Field Evaluation of Arbuscular Mycorrhizal Fungal Colonization in Miscanthus × giganteus and Seed-Based Miscanthus Hybrids Grown in Heavy-Metal-Polluted Areas
Abstract
1. Introduction
2. Materials and Methods
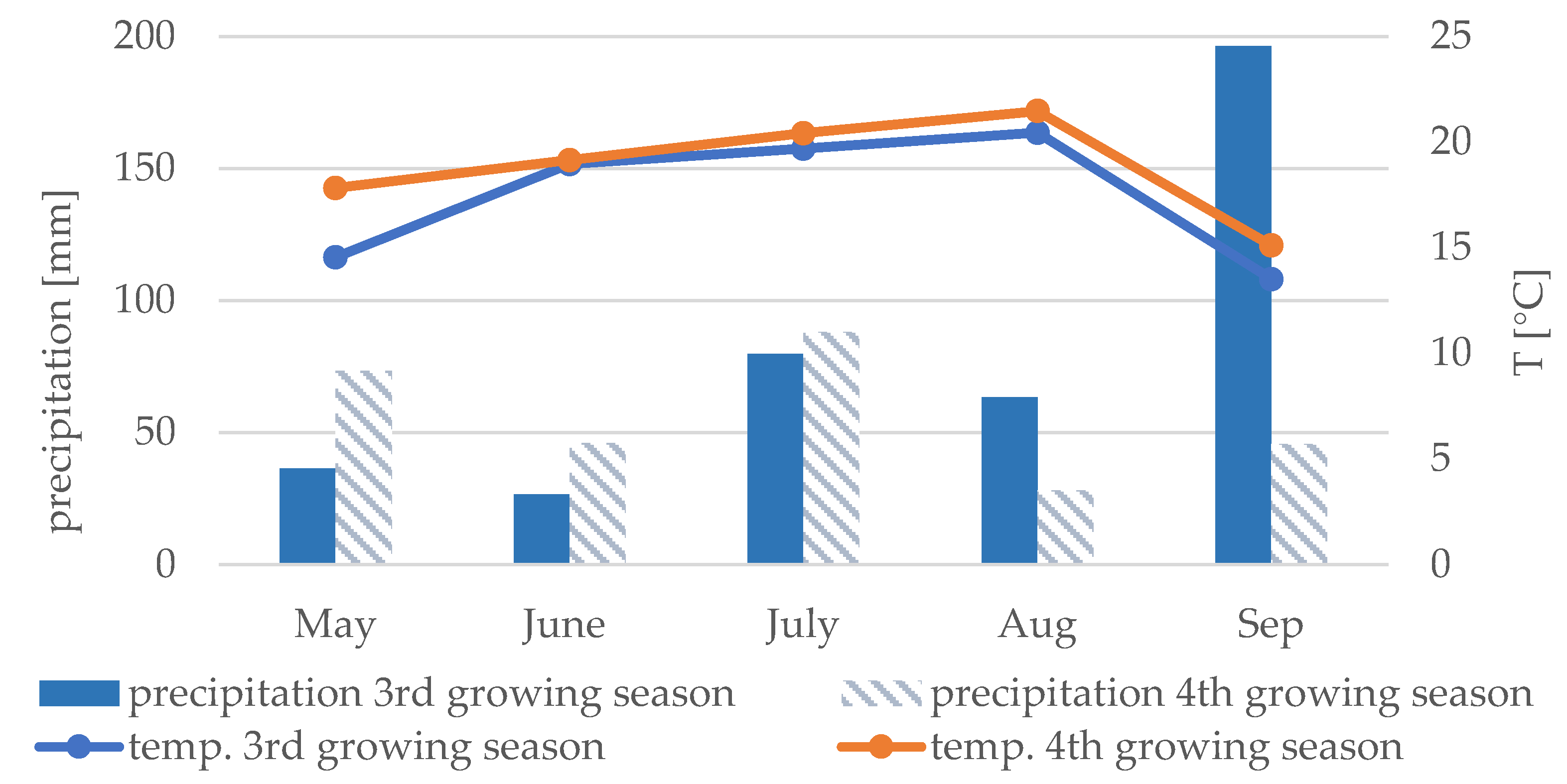
2.1. Experimental Design
2.2. Analysis of Soil Properties
2.3. Plant Sampling and Sample Preparation
2.4. Heavy Metal Content in Plant Tissues
2.5. Calculation of Translocation and Bioconcentration Factors
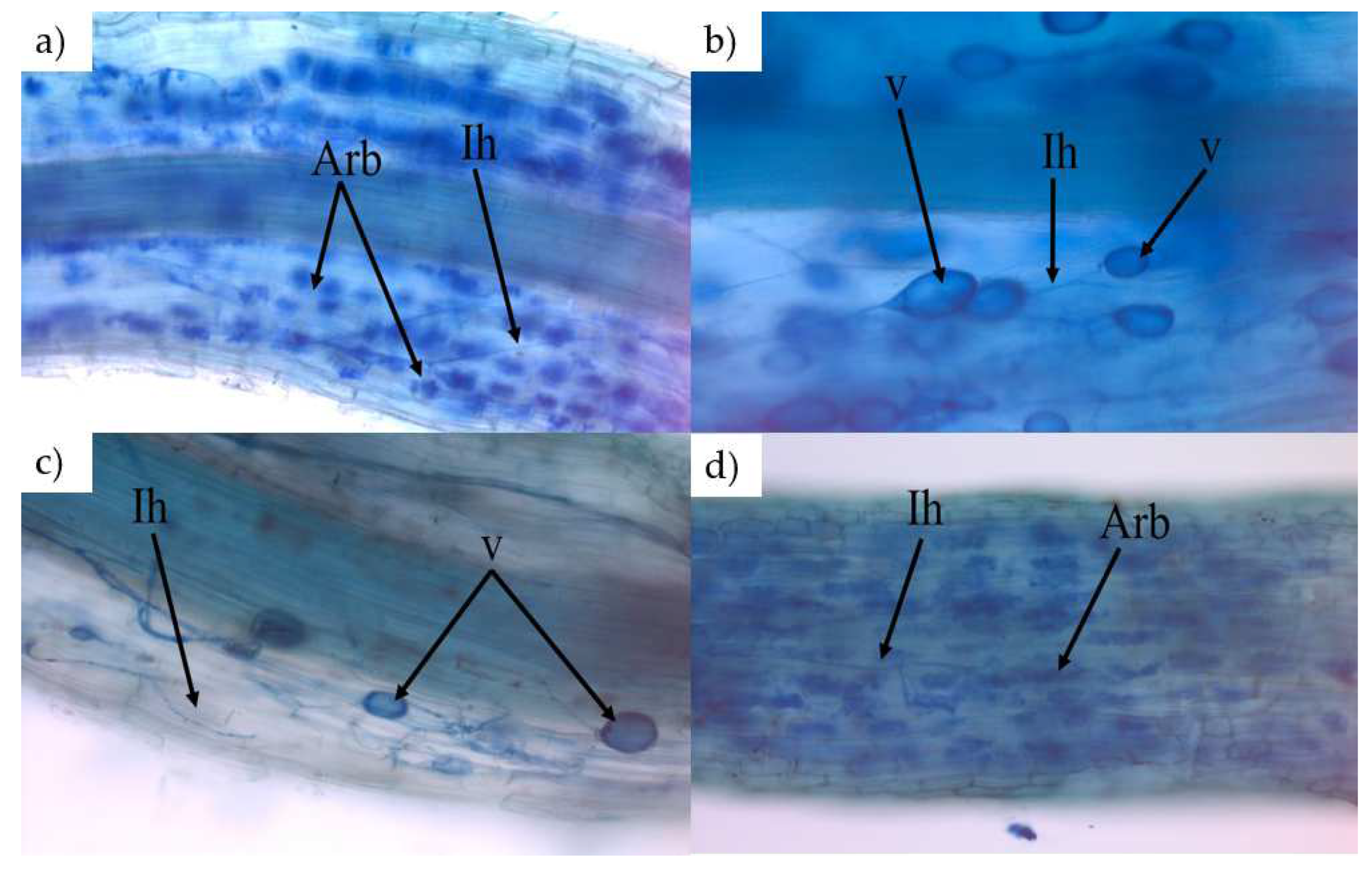
2.6. AMF Colonization Measurement
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Soil Properties and Heavy Metal Concentration
3.2. Concentration of Heavy Metals in Plant Roots and Shoots
3.3. Heavy Metal Bioconcentration (BCF) and Translocation Factors (TF)
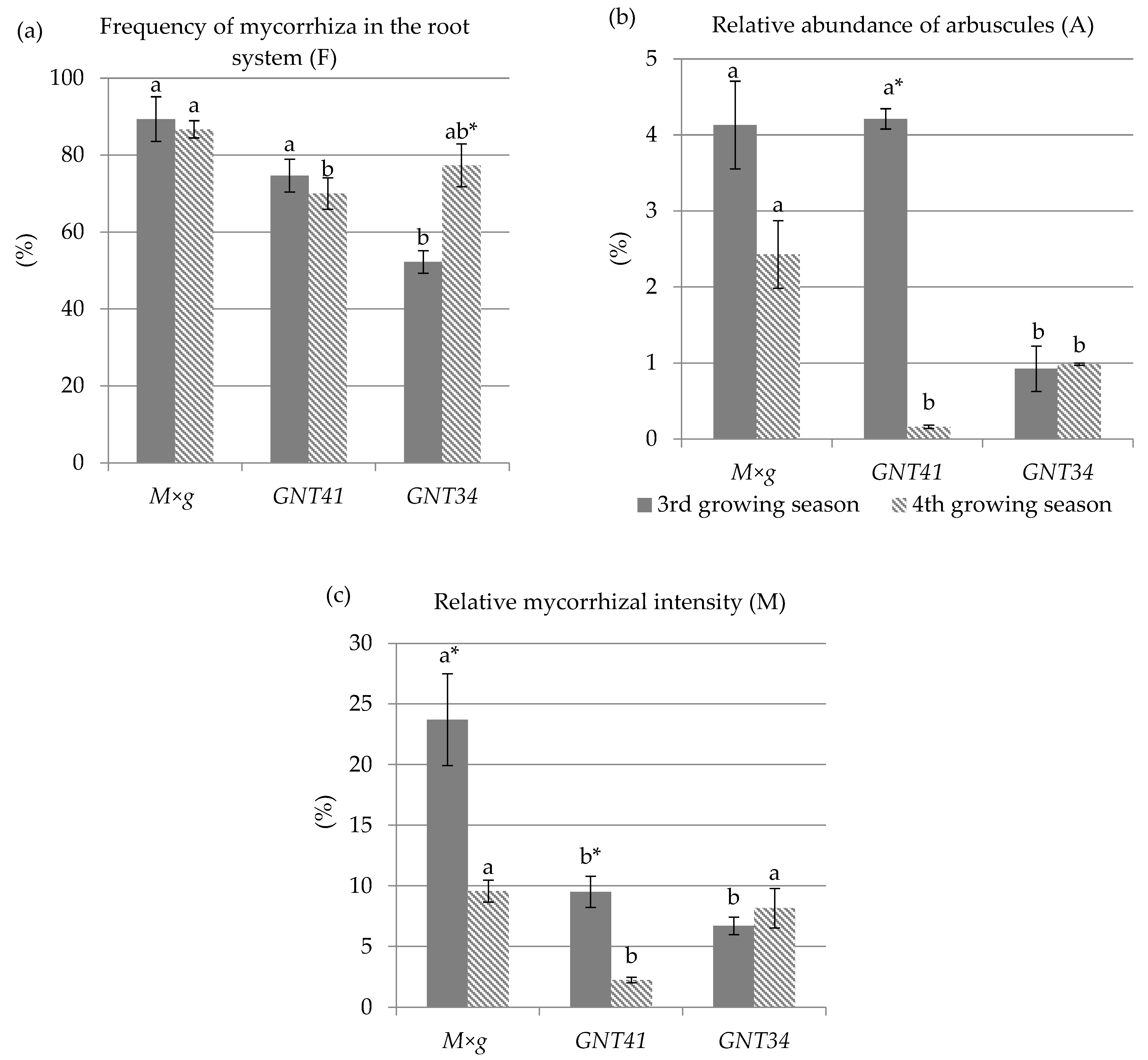
3.4. Mycorrhizal Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Favas, P.J.; Pratas, J.; Paul, M.S.; Prasad, M.N.V. Remediation of Uranium-Contaminated Sites by Phytoremediation and Natural Attenuation. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–300. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Singh, D.P.; Singh, N.K.; Pandey, V.C.; Rai, U.N. Sustainable Phytoremediation Strategies for River Water Rejuvenation. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 301–311. [Google Scholar] [CrossRef]
- Fu, W.; Huang, K.; Cai, H.H.; Li, J.; Zhai, D.L.; Dai, Z.C.; Du, D.L. Exploring the potential of naturalized plants for phytoremediation of heavy metal contamination. Int. Environ. Res. 2017, 11, 515–521. [Google Scholar] [CrossRef]
- Kumar, A.; Usmani, Z.; Ahirwal, J.; Rani, P. Phytomanagement of Chromium Contaminated Brown Fields. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–469. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisławska-Glubiak, E. Phytoremediation potential of Miscanthus× giganteus and Spartina pectinata in soil contaminated with heavy metals. Env. Sci. Pollut. Res. 2015, 22, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- Burges, A.; Epelde, L.; Benito, G.; Artetxe, U.; Becerril, J.M.; Garbisu, C. Enhancement of ecosystem services during endophyte-assisted aided phytostabilization of metal contaminated mine soil. Sci. Total. Environ. 2016, 562, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; Hebner, A.; Kopielski, K.; Werle, S.; Ratman-Kłosińska, I. Possibility of Using Energy Crops for Phytoremediation of Heavy Metals Contaminated Land—A Three-Year Experience. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2018; pp. 33–45. [Google Scholar]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; Werle, S.; Hebner, A.; Milandru, A. Case study on phytoremediation driven energy crop production using Sida hermaphrodita. Int. J. Phytoremediat. 2018, 20, 1194–1204. [Google Scholar] [CrossRef]
