) in landraces (5.36–40.00%) than in improved genotypes (5.95–24.00%). Six landraces (AC 35717, AC 36762, AC 36735, AC 35729, Moroberekan, and AC 36738) exhibited >30% RDR, while Ranbir Basmati 2 alone had a higher RDR of 24% in the category of improved genotypes (Table 2). Similarly, significant variation was observed for root dry weight (RDW) (p < 0.003) and total root number per plant (RN) (p < 0.041) between treatments, as well as between landraces and improved genotypes. However, a significant decrease in RDW was observed irrespective of groups under DS. The average decrease in RDW was lower in landraces (19%) than in improved genotypes (21%). On the contrary, the RN observed in improved genotypes (74) was much higher than that of landraces (61) under drought (Table 2).
2.4. Correlation between Different Gas Exchange Traits and Root Traits
The correlation among the different studied traits is presented in the form of a network to visualize the interrelationship among the traits. Under WW conditions, RN, RDW, and RDR were correlated with each other. Similarly, WUEi, Ci, and Tr were intercorrelated. SDW showed a relatively weak correlation with other traits. However, An had a strong positive correlation with WUEi and a weaker correlation with Ci, SDW, and RN (Figure 2A–C).
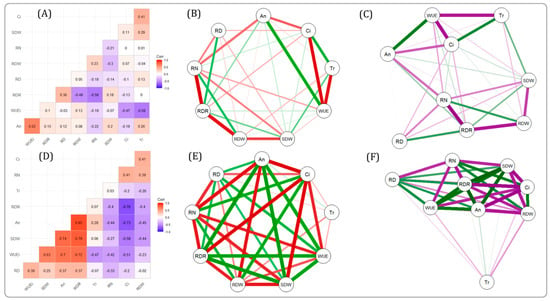
Figure 2.
Pairwise Pearson phenotypic correlation and network of morpho-physiological traits. The correlation (A,D) and network (B,C,F) of the nine traits evaluated in rice plants under well-watered (WW) (A–C) and drought stress (DS) (D–F) conditions. The width of the lines represents the strength of the correlation and the color of the lines represents the nature of the correlation. The red and the purple lines represent negative correlation and the green line represents positive correlation.
Under DS conditions, all the traits (WUEi, Ci, An, RDW, RDR, RN, and SDW) were strongly intercorrelated with each other except Tr. Tr had a comparatively weak correlation with the other traits (Figure 2D–F). RDR had a strong positive correlation with WUEi, SDW, and An. However, it had a strong negative correlation with RDW, Ci, and RN.
2.5. Instantaneous WUE (WUEi) Is Correlated with the Ratio of Deep Rooting and Biomass Accumulation
Instantaneous WUE is the ratio of photosynthetic rate to transpiration rate. High WUEi results from a higher photosynthetic rate with the same transpiration rate, the same photosynthetic rate with a low transpiration rate, or both. To analyze the factors that contribute more to WUEi, both transpiration rate and photosynthetic rate were regressed against WUEi (Figure 3). WUEi was strongly associated with photosynthesis (r = 0.701 **) and transpiration rate (r = −0.484 **) under drought conditions. This suggests that higher photosynthetic rates and lower transpiration rates contribute to higher WUEi. A higher photosynthetic rate is considered an indicator of high biomass accumulation that leads to higher WUEi, which is evident from the strong positive correlation of WUEi with shoot biomass accumulation (r = 0.634 **). At the same time, RDR showed a strong positive correlation with WUEi (r = 0.708 **) and shoot biomass (r = 0.788 **), thus indicating that deep-rooted genotypes efficiently use water to produce higher biomass under drought (Figure 3A,B). Further, the genotypes with high RDR had a lower decrease in shoot dry weight, which is an indicator of drought tolerance. For instance, traditional landraces AC 36738, AC 35729, AC 35717, AC 36762, AC 36735, and Moroberekan exhibited >30% RDR with a minimum reduction in SDW, ranging from 12.1% to 28.1%.
