Priming Treatments with Biostimulants to Cope the Short-Term Heat Stress Response: A Transcriptomic Profile Evaluation
Abstract
:1. Introduction
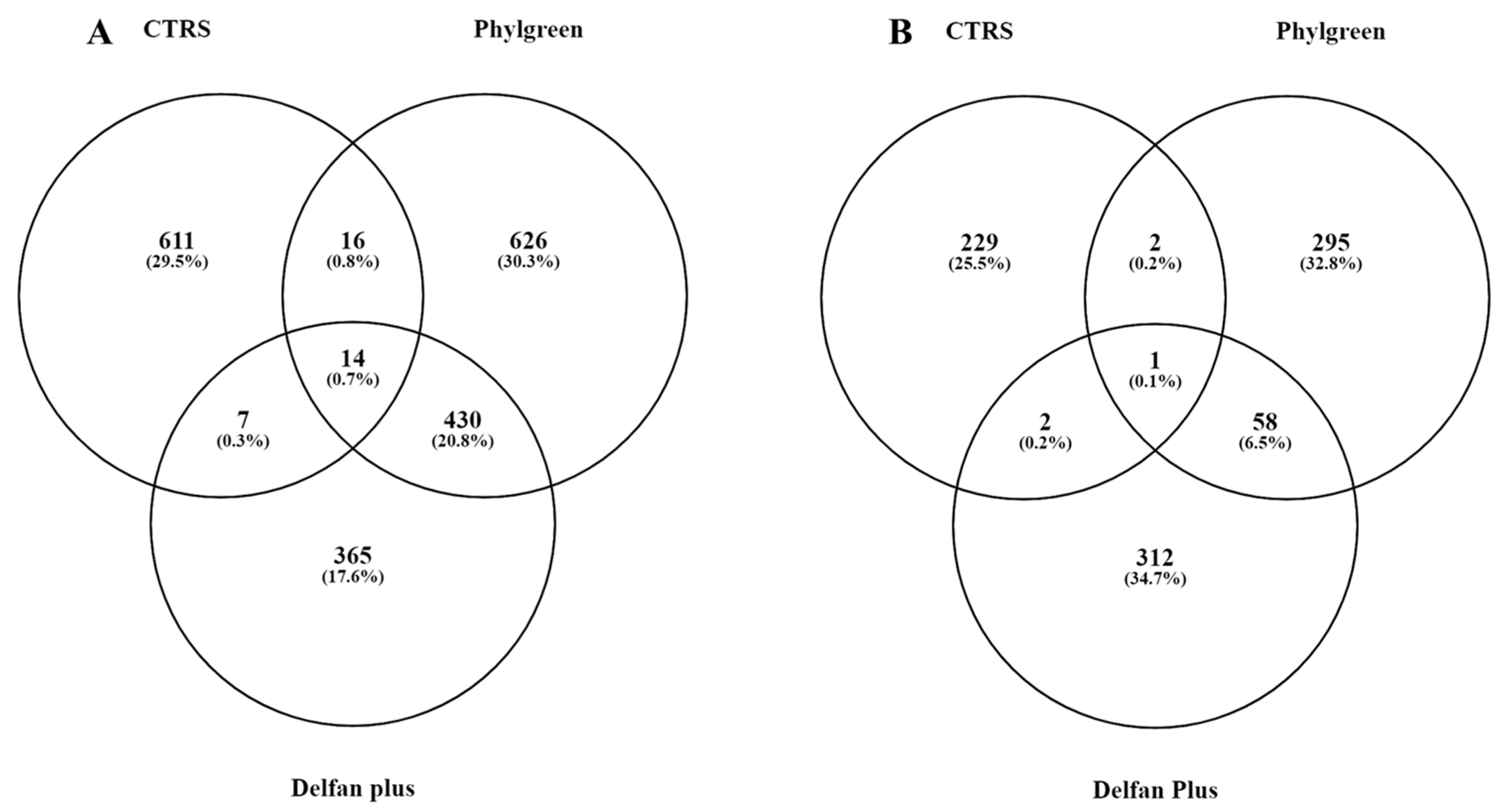
2. Results
2.1. Transcriptional Changes
2.2. Expression of Transcription Factors
2.3. Heat Stress Response-Associated Genes
2.4. Secondary Metabolism—Phenylpropanoid Pathway
2.5. DAVID Enrichment Analysis
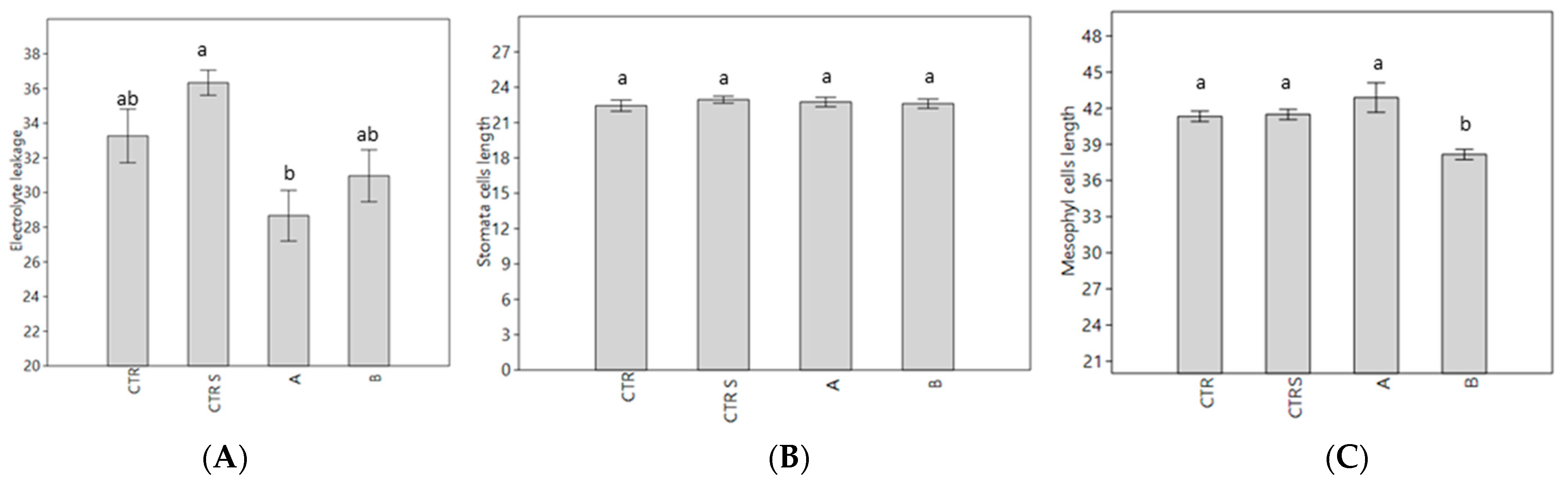
2.6. Histochemical Analysis
2.7. Physiological and Biochemical Changes
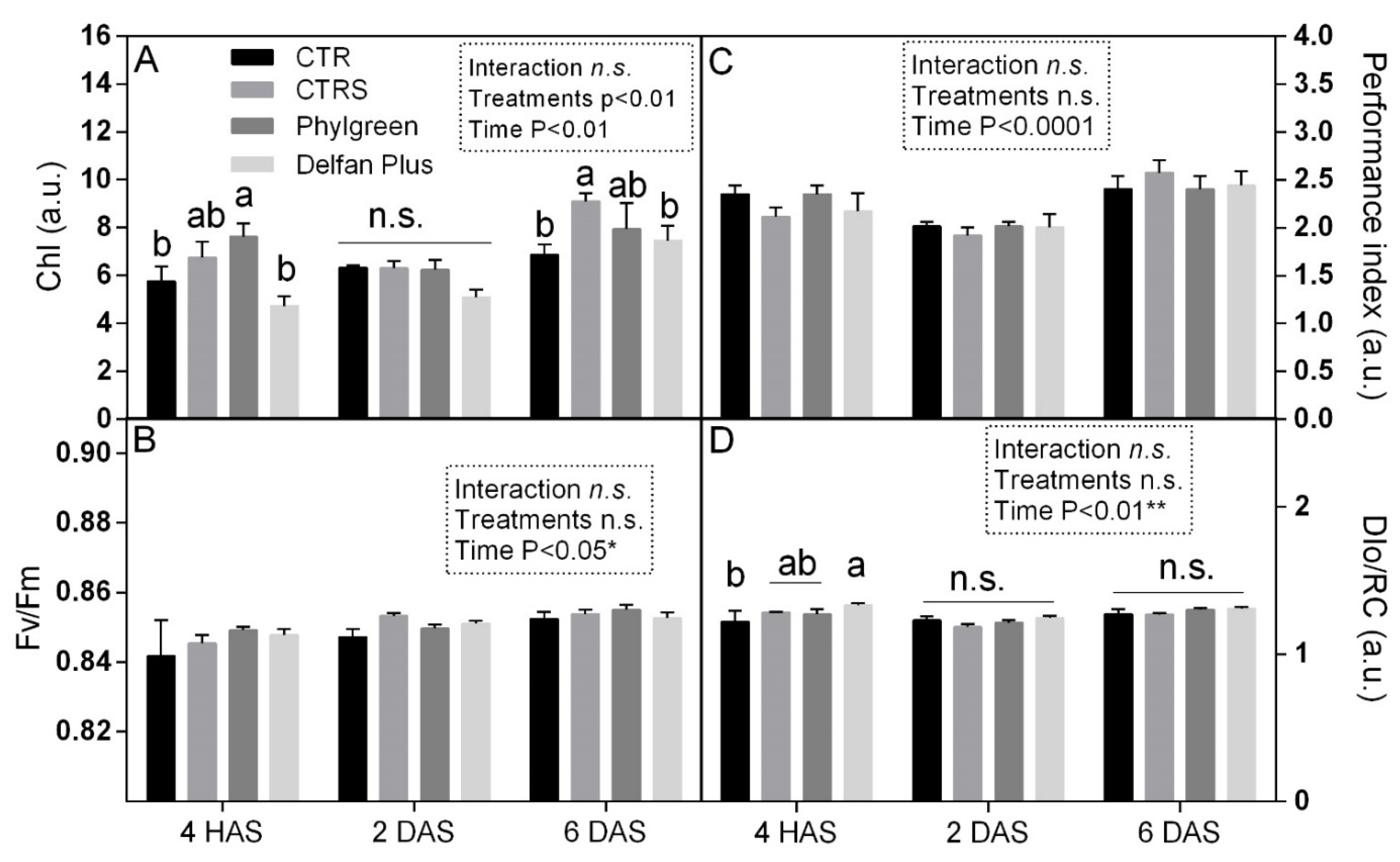
2.7.1. Chlorophyll and Chlorophyll a Fluorescence
2.7.2. Total Phenolics and Anthocyanins
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. RNA Isolation
4.3. RNA-Seq and Library Preparation
4.4. Bioinformatic Analysis
4.5. Non-Destructive Determination: Chlorophyll and Chlorophyll a Fluorescence
4.6. Phenolic Index and Anthocyanins Content
4.7. Histochemical Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2017, 162, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, A.; Mariani, L. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses: High and Low Values of Temperature, Light Intensity, and Relative Humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Shah, Z.; Shah, S.H.; Ali, G.S.; Munir, I.; Khan, R.S.; Iqbal, A.; Ahmed, N.; Jan, A. Introduction of Arabidopsis’s heat shock factor HsfA1d mitigates adverse effects of heat stress on potato (Solanum tuberosum L.) plant. Cell Stress Chaperon 2020, 25, 57–63. [Google Scholar] [CrossRef]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef]
- Karwa, S.; Arya, S.S.; Maurya, S.; Pal, M. Physiological characterization of reproductive stage heat stress tolerance in contrasting rice genotypes. Plant Physiol. Rep. 2020, 25, 157–162. [Google Scholar] [CrossRef]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Beckers, G.J.