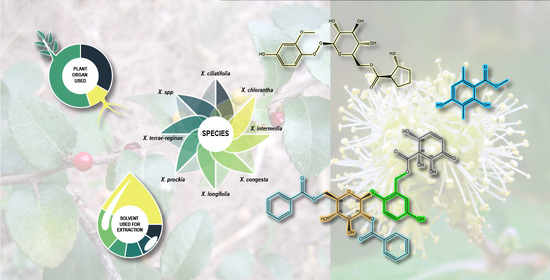
Xylosma G. Forst. Genus: Medicinal and Veterinary Use, Phytochemical Composition, and Biological Activity
Abstract
:1. Introduction
2. Genus
In shrubs or small trees, often with axillary spines, the branchlets commonly lenticellate. Leaves alternate, sometimes borne in fascicles, usually short-petiolate, estipulate, the blade is often ±coriaceous, usually glandular-dentate, penninerved, rarely entire-margined, without pellucid-glands. Inflorescences axillary, fasciculate or contracted-racemose, and are rarely racemose. Flowers are small, dioecious, or rarely polygamous; pedicels are articulated above the base, and the bracts are minute; sepals 4-5(-6), imbricate, usually scale-like, slightly connate at the base, often ciliolate along the margins, usually persistent; petals none; stamens ∞ (8–35 in Panamanian spp.), usually surrounded by an annular or glandular, fleshy disc, the filaments free, filiform, short- to usually long-exserted, the anthers minute, basifixed, extrose, longitudinally dehiscent; ovary sessile, inserted on an annular disc, 1-locular, with 2–3, rarely 4–6, parietal placentas, each placenta with 2, sometimes 4–6, ovules, the style entire or ±divided, sometimes very short, the stigmas scarcely dilated to dilated; rudimentary ovary wanting in male flowers. Fruits baccate, rather dry, indehiscent, surmounted by the persistent style, the pericarp rather thin-coriaceous, the seeds 2–8, +angular by mutual pressure, the testa thin; endosperm copious; embryo large, the cotyledons broad.