- Krzyżak, J.; Pogrzeba, M.; Rusinowski, S.; Clifton-Brown, J.; McCalmont, J.P.; Kiesel, A.; Mangold, A.; Mos, M. Heavy Metal Uptake by Novel Miscanthus Seed-Based Hybrids Cultivated in Heavy Metal Contaminated. Soil. Civ. Environ. Eng. Rep. 2017, 26, 121–132. [Google Scholar] [CrossRef][Green Version]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; McCalmont, J.P.; Jensen, E. Energy crop at heavy metal-contaminated arable land as an alternative for food and feed production: Biomass quantity and quality. In Plant Metallomics and Functional Omics; Springer: Cham, Switzerland, 2019; pp. 1–21. [Google Scholar]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. biomass production in a polluted area: Effects of two harvest timings on heavy metals uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Kocoń, A.; Jurga, B. The evaluation of growth and phytoextraction potential of Miscanthus × giganteus and Sida hermaphrodita on soil contaminated simultaneously with Cd, Cu, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2017, 24, 4990–5000. [Google Scholar] [CrossRef]
- Angelova, V.; Zapryanova, V. Miscanthus x giganteus as a biofuel crop for phytoremediation of heavy metal contaminated soils. Sci. Papers. Ser. E Land Reclam. Earth Obs. Surv. Environ. Eng. 2021, 10, 192–203. [Google Scholar]
- Chupakhin, E.; Babich, O.; Sukhikh, S.; Ivanova, S.; Budenkova, E.; Kalashnikova, O.; Kriger, O. Methods of Increasing Miscanthus Biomass Yield for Biofuel Production. Energies 2021, 14, 8368. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hu, X.; Zheng, P.; Hirota, M.; Kamijo, T. Photosynthetic Properties of Miscanthus condensatus at Volcanically Devastated Sites on Miyake-jima Island. Plants 2020, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sust. Energ. Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Firmin, S.; Labidi, S.; Fontaine, J.; Laruelle, F.; Tisserant, B.; Nsanganwimana, F.; Pourrut, B.; Dalpé, Y.; Grandmougin, A.; Douay, F.; et al. Arbuscular mycorrhizal fungal inoculation protects Miscanthus× giganteus against trace element toxicity in a highly metal-contaminated site. Sci. Total. Environ. 2015, 527, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gallos, A.; Paës, G.; Allais, F.; Beaugrand, J. Lignocellulosic fibers: A critical review of the extrusion process for enhancement of the properties of natural fiber composites. RSC Adv. 2017, 7, 34638–34654. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Schwarz, K.U.; Hastings, A. History of the development of Miscanthus as a bioenergy crop: From small beginnings to potential realization. Biol. Environ. Proc. R. Ir. Acad. 2015, 115, 45–57. [Google Scholar] [CrossRef]