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Sujeeth, N.; Gupta, S.; Omranian, N.; Guinan, K.J.; Brotman, Y.; Nikoloski, Z.; Fernie, A.R.; Mueller-Roeber, B.; Gechev, T.S. A Biostimulant Obtained from the Seaweed Ascophyllum nodosum Protects Arabidopsis thaliana from Severe Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 474. [Google Scholar] [CrossRef] [Green Version]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular under-standing of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Hidaka, T.; Fukamachi, H. Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci. Hortic. 1996, 67, 39–48. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu, R.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. In vitro antioxidant activities of sulfated poly-saccharide ascophyllan isolated from Ascophyllum nodosum. Int. J. Biol. Macromol. 2013, 59, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef]
- Prändl, R.; Hinderhofer, K.; Eggers-Schumacher, G.; Schöffl, F. HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol. Gen. Genet. MGG 1998, 258, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Eggers-Schumacher, G.; Wunderlich, M.; Schöffl, F. Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genom. 2004, 271, 11–21. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Sun, W.; Bernard, C.; Van De Cotte, B.; Van Montagu, M.; Verbruggen, N. At-HSP17. 6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J. 2001, 27, 407–415. [Google Scholar] [CrossRef]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Rojas, A.; Almoguera, C.; Jordano, J. Transcriptional activation of a heat shock gene promoter in sunflower embryos: Synergism between ABI3 and heat shock factors. Plant J. 1999, 20, 601–610. [Google Scholar] [CrossRef]
- Wehmeyer, N.; Vierling, E. The Expression of Small Heat Shock Proteins in Seeds Responds to Discrete Developmental Signals and Suggests a General Protective Role in Desiccation Tolerance. Plant Physiol. 2000, 122, 1099–1108. [Google Scholar] [CrossRef] [Green Version]
- Swindell, W.R.; Hu, W.; Hu, G.; Han, B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 2009, 176, 583–590. [Google Scholar]