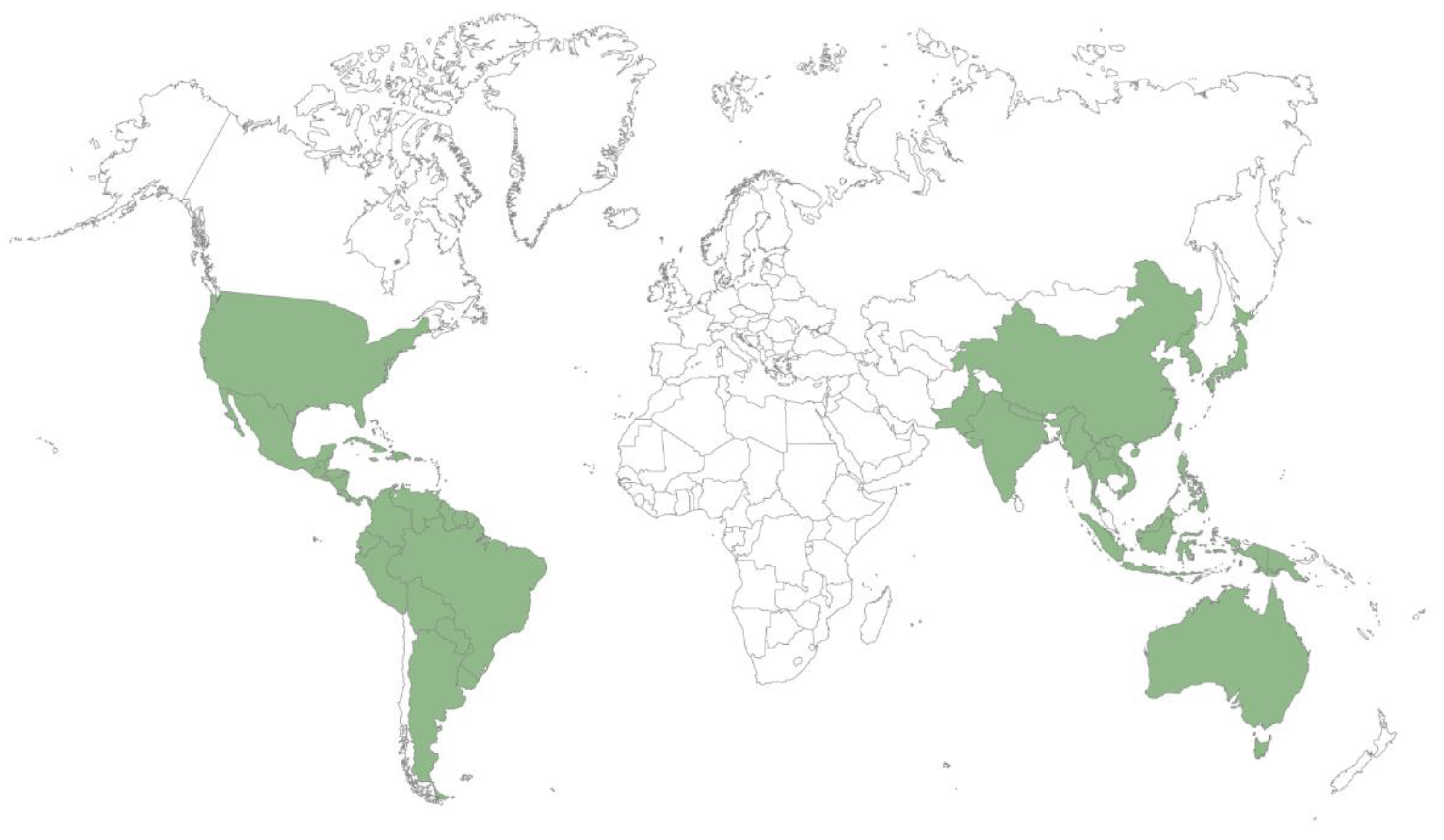
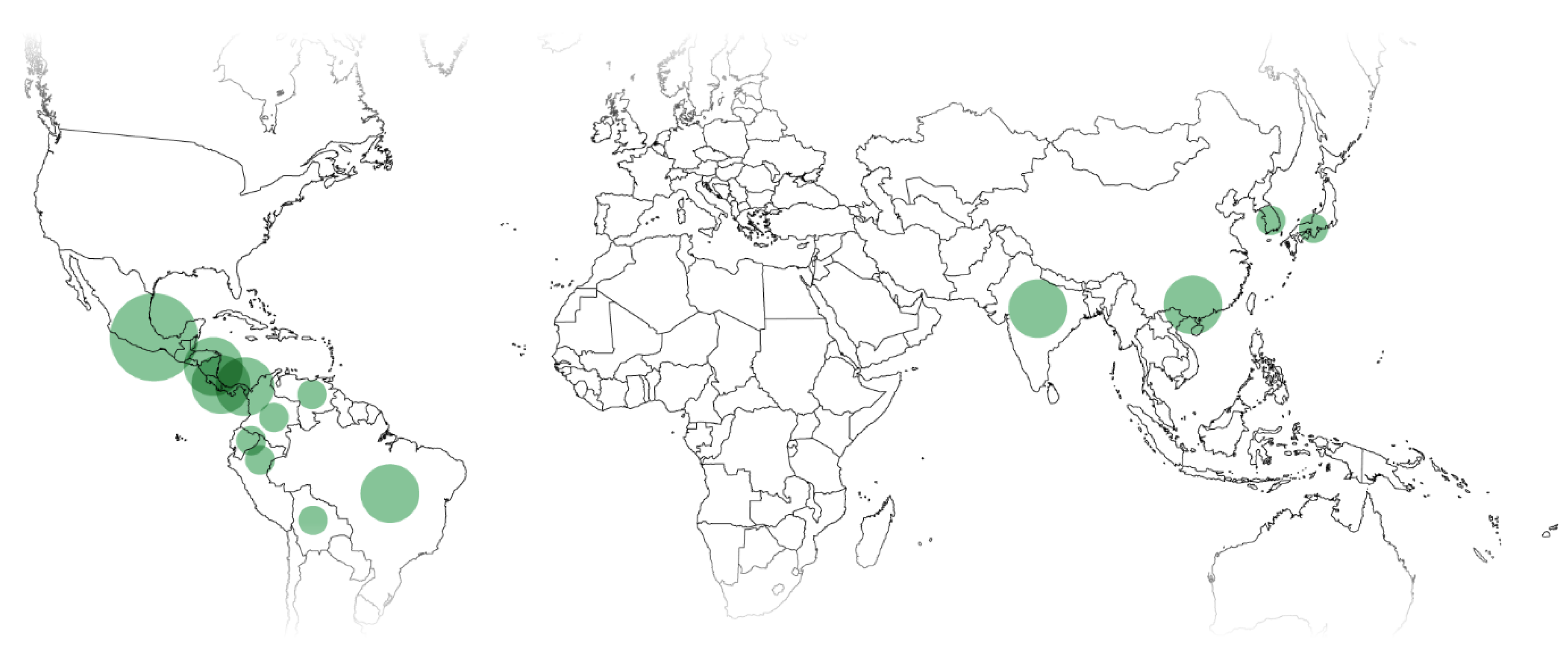
3. Distribution and Localization
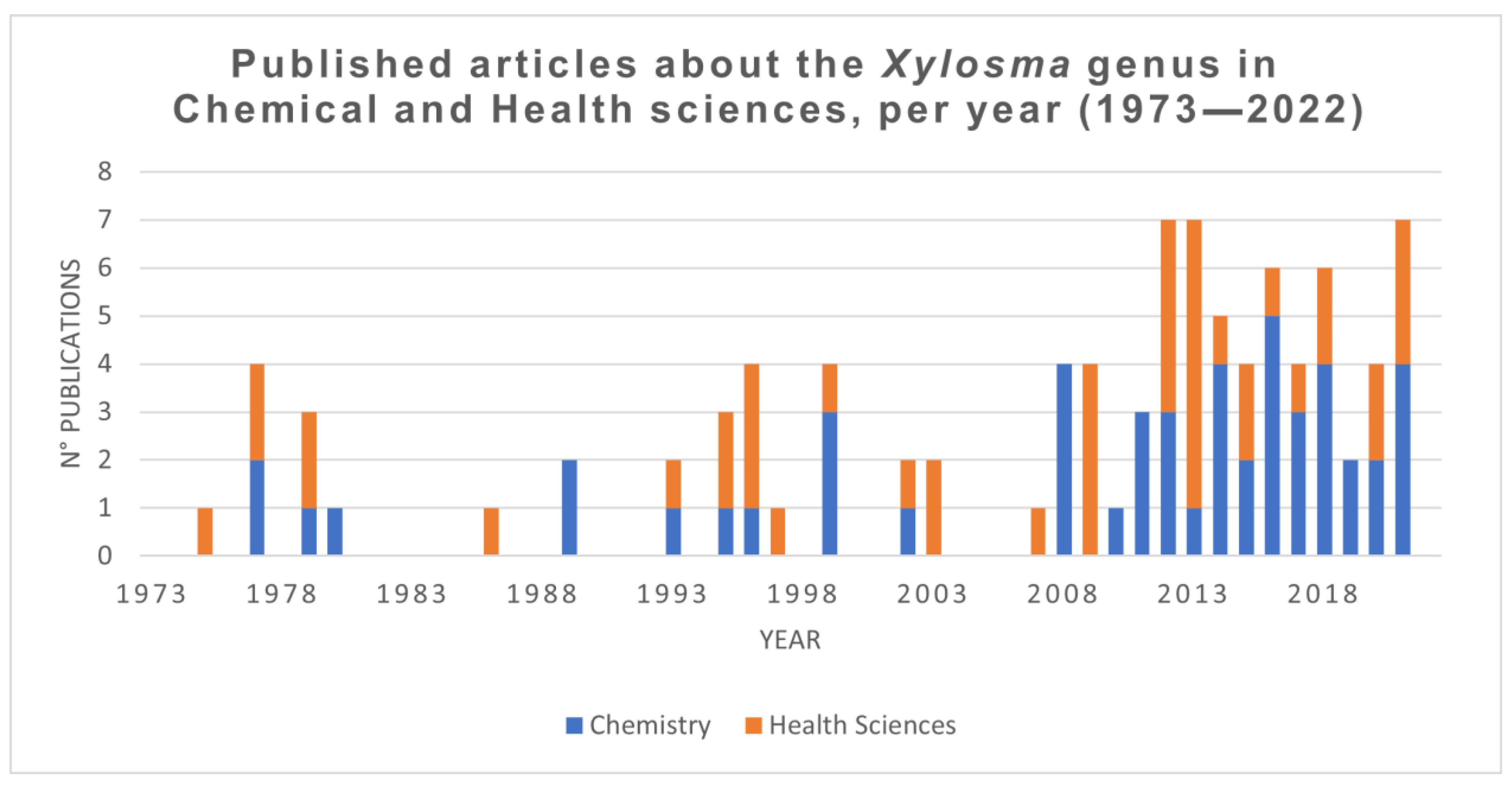
4. Methodology
5. Ethnopharmacological and Ethnoveterinary Usage
6. Biological Activity
In Vitro Activity
7. Phytochemical Composition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, K. Paleomedicine and the Evolutionary Context of Medicinal Plant Use. Rev. Bras. Farmacogn. 2021, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martín, E.M.; Tavares, J.; Ríjo, P.; Díaz-Lanza, A.M. Zoopharmacology: A Way to Discover New Cancer Treatments. Biomolecules 2020, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Solecki, R.S. Shanidar IV, a Neanderthal Flower Burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- ur Rehman, F.; Kalsoom, M.; Adnan, M.; Fazeli-Nasab, B.; Naz, N.; Ilahi, H.; Ali, M.F.; Ilyas, M.A.; Yousaf, G.; Toor, M.D.; et al. Importance of Medicinal Plants in Human and Plant Pathology: A Review. Int. J. Pharm. Biomed. Rese 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal Plants: Past History and Future Perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug Discovery from Plant Sources: An Integrated Approach. AYU Int. Q. J. Res. Ayurveda 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciênc. 2019, 91, e20190105. [Google Scholar] [CrossRef]
- Salicaceae Mirb.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002644-2 (accessed on 25 January 2022).