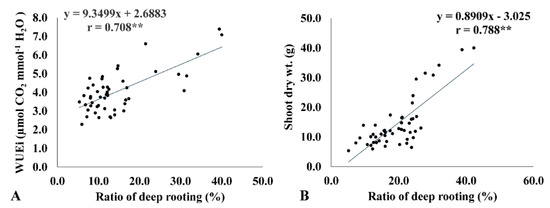
Figure 3.
Linear regression relationship between the ratio of deep rooting (RDR) with instantaneous water use efficiency (WUEi) (A) and shoot dry weight (B) under drought conditions. ** <0.01.
2.6. RDR Is Associated with Low Internal CO2 Concentration, Root Dry Weight, and Total Number of Roots
The ratio of deep rooting significantly affected the internal CO2 concentration (Table 3). RDR showed a strong negative association with Ci (r = −0.75 **) (Figure 4). The genotypes with more than 30% RDR had comparatively lower Ci (214−264 μmol CO2 mol−1 H2O) under drought. Similarly, RDW (r = −0.398 *) and RN (r = −0.386 *) revealed a negative association with RDR. The genotypes with greater RDR had relatively lower RDW and RN (Figure 5A,B).
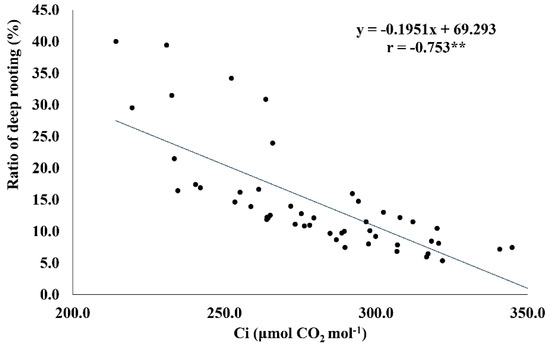
Figure 4.
Linear regression relationship between the ratio of deep rooting (RDR) and internal CO2 concentration under drought conditions. ** <0.01.
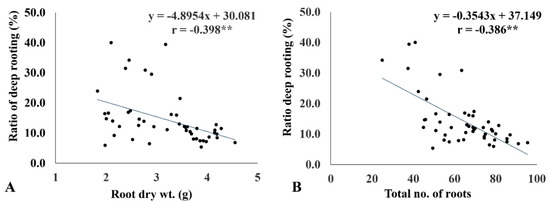
Figure 5.
Linear regression relationship between the ratio of deep rooting (RDR) and root dry weight (RDW) (A) and the total number of roots (RN) (B) under drought conditions. ** <0.01.
2.7. PCA with Root Traits Grouped the Genotypes into Distinct Categories Highlighting the Importance of RDR
To study the variance existing with these three root-related traits (RDR, RDW, and RN), the genotypes were plotted in a biplot that explained 80.11% of the total variance, according to the first two factors (F1 explained 41.59% and F2 explained 38.52% of the total variance). The biplot clearly separated the 49 genotypes into three groups. The first group included 25 genotypes showing high values for RDW and RN but low RDR. The second group included six genotypes with a high RDR and intermediate values for RDW and RN (AC 35717, AC 36762, AC 36735, AC 35729, AC 36738, and Moroberekan). The third group included 18 genotypes with low values for RDR, RDW, and RN (Figure 6).
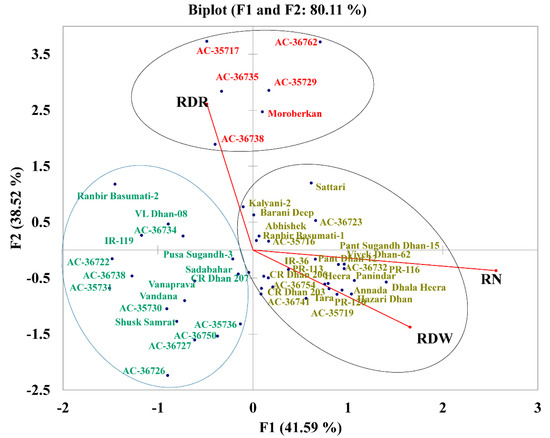
Figure 6.
Factorial plans with two principal components representing the ratio of deep rooting (RDR), total number of roots (RN), and root dry weight (RDW) averaged over three replications of 49 rice genotypes under drought stress conditions. The variance explained by each dimension is shown as a percentage of the total variance.