- Kim, D.H.; Xu, Z.-Y.; Hwang, I. AtHSP17.8 overexpression in transgenic lettuce gives rise to dehydration and salt stress resistance phenotypes through modulation of ABA-mediated signaling. Plant Cell Rep. 2013, 32, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.R.; Nguyen, S.L.; Eskandar, R.; Behan, J.; Sanders-Reed, Z. The recent evolution of a pseudogene: Diversity and divergence of a mitochondria-localized small heat shock protein in Arabidopsis thaliana. Genome 2008, 51, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Klemenz, R.; Gehring, W.J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell 1986, 44, 429–438. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Li, M.; Dong, X.; Peng, J.; Xu, W.; Ren, R.; Liu, J.; Cao, F.; Liu, Z. De novo transcriptome sequencing and gene expression analysis reveal potential mechanisms of seed abortion in dove tree (Davidia involucrata Baill.). BMC Plant Biol. 2016, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Murgia, I.; de Pinto, M.C.; Delledonne, M.; Soave, C.; De Gara, L. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J. Plant Physiol. 2004, 161, 777–783. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.; Tang, S.; Stallmann, A.; Dangl, J.L.; Bonardi, V. Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor. PLoS Genet. 2013, 9, e1003465. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Upregulated Genes | ||
| Accession n | Description—CTRS | Upreg |
| at5g12030 | Arabidopsis thaliana Heat Shock Protein 17.6A | 7 |
| at1g71000 | heat shock protein binding | 2.807 |
| at4g24190 | SHD (SHEPHERD), HSP90.7 | 2.585 |
| Description—Phylgreen | ||
| at3g46230 | AtHSP17.4 | 6.778 |
| at5g12020 | AtHSP17.6II (17.6 KDA CLASS II HEAT SHOCK PROTEIN) | 5.775 |
| at5g12030 | Arabidopsis thaliana Heat Shock Protein 17.6A | 5.547 |
| at2g29500 | class I small heat shock protein (HSP17.6B-CI) 17.6 kDa | 5.175 |
| at1g07400 | class I heat shock protein (HSP17.8-CI) 17.8 kDa | 5.018 |
| at2g21510 | DNAJ heat shock N-terminal domain-containing protein | 4.64 |
| at1g72070 | DNAJ heat shock N-terminal domain-containing protein | 3.858 |