- Xylosma G. Forst.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:332071-2 (accessed on 25 January 2022).
- WFO. Xylosma G. Forst. Available online: http://www.worldfloraonline.org/taxon/wfo-4000041044;jsessionid=402EBB0471E61A8E4D367F550003835E (accessed on 25 January 2022).
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Uphof, J. The Dictionary of Economic Plants; Cramer: Lehre, Germany, 1968; ISBN 978-3-7682-0001-1. [Google Scholar]
- Berry, S.S.; Chapman, J.; Jones, G.; Jones, J.; Pass, J.; Roper, C.F.E.; Wilkes, J. Encyclopaedia Londinensis, or, Universal Dictionary of Arts, Sciences, and Literature: Comprehending, under One General Alphabetical Arrangement, All the Words and Substance of Every Kind of Dictionary Extant in the English Language: In Which the Improved Departments of the Mechanical Compiled, Digested, and Arranged, by John Wilkes, of Milland House…; Assisted by Eminent Scholars of the English, Scotch, and Irish, Universities; Champante and Whitrow: London, UK, 1810. [Google Scholar]
- Woodson, R.E.; Schery, R.W.; Robyns, A. Flora of Panama. Family 128. Flacourtiaceae. Ann. Mo. Bot. Gard. 1968, 55, 93. [Google Scholar] [CrossRef]
- Vossler, F.G. Flower Visits, Nesting and Nest Defence Behaviour of Stingless Bees (Apidae: Meliponini): Suitability of the Bee Species for Meliponiculture in the Argentinean Chaco Region. Apidologie 2012, 43, 139–161. [Google Scholar] [CrossRef] [Green Version]
- García, R.; Pegüero, B.; Jiménez, F.; Veloz, A.; Clase, T. Lista Roja de La Flora Vascular en República Dominicana; Ministerio de Educación Superior Ciencia y Tecnología (MESCyT): Santo Domingo, Dominican Republic, 2016. [Google Scholar]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Ent, A. A Global Database for Plants That Hyperaccumulate Metal and Metalloid Trace Elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological Role of Nickel and Its Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Chaney, R.L.; Angle, J.S.; Broadhurst, C.L.; Peters, C.A.; Tappero, R.V.; Sparks, D.L. Improved Understanding of Hyperaccumulation Yields Commercial Phytoextraction and Phytomining Technologies. J. Environ. Qual. 2007, 36, 1429–1443. [Google Scholar] [CrossRef] [Green Version]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 1 February 2022).
- Digital Science Dimensions [Software]. Available online: https://app.dimensions.ai/analytics/publication/overview/timeline (accessed on 18 June 2021).
- Harzing, A.W. Publish or Perish; Version 8.2; Tarma Software Research, Ltd.: Hertfordshire, UK, 2021. [Google Scholar]
- Zhang, Z.-S.; Zeng, Q.-Y.; Liu, Y.-J. Frequent Ploidy Changes in Salicaceae Indicates Widespread Sharing of the Salicoid Whole Genome Duplication by the Relatives of Populus L. and Salix L. BMC Plant Biol. 2021, 21, 535. [Google Scholar] [CrossRef]
- Ye, H.; Li, C.; Ye, W.; Zeng, F.; Liu, F.; Liu, Y.; Wang, F.; Ye, Y.; Fu, L.; Li, J. Medicinal Angiosperms of Flacourtiaceae, Tamaricaceae, Passifloraceae, and Cucurbitaceae. In Common Chinese Materia Medica; Ye, H., Li, C., Ye, W., Zeng, F., Eds.; Springer: Singapore, 2021; pp. 51–130. ISBN 9789811658792. [Google Scholar]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 8 June 2021).
- Van den Berg, M.E.; da Silva, M.H.L.; da Silva, M.G. Plantas Aromáticas Da Amazônia. In Proceedings of the 1er Simpósio do Trópico Úmido, 1986; Volume II, p. 95. [Google Scholar]
- Rodríguez-Flores, O.; Torréz-Centeno, E.; Valenzuela-Betanco, R. Plantas Utilizadas Para el Tratamiento de Enfermedades en los Animales Domésticos, Reserva Natural El Tisey, Estelí. Ph.D. Thesis, Universidad Católica Agropecuaria del Trópico Seco Pbro, Francisco Luis Espinoza Pineda, Estelí, Nicaragua, 2005. [Google Scholar]
- Juep, A. Rescate del Conocimiento Tradicional y Biológico Para el Manejo de Productos Forestales no Maderables en la Comunidad Indígena Jameykari, Costa Rica. Master’s Thesis, Centro Agronómico Tropical de Educación y Enseñanza, Turrialba, Costa Rica, 2008. [Google Scholar]