- Sage, R.F.; Peixoto, M.D.M.; Friesen, P.; Deen, B. C4 bioenergy crops for cool climates, with special emphasis on perennial C4 grasses. J. Exp. Bot. 2015, 66, 4195–4212. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Ma, X.; Geng, Q.; Zhang, H.; Bian, C.; Chen, H.Y.; Jiang, D.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol. 2021, 229, 2957–2969. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; Tuinen, D.V.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Bitterlich, M.; Sandmann, M.; Graefe, J. Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant. Sci. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Cornejo, P.; Cartes, P.; Borie, F.; Medina, J.; Azcón, R. Interactive effect between Cu-adapted arbuscular mycorrhizal fungi and biotreated agrowaste residue to improve the nutritional status of Oenothera picensis growing in Cu-polluted soils. J. Plant. Nutr. Soil. Sc. 2015, 178, 126–135. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microb. Biot. 2015, 31, 1655–1664. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Sahraoui, A.L.-H.; Fourrier, H.; et al. Metal accumulation and shoot yield of Miscanthus× giganteus growing in contaminated agricultural soils: Insights into agronomic practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Sarkar, A.; Asaeda, T.; Wang, Q.; Kaneko, Y.; Rashid, M.H. Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora 2017, 234, 60–68. [Google Scholar] [CrossRef]
- Nowak, K.; Szada-Borzyszkowska, A.; Krzyżak, J.; Rusinowski, S.; Soja, M.; Pogrzeba, M. Comparison of root colonization by arbuscular mycorrhizal fungi in energy crop species cultivated on arable land contaminated with heavy metals. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012030. [Google Scholar] [CrossRef]
- Cieślińska, K.; Skalska, A.; Ciszek, D.; Krzyżak, J.; Pogrzeba, M. Mikoryza arbuskularna wybranych gatunków roślin energetycznych uprawianych na terenie zanieczyszczonym metalami ciężkimi. Interdyscyplinarne Zagadnienia w Inżynierii i Ochronie Srodowiska 2016, 7, 13–28. (In Polish) [Google Scholar]