2.8. Changes in Root Traits in Improved Genotypes from 1987 to 2016
To examine the effect of the selection pressure of grain yield on root traits, changes in RDR, the total number of roots in the crown region, and a root diameter within 10 cm of soil depth from the ground were analyzed in 24 genotypes released from 1987 to 2016. As the year of release of IR119, Phaninder, VL Dhan 08, IR36; and Ranbir Basumati (Basmati) 2 was unknown, these varieties were not included in this study. A gradual increase in RDR was observed from 8.5% to 13.0% within the past three decades, and a similar trend was observed for a root diameter present within 10 cm of soil depth from the ground (data not shown). The root diameter increased from 0.18 cm to 0.31 cm. In contrast, the number of roots in the crown region declined from 86 to 66 (Figure 7A,B). As shown in Figure 7A, there was no change in RDR from 1987 to 2006 but there was a considerable increase in RDR in the varieties released after 2009 (Figure 7B). Similarly, there was a sharper increase in root diameter from 2009 to 2016 than from 1987 to 2006.
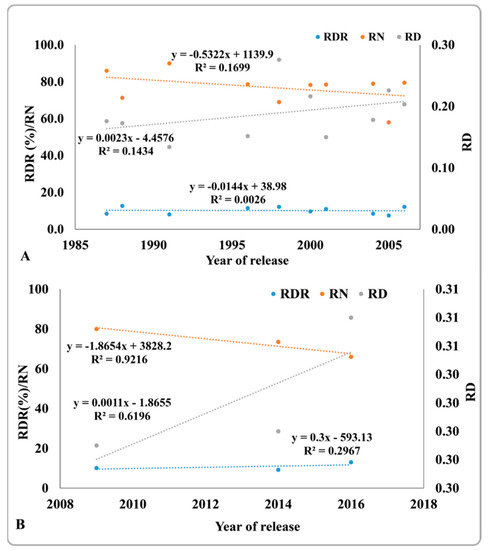
Figure 7.
Ratio of deep rooting (RDR), total number of roots in the crown region (RN), and root diameter (RD) of varieties released from 1987 to 2006 (A) and from 2009 to 2016 (B). Values are the mean of three replicates per variety per year of release. Genotypes 1 to 24 from Table 2 and Table 3 were used here.
3. Discussion
In the scenario of current climate change and in order to improve WUE in rice, deeper rooting is strongly required. In addition to water absorption, deeper rooting helps acquire nutrients from deeper soil layers. Nutrients such as nitrates are highly mobile and leach deep into the soil with percolating water, and these nutrients can be absorbed by deeper roots [33]. Maintaining a high rate of photosynthesis with a high biomass under decreased water is challenging in rice [34,35]. Efficient water use by the crop is accomplished through stomatal regulation and a reduction in stomatal density [14]. A reduction in stomatal density and transpiration check through stomatal regulation might decrease unnecessary water loss [17,36] but result in high canopy temperature under WW conditions, compared to those plants showing high stomatal density without any stomatal regulation [14,37]. Increased WUE might result from a high photosynthetic rate at the same transpiration rate or the same rate of photosynthesis from a low transpiration rate. The combination of both high photosynthetic rate and low transpiration rate can contribute to higher WUE in the active tillering stage. However, the significant contribution between these two factors toward high WUE is still debatable. During evolution, plants have maximized WUE through sustained photosynthetic rate and low transpiration rate [12]. We suggest that, although the contribution of both factors is significant, the rate of photosynthesis (r = 0.701 **) contributes more to WUEi than the transpiration rate (r = −0.484 **). Although this conservative water strategy is useful for survival under drought conditions, a decrease in transpiration rate restricts CO2 influx, resulting in decreased growth [13]. On the other hand, efficient water use is relevant with a higher growth rate [38]. In our opinion, for efficient water use, plants have to extract more water from the soil and diminish unnecessary water loss. Tolerant genotypes with restricted transpiration above a threshold VPD can maintain their photosynthetic rate without affecting biomass production, despite having partial stomatal closure under well-watered conditions. Under drought stress conditions, the tolerant genotypes with high RDR extract water from deeper soil layers and thus maintain the water status of the plant and restrict transpiration to decrease unnecessary water loss, thereby enabling them to photosynthesize for a longer period. However, the susceptible genotypes lack this mechanism. Our study found a strong correlation of WUEi with RDR under DS. Deeper rooting helps to siphon water from the deeper soil layers, thus maintaining turgidity in plants during drought and favoring comparatively higher photosynthesis. Usually, deeper root growth lowers water use efficiency, but in our study higher RDR favored higher WUE because of higher photosynthetic rates. The restriction in transpiration through stomata is independent of water extraction by roots, evident from the lack of correlation between transpiration rate and RDR (r = 0.074). Therefore, water use efficiency is a coordinated effort performed by both the root and stomata.