- Philippsen, A.F.; Miguel, O.G.; Miguel, M.D.; de Lima, C.P.; Kalegari, M.; Lordello, A.L.L. Validation of the antibacterial activity of root bark of Xylosma ciliatifolia (Clos) Eichler (Flacourtiaceae/Salicaceae sensu lato). Rev. Cuba. Plantas Med. 2013, 18, 258–267. [Google Scholar]
- Shizhen, L. Ben Cao Gang Mu, Volume II: Waters, Fires, Soils, Metals, Jades, Stones, Minerals, Salts; University of California Press: Berkeley, CA, USA, 2021; ISBN 978-0-520-97697-9. [Google Scholar]
- Lee, J.Y.; Ahn, E.-K.; Ko, H.-J.; Cho, Y.-R.; Ko, W.C.; Jung, Y.-H.; Choi, K.-M.; Choi, M.-R.; Oh, J.S. Anti-Melanogenic, Anti-Wrinkle, Anti-Inflammatory and Anti-Oxidant Effects of Xylosma congesta Leaf Ethanol Extract. J. Appl. Biol. Chem. 2014, 57, 365–371. [Google Scholar] [CrossRef]
- Yungui, W. A Disease Control Method for Suckling Piglets. Patent CN108235969A, 3 July 2018. [Google Scholar]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Medicinal Plants of the World; Reference Publications: Algonac, MI, USA, 1985; ISBN 978-0-917256-20-2. [Google Scholar]
- Xu, Z.-R.; Chai, X.-Y.; Bai, C.-C.; Ren, H.-Y.; Lu, Y.-N.; Shi, H.-M.; Tu, P.-F. Xylocosides A-G, Phenolic Glucosides from the Stems of Xylosma controversum. Helv. Chim. Acta 2008, 91, 1346–1354. [Google Scholar] [CrossRef]
- Cornejo-Báez, A. Evaluación de la actividad antibacteriana de los extractos y fracciones de Bidens pilosa L. y Xylosma flexuosum (H. B. & K.) Hemsl y estudio quimiométrico de la actividad antituberculosa de los perfiles cromatográficos de Bidens pilosa L. Master’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 2016. [Google Scholar]
- Grijalva Pineda, A. Flora útil: Etnobotánica de Nicaragua; Ministerio del Ambiente y Recursos Naturales de Nicaragua, Ed.; MARENA: Managua, Gobierno de Nicaragua, 2006; ISBN 978-99924-903-8-9. [Google Scholar]
- Pérez-Torres, F. Manual de Plantas Medicinales más Comunes del Occidente de Nicaragua; Universidad Autónoma de Nicaragua: León, Spain, 2007. [Google Scholar]
- Grandtner, M.M. Elsevier’s Dictionary of Trees: With Names in Latin, English, French, Spanish and Other Languages, 1st ed.; Elsevier: Amsterdam, The Netherlands; San Diego, CA, USA, 2005; ISBN 978-0-444-51784-5. [Google Scholar]
- Devi, W.R.; Singh, S.B.; Singh, C.B. Antioxidant and Anti-Dermatophytic Properties Leaf and Stem Bark of Xylosma longifolium Clos. BMC Complement. Altern. Med. 2013, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Truong, B.N.; Pham, V.C.; Mai, H.D.T.; Nguyen, V.H.; Nguyen, M.C.; Nguyen, T.H.; Zhang, H.; Fong, H.H.S.; Franzblau, S.G.; Soejarto, D.D.; et al. Chemical Constituents from Xylosma longifolia and Their Anti-Tubercular Activity. Phytochem. Lett. 2011, 4, 250–253. [Google Scholar] [CrossRef]
- Swapana, N.; Noji, M.; Nishiuma, R.; Izumi, M.; Imagawa, H.; Kasai, Y.; Okamoto, Y.; Iseki, K.; Singh, C.B.; Asakawa, Y.; et al. A New Diphenyl Ether Glycoside from Xylosma longifolium Collected from North-East India. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef] [Green Version]
- Salam, S. Medicinal Plant Used for the Treatment of Muscular Sprain by the Tangkhul Tribe of Ukhrul District, Manipur, India. Int. J. Adv. Res. 2020, 8, 167–170. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Reference; Springer: New York, NY, USA, 2007; ISBN 978-0-387-70637-5. [Google Scholar]
- Joly, L.G.; Guerra, S.; Séptimo, R.; Solís, P.N.; Correa, M.; Gupta, M.; Levy, S.; Sandberg, F. Ethnobotanical Inventory of Medicinal Plants Used by the Guaymi Indians in Western Panama. Part I. J. Ethnopharmacol. 1987, 20, 145–171. [Google Scholar] [CrossRef]
- García-Barriga, H. Flora Medicinal de Colombia: Botánica Médica; Instituto de Ciencias Naturales: Bogotá, Colombia, 1992. [Google Scholar]
- Cerón, C.; Montalvo, C. Reserva Biológica Limoncocha: Formaciones Vegetales, Diversidad y Etnobotánica. Cinchonia 2000, 1, 20. [Google Scholar]