| at5g51440 | mitochondrial small heat shock protein (HSP23.5-M) 23.5 kDa | 3.474 |
| at5g59720 | AtHSP18.2 (heat shock protein 18.2) | 2.545 |
| at5g47600 | heat shock protein-related | 2.459 |
| at1g44160 | DNAJ chaperone C-terminal domain-containing protein | 2.459 |
| at1g71000 | heat shock protein binding | 2.399 |
| at3g12580 | AtHSP70 (heat shock protein 70) | 2.142 |
| at5g52640 | HSP81-1, ATHS83, HSP81.1, HSP83, ATHSP90.1 | 2.129 |
| at1g28210 | ATJ1; heat shock protein binding/nucleic acid binding | 2.079 |
| at1g76770 | heat shock protein-related | 2 |
| Description—Delfan Plus | ||
| at3g46230 | AtHSP17.4 | 8.898 |
| at5g12030 | Arabidopsis thaliana Heat Shock Protein 17.6A | 7.79 |
| at5g12020 | AtHSP17.6II (17.6 KDA CLASS II HEAT SHOCK PROTEIN) | 7.562 |
| at2g29500 | class I small heat shock protein (HSP17.6B-CI) 17.6 kDa | 6.98 |
| at5g59720 | AtHSP18.2 (heat shock protein 18.2) | 6.523 |
| at1g07400 | class I heat shock protein (HSP17.8-CI) 17.8 kDa | 5.938 |
| at1g72070 | DNAJ heat shock N-terminal domain-containing protein | 4.585 |
| at5g51440 | mitochondrial small heat shock protein (HSP23.5-M) 23.5 kDa | 4.281 |
| at4g21320 | HSA32 (HEAT-STRESS-ASSOCIATED 32); | 4.138 |
| at5g52640 | HSP81-1, ATHS83, HSP81.1, HSP83, ATHSP90.1|ATHSP90.1 | 3.262 |
| at3g12580 | AtHSP70 (heat shock protein 70) | 2.716 |
| at2g32120 | HSP70T-2 (HEAT-SHOCK PROTEIN 70T-2); ATP binding | 2.413 |
| at1g59860 | 17.6 kDa class I heat shock protein (HSP17.6A-CI) | 2.409 |
| at2g26150 | AtHSFA2; DNA binding/transcription factor | 2.369 |
| at2g21510 | DNAJ heat shock N-terminal domain-containing protein | 2.259 |
| at1g71000 | heat shock protein binding | 2.142 |
| at1g28210 | AtJ1; heat shock protein binding | 2.036 |
| Downregulated genes | ||
| Description—Phylgreen | Downreg. | |
| at1g09080 | AtBIP3; ATP binding | −2.518 |
| at4g19590 | DNAJ heat shock N-terminal domain-containing protein | −2 |
| at2g03020 | heat shock protein-related | −2 |
| CTRS | |||||
| Category | Terms | p | F | Bonferroni | FDR |