The contribution of deeper rooting toward WUEi is also supported by the strong negative correlation between RDR and Ci. Low Ci is an indicator of high carboxylation efficiency. The genotypes registering high RDR had low Ci under drought, thus explaining higher carboxylation efficiency and WUEi. Under drought conditions, the rapid closure of stomata occurs, which leads to a decrease in Ci, suggested by some researchers [13]. According to this statement, Ci is supposed to be low in all genotypes because of the drought effect. However, stomata remain open in rolled leaves and leaf rolling is not directly related to drought response [39], which explained the variability in Ci in the studied genotypes under drought. Further, high RDR is expected to lower WUEi because of more open stomata and increase Ci; however, negative correlation of Ci with RDR and WUEi exists as WUEi is driven by a high photosynthetic rate.
Furthermore, RDR was negatively associated with RDW (r = −0.398*) and RN (r = −0.386*). In our study, the genotypes with high RDR were not the highest scorers in RDW and RN. This is observed clearly in Figure 5. The group that had high RDR exhibited intermediate values for RDW and RN. Contrary to this, genotypes that had high RDW and RN had low RDR. Many researchers reported that genotypes with high RDW are considered drought tolerant [24,40,41]. In contrast, deep rooting in a thinner root system has been observed to tolerate stronger drought conditions [42]. Our study found that genotypes with deeper rooting did not develop a thicker or denser root system at the cost of shoot growth. Rather, they meticulously diverted biomass toward the root to produce a moderately thick root with a lesser root number to continue shoot growth under drought stress. This is evident from the low rate of shoot biomass reduction in genotypes with high RDR. The RN recorded in improved genotypes was much higher than that of landraces under drought. This indicates that they have more surface roots developed for higher input (transplanted) conditions and are able to absorb more nutrients from the sub-surface soil profile [43]. Hence, they are not suitable for dry direct-seeded rice (rainfed upland), for which high RDR is necessary. The genotypes suitable for direct-seeded rice conditions should possess more than 30% RDR with surface rooting to absorb nutrients and to have grain yield on a par with that of transplanted rice.
Drought is a multigene-governed phenomenon for which no specific candidate gene has been identified. To study drought tolerance, researchers focus on particular traits such as stomatal density, osmotic regulation, root traits, etc. In past decades, the rice varieties developed had high grain yield with good grain quality. These varieties were targeted for irrigated areas and were thus highly susceptible to drought, causing substantial yield loss [44]. Owing to directional selection for grain yield, root traits were completely neglected in breeding programs, thus making improved varieties more vulnerable to drought stress [40]. Since the climate change scenario has worsened in the past two decades, breeders now emphasize root traits for developing drought-tolerant genotypes. The recent varieties developed from ICAR-NRRI, Cuttack, after 2014 for direct-seeded aerobic conditions such as CR Dhan 203, CR Dhan 206, and CR Dhan 207 have a high root biomass with fewer, thicker, and deeper roots (Figure 6). However, dense roots with high root numbers may rapidly deplete the soil water level at the early vegetative stage, causing terminal drought stress where water availability or rain occurs at early developmental stages. In contrast, an intermediate number of crown roots with deeper roots of thicker diameters may conserve water for the later developmental stage. This type of root system would be useful for direct-seeded rice systems and an intermediate number of crown roots would help to absorb the nutrients required for higher output in contrast to genotypes with a high RDR [45]. In our study, the landraces mostly have good and adaptive root traits compared with the improved genotypes because of our breeding program, which was oriented toward achieving higher grain yield. This led to a reduced root system compared with landraces and wild ancestors [46,47]. In addition, a higher metabolic cost (root respiration ~50% of the daily photosynthesis) for root growth and maintenance was involved [48]. The selection for high grain yield and harvest index has promoted genotypes with less biomass allocation toward the root [49]. However, a low-cost root system can be designed with fewer lateral roots and deep roots suitable for drought conditions [50]. Further, comparatively higher root growth would help to sequester more carbon from the atmosphere in this era of climate change [45].