- Caballero-George, C.; Gupta, M. A Quarter Century of Pharmacognostic Research on Panamanian Flora: A Review. Planta Med. 2011, 77, 1189–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M. Una paz Incierta: Comunidades Aguarunas Frente al Impacto de la Carretera Marginal; Serie Antroplógica; CAAAP: Magdalena, Perú, 1984; ISBN 84-89290-008-0. [Google Scholar]
- Vasquez, Y. Biological and Chemical Investigation of Panamanian Plants for Potential Utility against Metabolic Syndrome; University of Mississippi: Oxford, MS, USA, 2016. [Google Scholar]
- Setzer, M.C.; Moriarity, D.M.; Lawton, R.O.; Setzer, W.N.; Gentry, G.A.; Haber, W.A. Phytomedicinal Potential of Tropical Cloudforest Plants from Monteverde, Costa Rica. Rev. Biol. Trop. 2003, 51, 647–673. [Google Scholar]
- Folly, M.L.C.; Ferreira, G.F.; Salvador, M.R.; Sathler, A.A.; da Silva, G.F.; Santos, J.C.B.; dos Santos, J.R.A.; Nunes Neto, W.R.; Rodrigues, J.F.S.; Fernandes, E.S.; et al. Evaluation of In Vitro Antifungal Activity of Xylosma prockia (Turcz.) Turcz. (Salicaceae) Leaves Against Cryptococcus spp. Front. Microbiol. 2020, 10, 3114. [Google Scholar] [CrossRef] [Green Version]
- Mosaddik, M.A.; Banbury, L.; Forster, P.; Booth, R.; Markham, J.; Leach, D.; Waterman, P.G. Screening of Some Australian Flacourtiaceae Species for in vitro Antioxidant, Cytotoxic and Antimicrobial Activity. Phytomedicine 2004, 11, 461–466. [Google Scholar] [CrossRef]
- Castro, S.B.R.; Leal, C.A.G.; Freire, F.R.; Carvalho, D.A.; Oliveira, D.F.; Figueiredo, H.C.P. Antibacterial Activity of Plant Extracts from Brazil against Fish Pathogenic Bacteria. Braz. J. Microbiol. 2008, 39, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V.; Seo, E.-J.; Krusche, B.; Oswald, M.; Wiench, B.; Schröder, S.; Greten, H.J.; Lee, I.-S.; Efferth, T. Cytotoxicity and Pharmacogenomics of Medicinal Plants from Traditional Korean Medicine. Evid. Based Complement. Alternat. Med. 2013, 2013, 341724. [Google Scholar] [CrossRef]
- Jones, E.; Ekundayo, O.; Kingston, D.G.I. Plant Anticancer Agents. XI. 2,6-Dimethoxybenzoquinone as a Cytotoxic Constituent of Tibouchina pulchra. J. Nat. Prod. 1981, 44, 493–494. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Sáenz, J.A.; Galvez, N. Phytochemical Screening of Costa Rican Plants: Alkaloid Analysis. V. Rev. Biol. Trop. 1980, 28, 1–11. [Google Scholar]
- Bhattacharyya, R.; Boruah, J.; Medhi, K.; Borkataki, S. Phytochemical Analysis of Leaves of Xylosma longifolia Clos.: A Plant of Ethnomedicinal Importance. Int. J. Pharm. Sci. Res. 2020, 11, 2065–2074. [Google Scholar] [CrossRef]
- Bhaumik, P.K.; Guha, K.P.; Biswas, G.K.; Mukherjee, B. (−)Flacourtin, a Phenolic Glucoside Ester from Flacourtia indica. Phytochemistry 1987, 26, 3090–3091. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Singh, S.V.; Srivastava, R.K.; Srivastava, K.; Saxena, J.K.; Puri, S.K. Isolation and Identification of β-Hematin Inhibitors from Flacourtia indica as Promising Antiplasmodial Agents. Eur. J. Med. Chem. 2013, 60, 497–502. [Google Scholar] [CrossRef]
- Bourjot, M.; Leyssen, P.; Eydoux, C.; Guillemot, J.-C.; Canard, B.; Rasoanaivo, P.; Guéritte, F.; Litaudon, M. Flacourtosides A–F, Phenolic Glycosides Isolated from Flacourtia ramontchi. J. Nat. Prod. 2012, 75, 752–758. [Google Scholar] [CrossRef]
- Prangé, T.; Lavaud, C.; Massiot, G. A Reappraisal of the Structure of Xylosmin. Phytochem. Lett. 2021, 41, 123–124. [Google Scholar] [CrossRef]
- Ghavre, M.; Froese, J.; Murphy, B.; Simionescu, R.; Hudlicky, T. A Formal Approach to Xylosmin and Flacourtosides E and F: Chemoenzymatic Total Synthesis of the Hydroxylated Cyclohexenone Carboxylic Acid Moiety of Xylosmin. Org. Lett. 2017, 19, 1156–1159. [Google Scholar] [CrossRef]
- Xiao-Ping, P. Inhibition of Phosphodiesterase Activity by Chemical Constituents of Xylosma controversum Clos and Scolopia Chinensis(Lour.) Clos. Chin. J. New Drugs 2010, 19, 147–151. [Google Scholar]
- Yu, Y.; Zhou, L.; Sun, M.; Zhou, T.; Zhong, K.; Wang, H.; Liu, Y.; Liu, X.; Xiao, R.; Ge, J.; et al. Xylocoside G Reduces Amyloid-β Induced Neurotoxicity by Inhibiting NF-ΚB Signaling Pathway in Neuronal Cells. J. Alzheimers Dis. 2012, 30, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Chang, P.T.; Fong, H.H.S.; Farnsworth. Xylosmacin, a New Phenolic Glucoside Ester from Xylosma velutina (Flacourtiaceae). Lloydia-J. Nat. Prod. 1977, 40, 340–343. [Google Scholar]