| INTERPRO | IPR003614: Knottin, scorpion toxin-like | 3.1 × 1011 | 7.4 × 1014 | 2.2 × 1015 | 4.8 × 1016 |
| INTERPRO | IPR002472: Palmitoyl protein thioesterase | 6.3 × 1011 | 2.1 × 1015 | 4.0 × 1015 | 9.6 × 1015 |
| GOTERM_MF_DIRECT | GO:0016790~thiolester hydrolase activity | 6.5 × 1011 | 2.1 × 1015 | 2.3 × 1016 | 9 × 1015 |
| GOTERM_MF_DIRECT | GO:0008474~palmitoyl-(protein) hydrolase activity | 7.7 × 1011 | 1.1 × 1016 | 2.6 × 1015 | 1.1 × 1016 |
| SMART | SM00505:Knot1 | 2.7 × 1012 | 9.9 × 1015 | 3.1 × 1015 | 3.0 × 1016 |
| GOTERM_BP_DIRECT | GO:0002084~protein depalmitoylation | 6.1 × 1014 | 1.0 × 1016 | 9.8 × 1015 | 8.7 × 1015 |
| UP_SEQ_FEATURE | active site:Proton acceptor | 6.6 × 1015 | 1.7 × 1016 | 9.8 × 1015 | 9.3 × 1015 |
| GOTERM_CC_DIRECT | GO:0005829~cytosol | 7.0 × 1015 | 1.3 × 1016 | 7.4 × 1015 | 8.4 × 1015 |
| INTERPRO | IPR008176:Gamma thionin | 8.4 × 1015 | 9.3 × 1015 | 10 × 1015 | 12 × 1016 |
| KEGG_PATHWAY | ath00062:Fatty acid elongation | 3.9 × 1016 | 5.6 × 1015 | 3.0 × 1015 | 4.2 × 1015 |
| Phylgreen | |||||
| Category | Term | p | FE | Bonf. | FDR |
| GOTERM_BP_DIRECT | GO:0009611~response to wounding | 4.1 × 10−19 | 5.2 | 2.7 × 10−16 | 6.1 × 10−16 |
| GOTERM_BP_DIRECT | GO:0006952~defense response | 6.53 × 10−19 | 3.0 | 4.2 × 10−16 | 9.7 × 10−16 |
| UP_SEQ_FEATURE | DNA-binding region: AP2/ERF | 1.2 × 10−12 | 5.1 | 8.1 × 10−10 | 1.8 × 10−9 |
| INTERPRO | IPR001471:AP2/ERF domain | 1.5 × 10−11 | 4.7 | 1.2 × 10−8 | 2.3 × 10−8 |
| GOTERM_BP_DIRECT | GO:0010200~response to chitin | 2.2 × 10−13 | 5.3 | 1.4 × 10−10 | 3.3 × 10−10 |
| GOTERM_BP_DIRECT | GO:0009753~response to jasmonic acid | 4.7 × 10−13 | 4.8 | 3.1 × 10−10 | 7 × 10−10 |
| SMART | SM00380:AP2 | 3. × 10−11 | 4.4 | 4.5 × 10−9 | 4.1 × 10−8 |
| INTERPRO | IPR016177:DNA-binding, integrase-type | 1.1 × 10−10 | 4.3 | 8.5 × 10−8 | 1.6 × 10−7 |
| GOTERM_MF_DIRECT | GO:0043565~sequence-specific DNA binding | 3.9 × 10−9 | 2.3 | 1.7 × 10−6 | 5.5 × 10−6 |
| GOTERM_BP_DIRECT | GO:0006355~regulation of transcription, DNA-templated | 143 | 11.5 | 1.3 × 10−8 | 1.6 × 10−5 |