- Pogrzeba, M.; Rusinowski, S.; Sitko, K.; Krzyżak, J.; Skalska, A.; Małkowski, E.; Ciszek, D.; Werle, S.; McCalmont, J.P.; Mos, M.; et al. Relationships between soil parameters and physiological status of Miscanthus× giganteus cultivated on soil contaminated with trace elements under NPK fertilisation vs. microbial inoculation. Environ. Pollut. 2017, 225, 163–174. [Google Scholar] [CrossRef]
- PN-ISO 11265:1997; Soil quality—Determination of the specific electrical conductivity.
- ISO 11466:1995; Soil Quality d Extraction of Trace Elements Soluble in Aqua Regia.
- Peijnenburg, W.J.; Zablotskaja, M.; Vijver, M.G. Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol. Environ. Saf. 2007, 67, 163–179. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. (In French) [Google Scholar]
- Available online: http://www.dijon.inra.fr/mychintec/Mycocal-prg/download.html (accessed on 1 November 2018).
- Regulation of the Minister of Environment (Official Journal of Laws of the Republic of Poland of 2016 item 1395). Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001395/O/D20161395.pdf (accessed on 1 March 2022).
- Galal, T.M.; Shehata, H.S. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Kupper, H.; Parameswaran, A.; Leitenmaier, B.; Trtilek, M.; Setlik, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New. Phytol. 2007, 175, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, X.; Fang, J.; Xiao, Y. Physiological responses and metal uptake of Miscanthus under cadmium/arsenic stress. Environ. Sci. Pollut. Res. 2018, 25, 28275–28284. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.; Kuś, J.; Matyka, M. Uprawa roślin na cele energetyczne. Poradnik. W&B Wiesław Drzewiecki: Warszawa, Polska, 2009; pp. 1–32. (In Polish) [Google Scholar]
- Kołodziej, B.; Antonkiewicz, J.; Sugier, D. Miscanthus× giganteus as a biomass feedstock grown on municipal sewage sludge. Ind. Crops Prod. 2016, 81, 72–82. [Google Scholar] [CrossRef]
- Rusinowski, S.; Krzyżak, J.; Clifton-Brown, J.; Jensen, E.; Mos, M.; Webster, R.; Sitko, K.; Pogrzeba, M. New Miscanthus hybrids cultivated at a Polish metal-contaminated site demonstrate high stomatal regulation and reduced shoot Pb and Cd concentrations. Environ. Pollut. 2019, 252, 1377–1387. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, S.; Pervez, A.; Inoue, M. Phytoaccumulation of heavy metals in natural plants thriving on wastewater effluent at Hattar industrial estate, Pakistan. Int. J. Phytoremediat. 2015, 17, 154–158. [Google Scholar] [CrossRef]