- Wang, X.; Yu, W.; Lou, H. Antifungal Constituents from the Chinese Moss Homalia trichomanoides. Chem. Biodivers. 2005, 2, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-K.; Kang, K.-Y.; Jang, H.-Y.; Hwang, Y.-H.; Hong, S.-G.; Kim, S.-J.; Cho, H.-W.; Chang, D.-J.; Hur, J.-S.; Yee, S.-T. Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models. Int. J. Mol. Sci. 2020, 21, 7070. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.; Schleich, S.; Prade, I.; Degen, S.; Roell, D.; Schubert, U.; Tanner, T.; Claessens, F.; Matusch, R.; Baniahmad, A. The Natural Compound Atraric Acid Is an Antagonist of the Human Androgen Receptor Inhibiting Cellular Invasiveness and Prostate Cancer Cell Growth. J. Cell. Mol. Med. 2009, 13, 2210–2223. [Google Scholar] [CrossRef]
- Hoffmann, H.-R.; Matusch, R.; Baniahmad, A. Isolation of Atraric Acid, Synthesis of Atraric Acid Derivatives, and Use of Atraric Acid and the Derivatives Thereof for the Treatment of Benign Prostatic Hyperplasia, Prostate Carcinoma and Spinobulbar Muscular Atrophy. U.S. Patent 8,481,519, 9 July 2013. [Google Scholar]
- Chang, P.T.O.; Cordell, G.A.; Fong, H.H.S.; Farnsworth, N.R. Velutinic Acid, a New Friedelane Derivative from Xylosma velutina (Flacourtiaceae). Phytochemistry 1977, 16, 1443–1445. [Google Scholar] [CrossRef]
- Gibbons, S.; Gray, A.I.; Waterman, P.G.; Hockless, D.C.R.; Skelton, B.W.; White, A.H. Benzoylated Derivatives of 2-β-Glucopyranosyloxy-2,5-Dihydroxybenzyl Alcohol from Xylosma flexuosum: Structure and Relative Configuration of Xylosmin. J. Nat. Prod. 1995, 58, 554–559. [Google Scholar] [CrossRef]
- Nasr-Bouzaiene, N.; Sassi, A.; Bedoui, A.; Krifa, M.; Chekir-Ghedira, L.; Ghedira, K. Immunomodulatory and Cellular Antioxidant Activities of Pure Compounds from Teucrium ramosissimum Desf. Tumor Biol. 2016, 37, 7703–7712. [Google Scholar] [CrossRef]
- Parveen, M.; Ghalib, R.M. Flavonoids and Antimicrobial Activity of Leaves of Xylosma longifolium. J. Chil. Chem. Soc. 2012, 57, 989–991. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba Extract Kaempferol Inhibits Cell Proliferation and Induces Apoptosis in Pancreatic Cancer Cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, S.; Ali, M.; Jameel, M. Aliphatic Constituents from the Leaves of Dillenia indica L., Halothamus bottae Jaub. and Xylosma longifolium Clos. Chem. Res. J. 2018, 3, 109–117. [Google Scholar]
- Wilt, T.J.; Ishani, A.; MacDonald, R.; Stark, G.; Mulrow, C.D.; Lau, J. Beta-Sitosterols for Benign Prostatic Hyperplasia. Cochrane Database Syst. Rev. 1999, 2011, CD001043. [Google Scholar] [CrossRef] [PubMed]
- Acton, P.; Ashton, Q. Advances in Chlorophyll Research and Application; Scholarly Media LLC: Atlanta, GA, USA, 2012; ISBN 978-1-4649-9275-9. [Google Scholar]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and Its Derivatives as Anticancer and Anti-Inflammatory Agents: Molecular Mechanisms and Therapeutic Efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Hong-Yan, R.; Ya-Nan, L.; Xing-Yun, C.; Peng-Fei, T.; Zheng-Ren, X. Chemical Constituents from Xylosma controversum. J. Chin. Pharm. Sci. 2007, 16, 218–222. [Google Scholar]
- Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Antioxidant, Free Radical Scavenging and Liver Protective Effects of Friedelin Isolated from Azima tetracantha Lam. Leaves. Food Chem. 2013, 139, 860–865. [Google Scholar] [CrossRef]
- Levy, L. The Activity of Chaulmoogra Acids against Mycobacterium leprae. Am. Rev. Respir. Dis. 1975, 111, 703–705. [Google Scholar] [CrossRef]
- Cabot, M.C.; Goucher, C.R. Chaulmoogric Acid: Assimilation into the Complex Lipids of Mycobacteria. Lipids 1981, 16, 146–148. [Google Scholar] [CrossRef]
- Cher, C.; Tremblay, M.-H.; Barber, J.R.; Chung Ng, S.; Zhang, B. Identification of Chaulmoogric Acid as a Small Molecule Activator of Protein Phosphatase 5. Appl. Biochem. Biotechnol. 2010, 160, 1450–1459. [Google Scholar] [CrossRef]