| Delfan Plus | |||||
| Category | Term | p | FE | Bonf. | FDR |
| GOTERM_MF_DIRECT | GO:0003700 transcription factor activity, sequence-specific DNA binding | 1.7 × 1008 | 2.66 × 10−16 | 2.66 × 10−16 | 6.12 × 10−16 |
| GOTERM_BP_DIRECT | GO:0042542 response to hydrogen peroxide | 1.8 × 10−9 | 4.24 × 10−16 | 2.1 × 10−16 | 9.74 × 10−16 |
| UP_SEQ_FEATURE | region of interest: type E motif | 1.2 × 10−8 | 1.45 × 10−10 | 4.83 × 10−11 | 3.33 × 10−10 |
| GOTERM_BP_DIRECT | GO:0009408 response to heat | 2. × 10−10 | 3.06 × 10−10 | 7.64 × 10−11 | 7.02 × 10−10 |
| UP_KEYWORDS | Pyrrolidone carboxylic acid | 2.3 × 10−12 | 8.16 × 10−10 | 8.16 × 10−10 | 1.80 × 10−9 |
| UP_SEQ_FEATURE | DNA-binding region:WRKY | 5.9 × 10−9 | 3.55 × 10−9 | 3.55 × 10−9 | 1.17 × 10−8 |
| GOTERM_MF_DIRECT | GO:0044212 transcription regulatory region DNA binding | 6.7 × 10−9 | 1.21 × 10−8 | 1.21 × 10−8 | 2.31 × 10−8 |
| UP_SEQ_FEATURE | region of interest:Type E(+) motif | 8.1 × 10−9 | 4.52 × 10−9 | 4.52 × 10−9 | 4.13 × 10−8 |
| UP_KEYWORDS | Apoplast | 1.1 × 10−11 | 8.50 × 10−8 | 4.25 × 10−8 | 1.63 × 10−7 |
| GOTERM_BP_DIRECT | GO:0009751 response to salicylic acid | 2.0 × 10−10 | 1.67 × 10−6 | 8.33 × 10−7 | 5.50 × 10−6 |
| CTRS | |||||||
| Annotation Cluster 1—Enrichment Score: 2.2 | |||||||
| Category | Term | % | p | FE | Bonf | Benj | FDR |
| INTERPRO | IPR002472: Palmitoyl protein thioesterase | 0.5 | 6.3 × 10−4 | 21.2 | 0.40 | 0.22 | 0.96 |
| GOTERM_MF_DIRECT | GO:0016790~thiolester hydrolase activity | 0.5 | 6.5 × 10−4 | 20.9 | 0.20 | 0.22 | 0.90 |
| GOTERM_MF_DIRECT | GO:0008474~palmitoyl-(protein) hydrolase activity | 0.7 | 7.7 × 10−4 | 11.4 | 0.26 | 0.14 | 1.06 |
| KEGG_PATHWAY | ath00062: Fatty acid elongation | 0.8 | 0.004 | 5.6 | 0.30 | 0.30 | 4.15 |
| GOTERM_BP_DIRECT | GO:0002084~protein depalmitoylation | 0.5 | 0.006 | 10.3 | 0.98 | 0.98 | 8.71 |
| KEGG_PATHWAY | ath01212: Fatty acid metabolism | 0.7 | 0.17 | 2.2 | 1.0 | 0.93 | 88.32 |
| Phylgreen | |||||||
| Annotation Cluster 1—Enrichment Score: 8.6 | |||||||