- Pascual, I.; Antolín, M.C.; García, C.; Polo, A.; Sánchez-Díaz, M. Plant availability of heavy metals in a soil amended with a high dose of sewage sludge under drought conditions. Biol. Fertil. 2004, 40, 291–299. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M.; Gessler, A.; Kreuzwieser, J.; Simon, J.; Papen, H. Nitrogen balance in forest soils: Nutritional limitation of plants under climate change stresses. Plant Biol. 2009, 11, 4–23. [Google Scholar] [CrossRef]
- Shi, G.; Xia, S.; Ye, J.; Huang, Y.; Liu, C.; Zhang, Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ. Exp. Bot. 2015, 111, 127–134. [Google Scholar] [CrossRef]
- Xia, S.; Wang, X.; Su, G.; Shi, G. Effects of drought on cadmium accumulation in peanuts grown in a contaminated calcareous soil. Environ. Sci. Pollut. Res. 2015, 22, 18707–18717. [Google Scholar] [CrossRef] [PubMed]
- Bauddh, K.; Singh, R.P. Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol. Environ. Saf. 2012, 85, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Pidlisnyuk, V.; Abit, K.; Nurzhanov, C.; Kenessov, B.; Stefanovska, T.; Erickson, L. Comparative assessment of using Miscanthus× giganteus for remediation of soils contaminated by heavy metals: A case of military and mining sites. Environ. Sci. Pollut. Res. 2019, 26, 13320–13333. [Google Scholar] [CrossRef] [PubMed]
- Zaier, H.; Ghnaya, T.; Lakhdar, A.; Baioui, R.; Ghabriche, R.; Mnasri, M.; Sghair, S.; Lutts, S.; Abdelly, C. Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: Tolerance and accumulation. J. Hazard. Mater. 2010, 183, 609–615. [Google Scholar] [CrossRef]
- Zu, Y.Q.; Li, Y.; Chen, J.J.; Chen, H.Y.; Qin, L.; Schvartz, C. Hyper accumulation of Pb, Zn, and Cd in herbaceous grown on lead–zinc mining area in Yunnan, China. Environ. Int. 2005, 31, 755–762. [Google Scholar] [CrossRef]
- Murray, H.; Thompson, K.; Macfie, S.M. Site- and species-specific patterns of metal bioavailability in edible plants. Botany 2009, 87, 702–711. [Google Scholar] [CrossRef]
- Andrejić, G.; Šinžar-Sekulić, J.; Prica, M.; Dželetović, Ž.; Rakić, T. Phytoremediation potential and physiological response of Miscanthus× giganteus cultivated on fertilized and non-fertilized flotation tailings. Environ. Sci. Pollut. Res. 2019, 26, 34658–34669. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total. Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Gu, H.H.; Zhou, Z.; Gao, Y.Q.; Yuan, X.T.; Ai, Y.J.; Zhang, J.Y.; Zuo, W.-Z.; Taylor, A.A.; Nan, S.-Q.; Li, F.-P. The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Int. J. Phytoremediat. 2017, 19, 739–745. [Google Scholar] [CrossRef]
- Hesami, R.; Salimi, A.; Ghaderian, S.M. Lead, zinc, and cadmium uptake, accumulation, and phytoremediation by plants growing around Tang-e Douzan lead–zinc mine, Iran. Environ. Sci. Pollut. Res. 2018, 25, 8701–8714. [Google Scholar] [CrossRef] [PubMed]