4. Materials and Methods
4.1. Plant Materials
A total of 49 rice genotypes comprising 20 traditional landraces and 29 improved genotypes (Table 2) were collected from the gene bank of ICAR-National Rice Research Institute (NRRI), Cuttack, India. The experiment was conducted in a cemented tank at NRRI during the wet season of 2017. Each genotype was raised in a plastic basket repressed in the soil of cemented tanks in a randomized block design. The cemented tank was 1 × 4 × 2 m in size filled with sandy clay loam soil and had a penetration resistance of 11.12 to 1356.22 kPa with an average of 648.54 kPa (cone penetrometer; CP20, Rimik, Australia). The baskets were 10 cm in height. The baskets’ top and bottom diameters were 20 cm and 10 cm, respectively, with a mesh size of 3 mm. Four seeds of each genotype were sown per basket with three replications. Separate tanks were maintained for drought stress and well-watered treatments. Seven days after germination, a single plant was maintained in each basket. Thirty days after germination, drought stress was imposed by withdrawing irrigation. During the stress period, soil moisture content was measured using an ML3 Theta probe soil moisture sensor (Delta-T, Cambridge, UK) connected to a GP1 data logger. In addition, soil moisture tension was measured using a tensiometer (Soilspec, Australia) to monitor drought stress intensity. The measurements were taken at soil depths of 30 cm and 45 cm. Stress was continued until the soil moisture content dropped below 14–16% at 45 cm of soil depth, while the WW tank was supplied daily and necessary plant protection measures were taken. Fertilizer was applied as per the recommended doses of N:P:K at 80:40:40 kg ha−1 in the form of urea, single superphosphate, and muriate of potash, respectively.
4.1.1. Root Trait Measurements
After 20 days of stress (DAS) period (at tillering stage), one side of the cemented tank wall was broken and the soil around the baskets was removed carefully with the help of a jet pipe to minimize damage to the roots. The roots were excavated from a depth of 50 cm, amounting to 0.125 m3 of soil volume. The total number of roots and roots that emerged from the base of the baskets (i.e., 50° to 90° from the surface of the basket) were counted separately. The ratio of deep rooting was calculated as the ratio of the number of roots that emerged from the lower part of the baskets (the portion of the base of the baskets defined by an angle of 50° to 90° from the horizontal, taking the stem of the plant as the central axis) to the total number of roots emerged from the whole basket [31]. In addition, shoot dry weight (SDW) and other root-related traits such as root diameter (RD) (using a digital Vernier caliper), root dry weight (RDW), and total number of roots (RN) were measured. The samples were dried in a hot-air oven at 60 °C for 5 days to record RDW.
4.1.2. Gas Exchange Measurements
Gas exchange was measured with the help of an infrared gas analyzer (IRGA) LI-6400 XT system (Li-Cor, Lincoln, NE, USA). This occurred under standard conditions at active tillering stage 10 days after stress imposition to avoid inhibition of photosynthesis due to leaf rolling. To avoid environmental fluctuations, 1500 μmol quanta m−2 s−1 of light was supplied through an RGB LED light source and a constant flow of 400 μmol CO2 mol−1 was supplied through a carbon dioxide cylinder attached to the system. The temperature of the leaf chamber was maintained at 25 °C with a flow rate of 500 μmol s−1. Measurements were made on the second fully expanded leaf after steady-state conditions were obtained (indicated by steady stomatal conductance and assimilation rate) from each replication. The photosynthesis rate (An, μmol CO2 m−2 s−1), transpiration rate (Tr, mmol H2O m−2 s−1), vapor pressure deficit (VPD), stomatal conductance (gs, mol H2O m−2 s−1), and internal CO2 concentration (Ci, μmol CO2 mol−1) were calculated by the manufacturer’s software. Instantaneous water use efficiency (WUEi) or leaf-level water use efficiency was calculated as the ratio between net photosynthetic rate (An) and transpiration rate (Tr).