- Miyazawa, M.; Utsunomiya, H.; Inada, K.; Yamada, T.; Okuno, Y.; Tanaka, H.; Tatematsu, M. Inhibition of Helicobacter pylori Motility by (+)-Syringaresinol from Unripe Japanese Apricot. Biol. Pharm. Bull. 2006, 29, 172–173. [Google Scholar] [CrossRef] [Green Version]


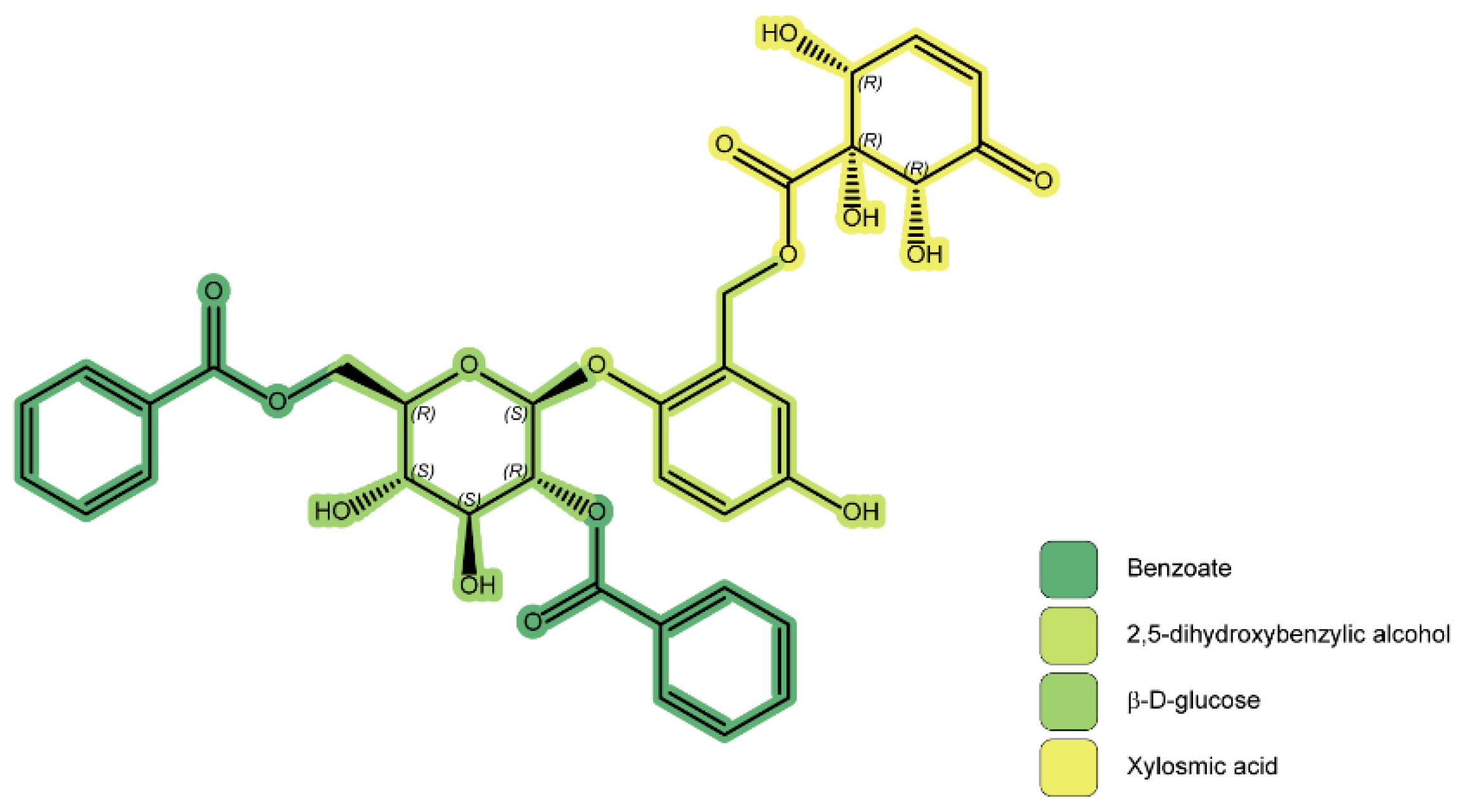
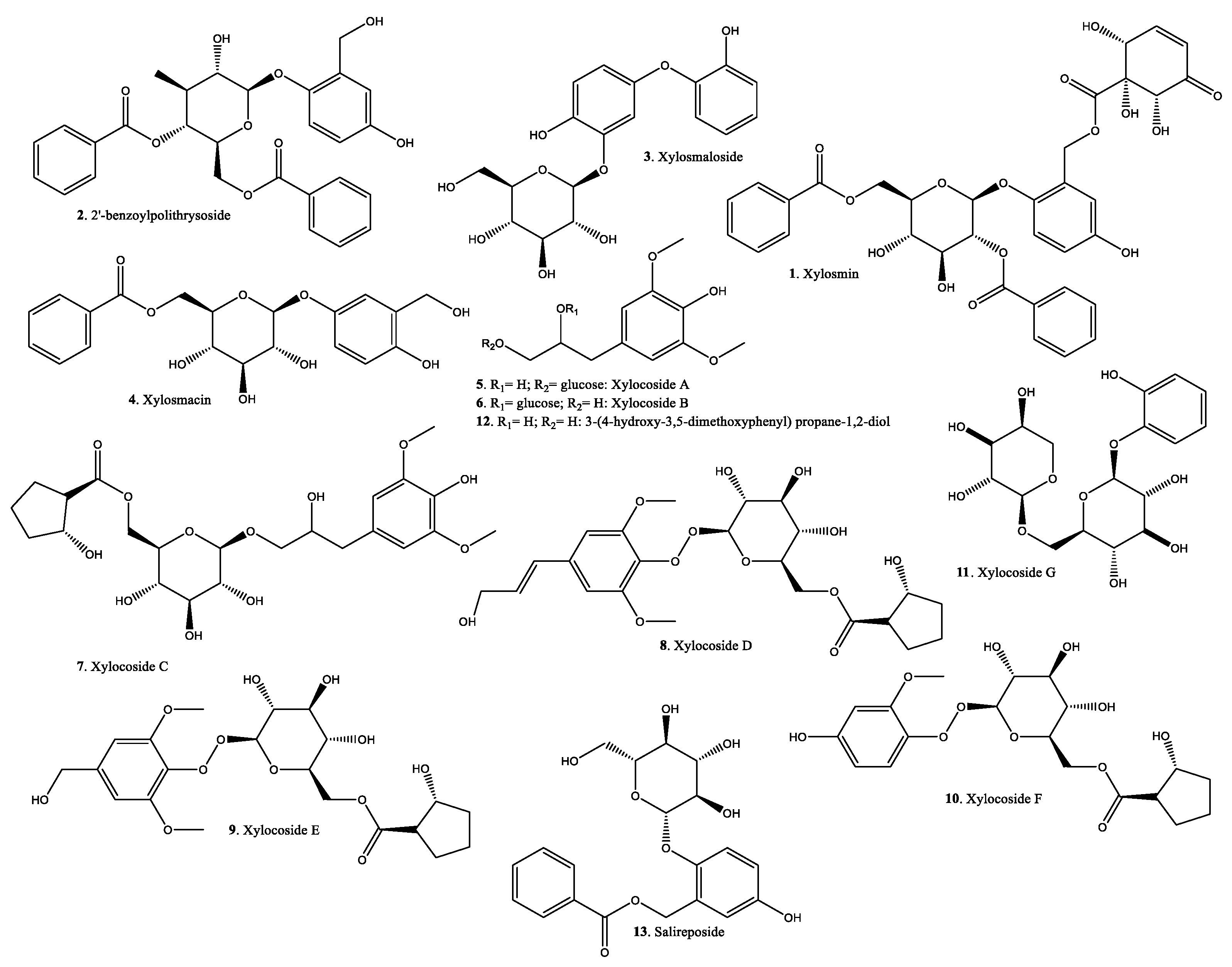
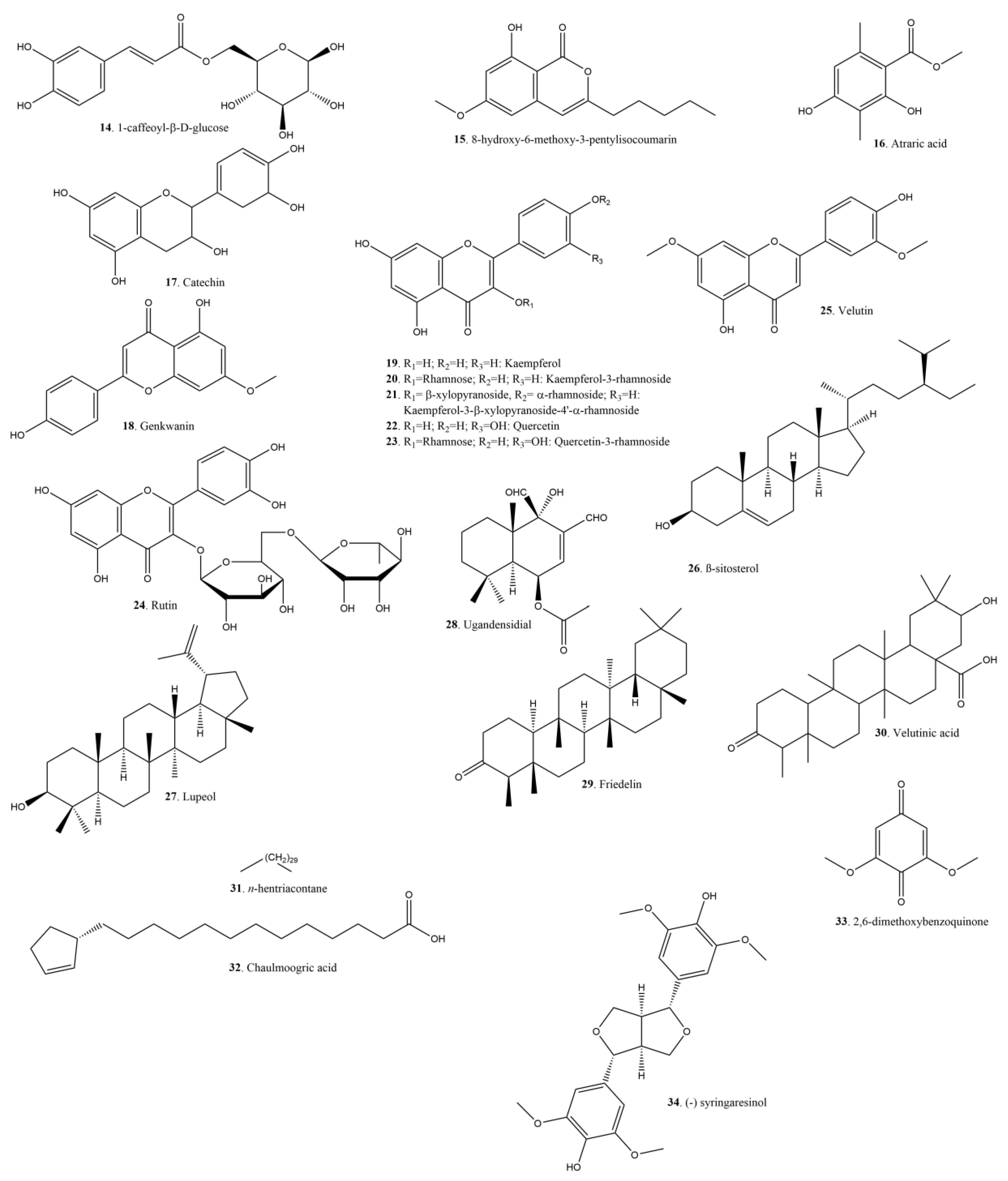
| No. | Species | Region | Plant Organs Used | Use | Form of Usage | ATC Category | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Xylosma benthamii (Tul.) Triana and Planch. | Brazil | NS | Medicinal (not specified) | NS | NS | [27] |
| 2 | Xylosma characantha Standl. | Nicaragua | Leaves | Placentary retention in cattle | Decoction | Vet. | [28] |
| 3 | Xylosma chlorantha Donn. Sm. | Costa Rica | Bark | Medicinal (not specified) | NS | NS | [29] |
| 4 | Xylosma ciliatifolia (Clos) Eichler | Brazil | Root bark | Antibacterial | NS | V | [30] |
| 5 | Xylosma congesta (Lour.) Merr. | China Japan Korea | Bark Leaves | NS Anti-inflammatory Disease prevention in suckling piglets Birthing aid | Bark ashes Poultice | NS D G Vet. | [31] [32] [33] [34] |
| 6 | Xylosma controversa Clos. | Guangxi, China | Roots Leaves | NS | NS | NS | [35] |
| 7 | Xylosma flexuosa (Kunth) Hemsl. | Mexico | NS | Antipyretic Anti-tuberculosis | NS | N R | [36,37] |
| 8 | Xylosma horrida Rose. | Mexico Nicaragua Costa Rica | Bark | Kidneys | Decoction | G | [38] |
| 9 | Xylosma intermedia (Seem.) Triana and Planch. | Bolivia | Bark | Toothache | NS | N | [39] |
| 10 | Xylosma longifolia Clos | India China | Leaves Stem bark | Antifungal Antispasmodic Antidiarrheic Anti-tuberculosis Muscle sprains Narcotic | Paste Decoction Extract | D A A R M N | [40] [41] [42] [43] [44] |
| 11 | Xylosma panamensis (Turcz) | Panama Mexico | Bark Leaves | Cough Bronchitis | Dried | R | [45] |
| 12 | Xylosma spiculifera (Tul.) Triana and Planch | Colombia, Venezuela | Leaves | Ulcers, Dermatitis | Decoction | D | [46] |
| 13 | Xylosma tessmanii Sleumer | Ecuador | Leaves | Medicinal (NS) | NS | NS | [47] |
| 14 | Xylosma sp. (not specified) | Panama | Stem Root | Spider bites | Infusion | V | [48] |
| 15 | Xylosma sp. (not specified) | Perú | Bark | Bronchitis (with other plant species) | Decoction | R | [49] |
| Species | Extract | Plant Organs Used | Biological Activity | Biological Model | Effect | Methodology | Ref. |
|---|---|---|---|---|---|---|---|
| X. ciliatifolia | Ethanol/Hexane partition | Root bark | Antibacterial | S. aureus S. epidermis S. typihimurium E. coli | Effective against S. aureus S. epidermis MIC (µg/mL) 250, 500 | Disk diffusion assay | [30] |
| X. clorantha | Ethanol | Leaves | Metabolic syndrome | HepG2 cells | LXR 2.14 ± 0.11: 100 µg/mL | LXR transcriptional activity | [50] |
| X. congesta | Ethanol | Leaves | Anti-melanogenic | B16F10 cells | Melanin synthesis inhibition: up to 57.9% | α-MSH | [32] |
| X. intermedia | DCM/Ethanol | Bark | Antibacterial | Bacillus cereus S. aureus | MIC (ppm) 156 512 | Microbroth dilution | [51] |
| X. longifolia | Petroleum ether Chloroform Methanol | Leaves, Stem bark | Antifungal | Microsporum boullardii,
M. canis, M. gypseum Trichophyton ajelloi T. rubrum | MIC (mg/mL) 0.141–9.0 | Agar diffusion Micro wells diffusion | [40] |
| X. prockia | Ethanol | Leaves | Antifungal | Cryptococcus spp. | MIC (ppm) 8–64 | Antifungal microdilution susceptibility standard test | [52] |
| X. terrae reginae | Methanol | Root | Antibacterial Antifungal | S. aureus C. albicans | MIC (mg/mL) 2.5 1.2 | Dilution method | [53] |
| X. sp II | Methanol | Leaves | Antibacterial | Flavobacterium columnae | MIC 375 µg/mL | Agar diffusion assay | [54] |
| No. | Compound | Identified/Isolated | Species | Collection Area | Plant Organ Used | Use | Effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Xylosmin | Y/Y | X. velutina X. flexuosa | Colombia Guanacaste, Costa Rica | Aerial parts | Antiviral Anti venom | RNA polymerase inhibition PDE inhibition | [72] [73] [62] [65] |