- Ladislas, S.; El-Mufleh, A.; Gérente, C.; Chazarenc, F.; Andrès, Y.; Béchet, B. Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban stormwater runoff. Water Air Soil Pollut. 2012, 223, 877–888. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soil Sediment. 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a productive biofuel crop for phytoremediation. Criti. Rev. Plant. Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- Pavel, P.B.; Puschenreiter, M.; Wenzel, W.W.; Diacu, E.; Barbu, C.H. Aided phytostabilization using Miscanthus sinensis× giganteus on heavy metal-contaminated soils. Sci. Total. Environ. 2014, 479, 125–131. [Google Scholar] [CrossRef]
- Kirk, A.; Fox, S.; Entz, M.; Tenuta, M. Preliminary findings on the arbuscular mycorrhizal colonization of organic wheat. In Proceedings of the 16th IFOAM Organic World Congress, Modena, Italy, 18–20 June 2008. [Google Scholar]
- Castellanos-Morales, V.; Keiser, C.; Cárdenas-Navarro, R.; Grausgruber, H.; Glauninger, J.; García-Garrido, J.M.; Steinkellner, S.; Sampedro, I.; Hage-Ahmed, K.; Illana, A.; et al. The bioprotective effect of AM root colonization against the soilborne fungal pathogen Gaeumannomyces graminis var. tritici in barley depends on the barley variety. Soil Biol. Biochem. 2011, 43, 831–834. [Google Scholar] [CrossRef]
- Estaún, V.; Calvet, C.; Camprubí, A. Effect of differences among crop species and cultivars on the arbuscular mycorrhizal symbiosis. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Heidelberg, Germany, 2010; pp. 279–295. [Google Scholar]
- Chatzistathis, T.; Orfanoudakis, M.; Alifragis, D.; Therios, I. Colonization of Greek olive cultivars’ root system by arbuscular mycorrhiza fungus: Root morphology, growth, and mineral nutrition of olive plants. Sci. Agric. 2013, 70, 185–194. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Anwar, G. Arbuscular Mycorrhizal Fungi of Northern White Cedar (Thuja Occidentalis L.): Habitat Effects on Fungal Communities and Inoculum Effects on Plant Growth on Acid Peat Soils. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, January 2016. [Google Scholar]