4.2. Statistical Analysis
Significant differences between treatments and improved genotypes vs. landraces for the studied traits were estimated through a t-test using XLSTAT 2014 version (https://www.xlstat.com accessed on 6 July 2021) to assess the variability among the genotypes and the treatments. The interaction between traits and genotypes was established through principal component analysis and the scatter plots for correlation were constructed using XLSTAT 2014. Pearson phenotypic correlation and the network of morpho-physiological traits were assessed using the packages “ggcorrplot” and “qgraph” in R version 4.1.3 (R Core Team 2022).
5. Conclusions
Our study revealed sufficient variability in root traits and gas exchange parameters among genotypes in response to drought. The study found that deeper rooting helps to increase water use efficiency by lowering Ci under drought. The study also suggests that genotypes that are suitable for dry direct-seeded rice conditions should have a higher RDR and root diameter with a moderate number of roots. Previously, little attention was given to root traits in rice breeding programs. For the past decade, the rice varieties released have had deep roots with a comparatively low root number and high root diameter. These are more suitable to drought conditions in the current scenario of climate change.
Author Contributions
Conceptualization: G.K.D., R.G., A.A., P.S. and J.A. Conducting of the experiment and the methodology: R.G., A.A. and G.K.D. Data curation, phenotypic trait characterization, data analysis, and validation: G.K.D. and A.M. Writing (original draft preparation): GKD. Writing (review and editing): G.K.D., A.A., A.M., J.P.L. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ICAR-NICRA, India; the Bill & Melinda Gates Foundation provided a research grant in the Green Super Rice Project under ID OPP1130530; and the Department of Agriculture, Philippines, provided funds to J.A. under the Next-Gen Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the director, ICAR-National Rice Research Institute, Cuttack, for providing field and laboratory facilities to carry out the research activities. We extend special thanks to the administrative staff of both institutes that supported carrying out this experiment in official terms.
Conflicts of Interest
The authors declare that the research review was conducted in the absence of any commercial or economic associations that could be construed as a potential conflict of interest.
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Girma, B., Kissel, E.S., Levy, A.N., MacCracken, S., Mastrandrea, P.R., White, L.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 485–533. [Google Scholar]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought susceptibility of modern rice varieties: An effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015, 13, 14799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korres, N.E.; Norsworthy, J.K.; Burgos, N.R.; Oosterhuis, D.M. Temperature and drought impacts on rice production: An agronomic perspective regarding short- and long-term adaptation measures. Water Resour. Rural. Dev. 2017, 9, 12–27. [Google Scholar] [CrossRef]
- Bacom, M. Water Use Efficiency in Plant Biology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; Available online: https://www.routledge.com/Water-Use-Efficiency-in-Plant-Biology/Bacon/p/book/9780849323546 (accessed on 13 May 2021).