| Category | Term | % | p | FE | Bonf. | Benj. | FDR |
| UP_SEQ_FEATURE | DNA-binding region:AP2/ERF | 2.4 | 1.1 × 10−12 | 5.1 | 8.1 × 10−10 | 8.1 × 10−10 | 1.8 × 10−9 |
| INTERPRO | IPR001471:AP2/ERF domain | 2.4 | 1.5 × 10−11 | 4.7 | 1.2 × 10−8 | 1.2 × 10−8 | 2.3 × 10−8 |
| SMART | SM00380:AP2 | 2.4 | 3.6 × 10−11 | 4.4 | 4.5 × 10−9 | 4.5 × 10−9 | 4.1 × 10−8 |
| INTERPRO | IPR016177:DNA-binding, integrase-type | 2.4 | 1.1 × 10−10 | 4.3 | 8.5 × 10−8 | 4.2 × 10−8 | 1.6 × 10−7 |
| UP_KEYWORDS | Ethylene signalling pathway | 1.9 | 8.3 × 10−7 | 3.3 | 2.0 × 10−4 | 5.0 × 10−5 | 0.001 |
| GOTERM_BP_DIRECT | GO:0009873~ethylene-activated signalling pathway | 1.9 | 2.1 × 10−6 | 3.2 | 0.001 | 1.3 × 10−4 | 0.003 |
| UP_KEYWORDS | Activator | 3.5 | 6.9 × 10−6 | 2.1 | 0.001 | 3.3 × 10−4 | 0.008 |
| Annotation Cluster 2—Enrichment Score: 6.3 | |||||||
| Category | Term | % | p | FE | Bonf. | Benjamini | FDR |
| GOTERM_MF_DIRECT | GO:0003700~transcription factor activity, sequence-specific DNA binding | 10.3 | 8.3 × 10−12 | 1.8 | 3.6 × 10−9 | 3.6 × 10−9 | 1.2 × 10−8 |
| GOTERM_MF_DIRECT | GO:0043565~sequence-specific DNA binding | 5.0 | 3.9 × 10−9 | 2.3 | 1.7 × 10−6 | 8.3 × 10−7 | 5.5 × 10−6 |
| GOTERM_BP_DIRECT | GO:0006355~regulation of transcription, DNA-templated | 11.6 | 1.3 × 10−8 | 1.6 | 8.5 × 10−8 | 1.7 × 10−6 | 2.0 × 10−5 |
| GOTERM_BP_DIRECT | GO:0006351~transcription, DNA-templated | 10.5 | 2.1 × 10−8 | 1.6 | 1.4 × 10−5 | 2.3 × 10−6 | 3.2 × 10−5 |
| UP_KEYWORDS | Transcription regulation | 10.7 | 1.2 × 10−7 | 1.6 | 2.9 × 10−5 | 1.5 × 10−5 | 1.6 × 10−4 |
| UP_KEYWORDS | Transcription | 10.7 | 4.5 × 10−7 | 1.6 | 1.1 × 10−4 | 3.6 × 10−5 | 5.8 × 10−4 |
| UP_KEYWORDS | DNA-binding | 8.9 | 8.4 × 10−5 | 1.4 | 0.02 | 0.003 | 0.11 |
| GOTERM_MF_DIRECT | GO:0003677~DNA binding | 8.9 | 0.002 | 1.3 | 0.49 | 0.109 | 2.26 |
| Delfan Plus | |||||||
| Annotation Cluster 1—Enrichment Score: 5.5 | |||||||
| Category | Term | % | p | FE | Bonf. | Benj. | FDR |