- Klironomos, J.N.; Hart, M.M.; Gurney, J.E.; Moutoglis, P. Interspecific differences in the tolerance of arbuscular mycorrhizal fungi to freezing and drying. Can. J. Bot. 2001, 79, 1161–1166. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Env. Sci. Tec. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M.; Donnison, I.S.; Robson, P.R. Phenomics analysis of drought responses in Miscanthus collected from different geographical locations. Gcb Bioenergy 2017, 9, 78–91. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Muszyńska, E.; Giemzik, A. Review on studies of zinc-lead waste heaps microbiota. Arch. Waste Manag. Environ. Prot. 2015, 17, 59–68. [Google Scholar]
- Loth, F.G.; Höfner, W. Einfluß der VA-Mykorrhiza auf die Schwermetallaufnahme von Hafer (Avena sativa L.) in Abhängigkeit vom Kontaminationsgrad der Böden. Zeitschrift für Pflanzenernährung und Bodenkunde 1995, 158, 339–345. [Google Scholar] [CrossRef]
- Lin, A.J.; Zhang, X.H.; Wong, M.H.; Ye, Z.H.; Lou, L.Q.; Wang, Y.S.; Zhu, Y.G. Increase of multi-metal tolerance of three leguminous plants by arbuscular mycorrhizal fungi colonization. Environ. Geochem. Health 2007, 29, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Borymski, S.; Piotrowska-Seget, Z. Ryzosfera metalofitów i jej rola w procesie bioremediacji metali ciężkich. Chemik 2014, 68, 554–559. (In Polish) [Google Scholar]
| Genotype | |||
|---|---|---|---|
| M × g | GNT41 | GNT34 | |
| Physicochemical Soil Parameters | |||
| pH (H2O) | 6.50 ± 0.02 a | 6.52 ± 0.04 a | 6.54 ± 0.06 a |
| pH (KCl) | 5.99 ± 0.02 a | 5.99 ± 0.04 a | 6.03 ± 0.04 a |
| EC (µS cm−1) | 83.08 ± 0.26 a | 80.90 ± 3.19 a | 75.45 ± 1.94 a |
| Heavy Metal Concentration in Soil | |||
| Pb (mg kg−1) | 689.50 ± 25.20 ab | 621.36 ± 3.56 b | 719.63 ± 20.47 a |
| Cd (mg kg−1) | 26.5 ± 1.93 a | 23.17 ± 0.53 a | 26.50 ± 0.66 a |
| Zn (mg kg−1) | 2805.4 ± 37.6 b | 2590 ± 47.1 b | 3568.9 ± 80.8 a |
| Bioavailable Forms of Heavy Metals in Soil | |||
| Pb (mg kg−1) | LOQ | LOQ | LOQ |
| Cd (mg kg−1) | 1.48 ± 0.06 a | 1.23 ± 0.03 a | 1.57 ± 0.15 a |
| Zn (mg kg−1) | 96.95 ± 0.71 b | 86.59 ± 2.89 b | 127.75 ± 3.45 a |
| Concentration of HM | ||||||
|---|---|---|---|---|---|---|
| Pb (mg kg −1) | Cd (mg kg −1) | Zn (mg kg −1) | ||||
| 3rd Growing Season | 4th Growing Season | 3rd Growing Season | 4th Growing Season | 3rd Growing Season | 4th Growing Season | |
| Root | ||||||
| M × g | 71.19 ± 7.84 a | 48.17 ± 3.79 b | 43.94 ± 2.2 ab * | 25.37 ± 2.10 a | 1534.26 ± 163 a | 1033.8 ± 54.48 a |
| GNT41 | 41.46 ± 7.92 a | 64.63 ± 2.08 a | 36.29 ± 2.59 b | 32.61 ± 0.64 a | 1243.21 ± 90.6 a | 946.1 ± 65.52 a |
| GNT34 | 61.44 ± 14.90 a | 41.72 ± 0.65 b | 53.60 ± 2.32 a * | 31.2 ± 2.35 a | 1065.6 ± 59.7 a | 1123.6 ± 108.2 a |
| Shoot | ||||||
| M × g | 11.92 ± 0.89 b * | 7.46 ± 0.97 a | 0.66 ± 0.06 a | 0.70 ± 0.04 a | 135.1 ± 5.29 a | 123.95 ± 5.18 a |