- Elert, E. Rice by the numbers: A good grain. Nature 2014, 514, S50–S51. [Google Scholar] [CrossRef] [Green Version]
- Surendran, U.; Raja, P.; Jayakumar, M.; Subramoniam, S.R. Use of efficient water saving techniques for production of rice in india under climate change scenario: A critical review. J. Clean. Prod. 2021, 309, 127272. [Google Scholar] [CrossRef]
- Matsuda, S.; Takano, S.; Sato, M.; Furukawa, K.; Nagasawa, H.; Yoshikawa, S.; Kasuga, J.; Tokuji, Y.; Yazaki, K.; Nakazono, M.; et al. Rice stomatal closure requires guard cell plasma membrane ATP-binding cassette transporter RCN1/OsABCG5. Mol. Plant 2016, 9, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Maria, J.; Parfitt, B.; Concenço, G.; Scivittaro, W.B.; Andres, A.; Trombetta Da Silva, J.; Alves, M.; Pinto, B. Soil and water management for sprinkler irrigated rice in southern Brazil. In Advances in International Rice Research; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Shareef, T.M.E.; Ma, Z.; Zhao, B.; Shareef, T.M.E.; Ma, Z.; Zhao, B. Essentials of drip irrigation system for saving water and nutrients to plant roots: As a guide for growers. J. Water Resour. Prot. 2019, 11, 1129–1145. [Google Scholar] [CrossRef] [Green Version]
- Patek, R.; Raha, P.; Laxmi, S.; Paul, A. Effect of antitranspirants on rice (Oryza sativa) grown under submerged and water stressed condition in an Inceptisol of Varanasi, Uttar Pradesh. J. Crop Weed 2019, 15, 100–106. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; El-Bassiouny, H.M.S.; AbouSeeda, M.A. Potential role of kaolin or potassium sulfate as anti-transpirant on improving physiological, biochemical aspects and yield of wheat plants under different watering regimes. Bull. Natl. Res. Cent. 2019, 43, 134. [Google Scholar] [CrossRef]
- Dharmappa, P.M.; Doddaraju, P.; Malagondanahalli, M.V.; Rangappa, R.B.; Mallikarjuna, N.M.; Rajendrareddy, S.H.; Ramanjinappa, R.; Mavinahalli, R.P.; Prasad, T.G.; Udayakumar, M.; et al. Introgression of root and water use efficiency traits enhances water productivity: An evidence for physiological breeding in rice (Oryza sativa L.). Rice 2019, 12, 14. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- Kholová, J.; Hash, C.T.; Kakkera, A.; Koová, M.; Vadez, V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. J. Exp. Bot. 2010, 61, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Jewel, Z.A.; Mahender, A.; Anandan, A.; Hernandez, J.; Li, Z. Molecular genetics and breeding for nutrient use efficiency in rice. Int. J. Mol. Sci. 2018, 19, 1762. [Google Scholar] [CrossRef] [Green Version]
- Kitomi, Y.; Kanno, N.; Kawai, S.; Mizubayashi, T.; Fukuoka, S.; Uga, Y. QTLs underlying natural variation of root growth angle among rice cultivars with the same functional allele of deeper rooting 1. Rice 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, X.; Zhan, X.; Zhai, R.; Wu, W.; Shen, X.; Dai, G.; Cao, L.; Cheng, S. Identification of QRL7, a major quantitative trait locus associated with rice root length in hydroponic conditions. Breed. Sci. 2013, 63, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Obara, M.; Tamura, W.; Ebitani, T.; Yano, M.; Sato, T.; Yamaya, T. Fine-mapping of QRL6.1, a Major QTL for root length of rice seedlings grown under a wide range of NH4+ concentrations in hydroponic conditions. Theor. Appl. Genet. 2010, 121, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Han, Y.; Liu, L.; Chen, Y.; Du, Y.; Zhang, J.; Sun, H.; Zhao, Q. QRT9, a quantitative trait locus controlling root thickness and root length in upland rice. J. Exp. Bot. 2015, 66, 2723–2732. [Google Scholar] [CrossRef] [Green Version]
- Catolos, M.; Sandhu, N.; Dixit, S.; Shamsudin, N.A.A.; Naredo, M.E.B.; McNally, K.L.; Henry, A.; Diaz, M.G.; Kumar, A. Genetic loci governing grain yield and root development under variable rice cultivation conditions. Front. Plant Sci. 2017, 8, 1763. [Google Scholar] [CrossRef] [Green Version]
- Dash, G.K.; Barik, M.; Debata, A.K.; Baig, M.J.; Swain, P. Identification of most important rice root morphological markers in response to contrasting moisture regimes under vegetative stage drought. Acta Physiol. Plant. 2017, 39, 8. [Google Scholar] [CrossRef]