| GOTERM_BP_DIRECT | GO:0042542~response to hydrogen peroxide | 1.2 | 1.9 × 10−7 | 7.1 | 1.1 × 10−4 | 5.7 × 10−5 | 2.8 × 10−4 |
| GOTERM_BP_DIRECT | GO:0009408~response to heat | 2.0 | 2.0 × 10−6 | 3.7 | 0.001 | 4.2 × 10−4 | 0.003 |
| GOTERM_BP_DIRECT | GO:0009644~response to high light intensity | 1.0 | 8.6 × 10−5 | 5. 4 | 0.05 | 0.007 | 0.12 |
| Annotation Cluster 2—Enrichment Score: 4.5 | |||||||
| Category | Term | % | p | FE | Bonf. | Benj. | FDR |
| UP_SEQ_FEATURE | DNA-binding region:WRKY | 1.1 | 5.9 × 10−6 | 5.7 | 0.003 | 0.001 | 0.009 |
| SMART | SM00774:WRKY | 1.2 | 2.6 × 10−5 | 4.9 | 0.003 | 0.003 | 0.03 |
| INTERPRO | IPR003657:DNA-binding WRKY | 1.2 | 3.5 × 10−5 | 4.0 | 0.02 | 0.02 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocetta, G.; Landoni, M.; Pilu, R.; Repiso, C.; Nolasco, J.; Alajarin, M.; Ugena, L.; Levy, C.C.B.; Scatolino, G.; Villa, D.; et al. Priming Treatments with Biostimulants to Cope the Short-Term Heat Stress Response: A Transcriptomic Profile Evaluation. Plants 2022, 11, 1130. https://doi.org/10.3390/plants11091130
Cocetta G, Landoni M, Pilu R, Repiso C, Nolasco J, Alajarin M, Ugena L, Levy CCB, Scatolino G, Villa D, et al. Priming Treatments with Biostimulants to Cope the Short-Term Heat Stress Response: A Transcriptomic Profile Evaluation. Plants. 2022; 11(9):1130. https://doi.org/10.3390/plants11091130
Chicago/Turabian StyleCocetta, Giacomo, Michela Landoni, Roberto Pilu, Carlos Repiso, José Nolasco, Marcos Alajarin, Lydia Ugena, Camila C. B. Levy, Giacomo Scatolino, Daniele Villa, and et al. 2022. "Priming Treatments with Biostimulants to Cope the Short-Term Heat Stress Response: A Transcriptomic Profile Evaluation" Plants 11, no. 9: 1130. https://doi.org/10.3390/plants11091130
APA StyleCocetta, G., Landoni, M., Pilu, R., Repiso, C., Nolasco, J., Alajarin, M., Ugena, L., Levy, C. C. B., Scatolino, G., Villa, D., & Ferrante, A. (2022). Priming Treatments with Biostimulants to Cope the Short-Term Heat Stress Response: A Transcriptomic Profile Evaluation. Plants, 11(9), 1130. https://doi.org/10.3390/plants11091130