| GNT41 | 17.03 ± 0.45 a * | 7.70 ± 0.64 a | 0.52 ± 0.05 a * | 0.31 ± 0.04 b | 154.0 ± 9.64 a * | 103.97 ± 4.16 b |
| GNT34 | 11.92 ± 0.89 b * | 6.02 ± 0.19 a | 0.66 ± 0.07 a * | 0.26 ± 0.03 b | 133.1 ± 5.98 a | 140.25 ± 7.81 a |
| Translocation Factor | ||||||
|---|---|---|---|---|---|---|
| TFPb | TFCd | TFZn | ||||
| 3rd growing season | 4th growing season | 3rd growing season | 4th growing season | 3rd growing season | 4th growing season | |
| M × g | 0.17 ± 0.02 b | 0.16 ± 0.03 a | 0.02 ± 0.0 a | 0.03 ± 0.0 a* | 0.09 ± 0.01 b | 0.12 ± 0.01 a |
| GNT41 | 0.33 ± 0.0 a* | 0.12 ± 0.02 a | 0.01 ± 0.0a | 0.01 ± 0.0 b | 0.12 ± 0.0 a | 0.11 ± 0.01 a |
| GNT34 | 0.25 ± 0.04 a* | 0.14 ± 0.01 a | 0.01 ± 0.0 a | 0.01 ± 0.0 b | 0.13 ± 0.01 a | 0.13 ± 0.01 a |
| Bioconcentration factor | ||||||
| BCFPb | BCFCd | BCFZn | ||||
| 3rd growing season | 4th growing season | 3rd growing season | 4th growing season | 3rd growing season | 4th growing season | |
| M × g | 0.02 ± 0.0 b | 0.01 ± 0.0 a | 0.03 ± 0.0 a | 0.03 ± 0.0 a | 0.05 ± 0.0 a | 0.04 ± 0.0 a |
| GNT41 | 0.03 ± 0.0 a* | 0.01 ± 0.0 a | 0.02 ± 0.0 a | 0.01 ± 0.0 b | 0.06 ± 0.01 a* | 0.04 ± 0.0 a |
| GNT34 | 0.02 ± 0.0 b | 0.01 ± 0.0 a | 0.03 ± 0.0 a* | 0.01 ± 0.0 b | 0.04 ± 0.0 b | 0.04 ± 0.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szada-Borzyszkowska, A.; Krzyżak, J.; Rusinowski, S.; Sitko, K.; Pogrzeba, M. Field Evaluation of Arbuscular Mycorrhizal Fungal Colonization in Miscanthus × giganteus and Seed-Based Miscanthus Hybrids Grown in Heavy-Metal-Polluted Areas. Plants 2022, 11, 1216. https://doi.org/10.3390/plants11091216
Szada-Borzyszkowska A, Krzyżak J, Rusinowski S, Sitko K, Pogrzeba M. Field Evaluation of Arbuscular Mycorrhizal Fungal Colonization in Miscanthus × giganteus and Seed-Based Miscanthus Hybrids Grown in Heavy-Metal-Polluted Areas. Plants. 2022; 11(9):1216. https://doi.org/10.3390/plants11091216
Chicago/Turabian StyleSzada-Borzyszkowska, Alicja, Jacek Krzyżak, Szymon Rusinowski, Krzysztof Sitko, and Marta Pogrzeba. 2022. "Field Evaluation of Arbuscular Mycorrhizal Fungal Colonization in Miscanthus × giganteus and Seed-Based Miscanthus Hybrids Grown in Heavy-Metal-Polluted Areas" Plants 11, no. 9: 1216. https://doi.org/10.3390/plants11091216
APA StyleSzada-Borzyszkowska, A., Krzyżak, J., Rusinowski, S., Sitko, K., & Pogrzeba, M. (2022). Field Evaluation of Arbuscular Mycorrhizal Fungal Colonization in Miscanthus × giganteus and Seed-Based Miscanthus Hybrids Grown in Heavy-Metal-Polluted Areas. Plants, 11(9), 1216. https://doi.org/10.3390/plants11091216