- Lou, Q.; Chen, L.; Mei, H.; Wei, H.; Feng, F.; Wang, P.; Xia, H.; Li, T.; Luo, L. Quantitative trait locus mapping of deep rooting by linkage and association analysis in rice. J. Exp. Bot. 2015, 66, 4749–4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uga, Y.; Kitomi, Y.; Yamamoto, E.; Kanno, N.; Kawai, S.; Mizubayashi, T.; Fukuoka, S. A QTL for Root growth angle on rice chromosome 7 is involved in the genetic pathway of deeper rooting 1. Rice 2015, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitomi, Y.; Nakao, E.; Kawai, S.; Kanno, N.; Ando, T.; Fukuoka, S.; Irie, K.; Uga, Y. Fine mapping of QUICK ROOTING 1 and 2, quantitative trait loci increasing root length in rice. G3 Genes Genomes Genet. 2018, 8, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guo, Z.; Lv, Y.; Cen, X.; Ding, X.; Wu, H.; Li, X.; Huang, J.; Xiong, L. Genetic control of the root system in rice under normal and drought stress conditions by genome-wide association study. PLoS Genet. 2017, 13, e1006889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corales, M.; Nguyen, N.T.A.; Abiko, T.; Mochizuki, T. Mapping quantitative trait loci for water uptake of rice under aerobic conditions. Plant Prod. Sci. 2020, 23, 436–451. [Google Scholar] [CrossRef]
- Sandhu, N.; Anitha Raman, K.; Torres, R.O.; Audebert, A.; Dardou, A.; Kumar, A.; Henry, A. Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol. 2016, 171, 2562–2576. [Google Scholar] [CrossRef] [Green Version]
- Uga, Y.; Okuno, K.; Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 2011, 62, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Qun, Z.; Ju, C.X.; Wang, Z.Q.; Zhang, H.; Liu, L.J.; Yang, J.C.; Zhang, J. Grain yield and water use efficiency of super rice under soil water deficit and alternate wetting and drying irrigation. J. Integr. Agric. 2017, 16, 1028–1043. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Halberg, N.; Nicolaisen, M.; Olesen, J.E.; Crews, T.E.; Hinsinger, P.; Kirkegaard, J.; Pierret, A.; Bodin Dresbøll, D. Digging deeper for agricultural resources, the value of deep rooting. Trends Plant Sci. 2020, 25, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice responses to rising temperatures—Challenges, perspectives and future directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, F.; Zhou, J.; Chen, F.; Wang, B.; Xie, X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-Responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Majhi, P.K.; Anandan, A.; Mahender, A.; Veludandi, S.; Bastia, D.; Guttala, S.B.; Singh, S.K.; Saha, S.; Ali, J. Proofing direct-seeded rice with better root plasticity and architecture. Int. J. Mol. Sci. 2021, 22, 6058. [Google Scholar] [CrossRef]
- Mallikarjuna Swamy, B.P.; Kumar, A. Sustainable rice yield in water-short drought-prone environments: Conventional and molecular approaches. In Irrigation Systems and Practices in Challenging Environments; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Anandan, A.; Meher, J.; Sah, R.P.; Samantaray, S.; Parameswaran, C.; Panneerselvam, P.; Dash, S.K.; Swain, P.; Kartikeyan, P.; Annamalai, M.; et al. Enhancing input use efficiency in direct-seeded rice with classical and molecular breeding. In Rice Research for Enhancing Productivity, Profitability and Climate Resilience; Pathak, H., Nayak, A.K., Jena, M., Singh, O.N., Samal, P., Sharma, S.G., Eds.; ICAR-NRRI: Cuttack, Indian, 2018; pp. 73–89. ISBN 81-88409-04-09. [Google Scholar]
- Chen, X.; Zhang, J.; Chen, Y.; Li, Q.; Chen, F.; Yuan, L.; Mi, G. Changes in root size and distribution in relation to nitrogen accumulation during maize breeding in China. Plant Soil 2014, 374, 121–130. [Google Scholar] [CrossRef]
- Isaac, M.E.; Nimmo, V.; Gaudin, A.C.M.; Leptin, A.; Schmidt, J.E.; Kallenbach, C.M.; Martin, A.; Entz, M.; Carkner, M.; Rajcan, I.; et al. Crop domestication, root trait syndromes, and soil nutrient acquisition in organic agroecosystems: A systematic review. Front. Sustain. Food Syst. 2021, 5, 317. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes that reduce the metabolic costs of soil exploration: Opportunities for 21st century agriculture. Plant Cell Environ. 2015, 38, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Mathew, I.; Shimelis, H.; Mutema, M.; Clulow, A.; Zengeni, R.; Mbava, N.; Chaplot, V. Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 2019, 205, 385–400. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and n acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).