| 2 | 2′-benzoylpoliothrysoside | Y/Y | X. flexuosa | Guanacaste, Costa Rica | Aerial parts | [73] | ||
| 3 | Xylosmaloside | Y/Y | X. longifolia | North-east India | NS | Antioxidant | [42] | |
| 4 | Xylosmacin | Y/Y | X. velutina | NS | Stem bark | [67] | ||
| 5 6 7 8 9 10 | Xylocosides A-F | Y/Y | X. controversa | Guangxi, China | Stems | [35] | ||
| 11 | Xylocoside G | Y/Y | X. controversa | Guangxi, China | Stems | Neuroprotective | [35] [66] | |
| 12 | 3-(4-hydroxy-3,5-dimethoxyphenyl)propane-1,2-diol | Y/Y | X. controversa | Guangxi, China | Stems | [35] | ||
| 13 | Salireposide | Y/Y | X. flexuosa | Guanacaste, Costa Rica | Aerial parts | [73] | ||
| 14 | 1-caffeoyl-β-d-glucose | Y/ | X. prockia | Minas Gerais, Brazil | Leaves | Antifungal | [52] | |
| 15 | 8-hydroxy-6-methoxy-3-pentylisocoumarin | Y/Y | X. longifolia | Cuc Phuong, Vietnam | Stem bark | Antituberculosis | MIC: 40.5 µg/mL | [41] |
| 16 | Atraric acid | Y/Y | X. longifolia X. velutina | Manipur, India NS | Leaves Bark | Antifungal Antiproliferative | [40] [70] | |
| 17 | Catechin | Y/Y | X. longifolia X. controversa | Manipur, India China | Leaves | Antifungal PDE inhibitor | [40] [65] | |
| 18 | Genkwanin | Y/Y | X. velutina | Colombia | leaves, twigs and inflorescences | Immunomodulator | [72] [74] | |
| 19 | Kaempferol | Y/Y | X. longifolia | Dehradun, India | Leaves | Antiproliferative | [75] [76] | |
| 20 | Kaempferol-3-rhamnoside | Y/Y | X. longifolia | Dehradun, India | Leaves | Antioxidant | [75] | |
| 21 | Kaempferol-3-β-xylopyranoside-4′-α-rhamnoside | Y/Y | X. longifolia | Dehradun, India | Leaves | Antioxidant | [75] | |
| 22 | Quercetin | Y/Y | X. longifolia | Dehradun, India | Leaves | Antioxidant | [75] [77] | |
| 23 | Quercetrin-3-rhamnoside | Y/Y | X. longifolia | Dehradun, India | Leaves | Antioxidant | [75] | |
| 23 | Rutin | Y/ | X. longifolia | Manipur, India | Leaves | Antifungal Antioxidant | [40] | |
| 25 | Velutin | Y/Y | X. velutina | Colombia | Leaves, twigs and inflorescences | [72] | ||
| 26 | β-sitosterol | Y/Y | X. longifolia | Delhi, India | Leaves | Benign prostate hyperplasia symptom relief | [78] [79] | |
| 27 | Lupeol | Y/Y | X.flexuosa | Guerrero, Mexico | Leaves | Anti-inflammatory | [80] [81] | |
| 28 | Ugandensidial | Y/Y | X. ciliatifolia | Curitiba, Brazil | Root bark | Antibacterial S. aureus S. epidermis | MIC 62.5µg/mL | [30] |
| 29 | Friedelin | Y/Y | X. controversa | Guangxi, China | Stems | Antioxidant Hepatoprotective | [82] [83] | |
| 30 | Velutinic acid | Y/Y | X. velutina | Colombia | leaves, twigs and inflorescences | [72] | ||
| 31 | n-hentriacontane | Y/Y | X. longifolia | Delhi, India | Leaves | [78] | ||
| 32 | Chaulmoogric acid | Y/Y | X. controversa | Guangxi, China | Stems | Antibacterial (leprosy) Neuroprotective | [82] [84] [85] [86] | |
| 33 | 2,6-dimethoxybenzoquinone | Y/Y | X. velutina | Colombia | Stem bark | Antibacterial Cytotoxic | [67] | |
| 34 | (−) Syringaresinol | Y/Y | X. controversa | Guangxi, China | Stems | Bacteriostatic (H. pylori) | [82] [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte-Casar, R.; Romero-Benavides, J.C. Xylosma G. Forst. Genus: Medicinal and Veterinary Use, Phytochemical Composition, and Biological Activity. Plants 2022, 11, 1252. https://doi.org/10.3390/plants11091252
Duarte-Casar R, Romero-Benavides JC. Xylosma G. Forst. Genus: Medicinal and Veterinary Use, Phytochemical Composition, and Biological Activity. Plants. 2022; 11(9):1252. https://doi.org/10.3390/plants11091252
Chicago/Turabian StyleDuarte-Casar, Rodrigo, and Juan Carlos Romero-Benavides. 2022. "Xylosma G. Forst. Genus: Medicinal and Veterinary Use, Phytochemical Composition, and Biological Activity" Plants 11, no. 9: 1252. https://doi.org/10.3390/plants11091252
APA StyleDuarte-Casar, R., & Romero-Benavides, J. C. (2022). Xylosma G. Forst. Genus: Medicinal and Veterinary Use, Phytochemical Composition, and Biological Activity. Plants, 11(9), 1252. https://doi.org/10.3390/plants11091252